Abstract
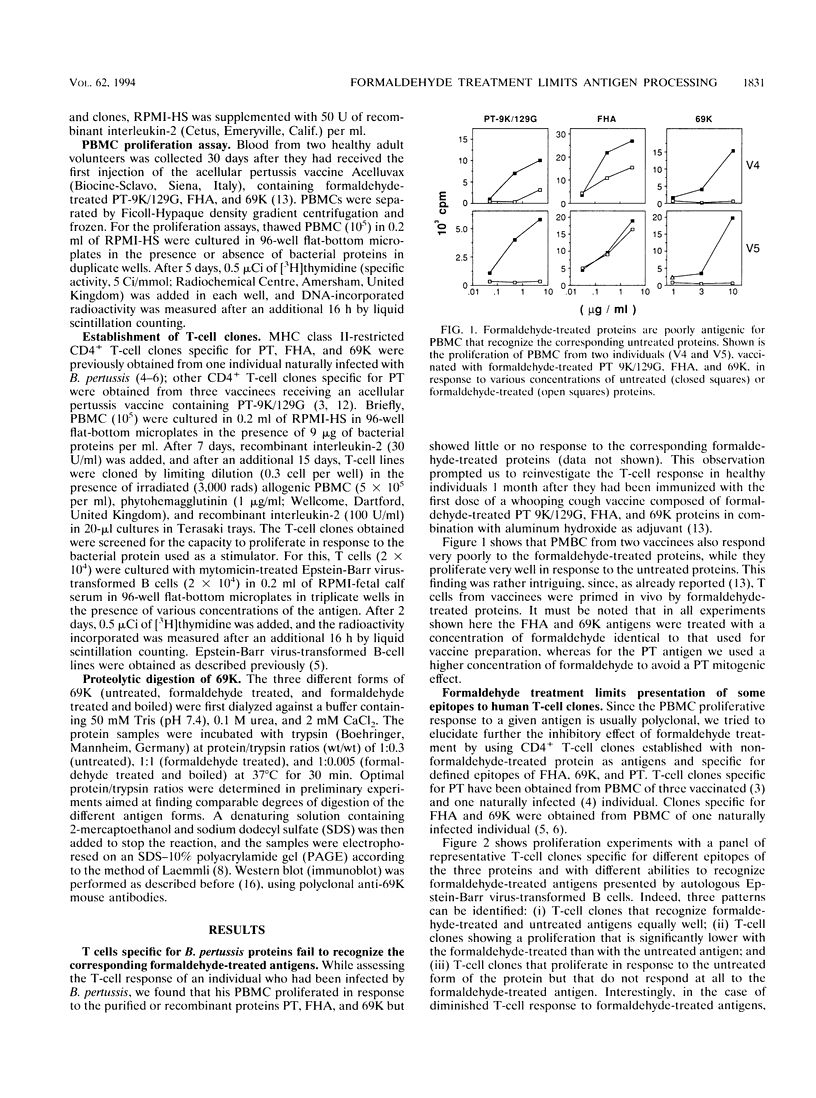
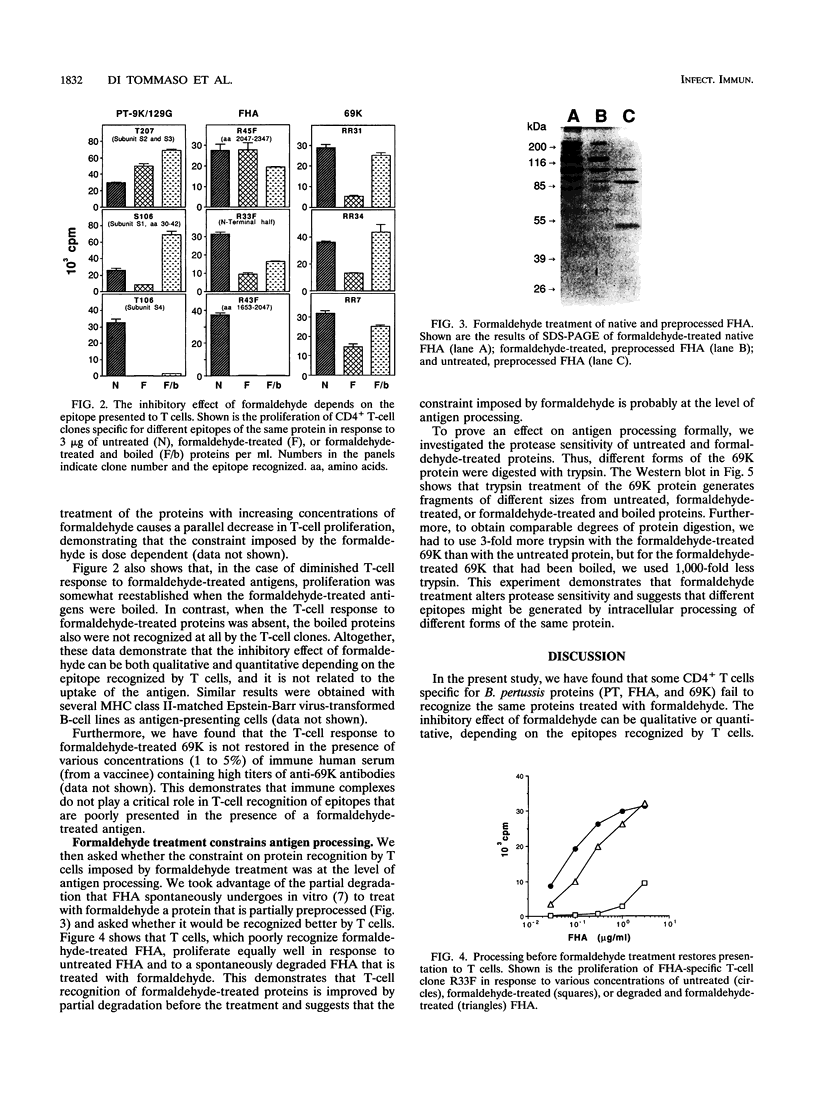
Proteins to be used as vaccines are frequently treated with formaldehyde, although little is known about the effects of this treatment on protein antigenicity. To investigate the effect of formaldehyde treatment on antigen recognition by T cells, we compared the in vitro T-cell response to proteins that have been formaldehyde treated with the response to untreated proteins. We found that peripheral blood mononuclear cells from individuals vaccinated with three formaldehyde-treated proteins (pertussis toxin, filamentous hemagglutinin, pertactin) of Bordetella pertussis showed little or no response to the formaldehyde-treated proteins but proliferated very well in response to the corresponding untreated protein. These findings were further confirmed with CD4+ T-cell clones specific for defined epitopes of the bacterial proteins. We found that some epitopes are presented poorly or not at all when formaldehyde-treated proteins are used, whereas other epitopes are equally presented to T-cell clones when either formaldehyde-treated or untreated antigens are used. However, T-cell recognition could be restored by either antigen degradation before formaldehyde treatment or heat denaturation after such treatment. Parallel digestion with trypsin of both formaldehyde-treated and untreated proteins showed that fragments generated from the two forms of the same antigen were different in size. These results demonstrate that formaldehyde treatment can constrain antigen presentation to T cells and that this may be due to an altered proteolytic processing of formaldehyde-treated proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botarelli P., Houlden B. A., Haigwood N. L., Servis C., Montagna D., Abrignani S. N-glycosylation of HIV-gp120 may constrain recognition by T lymphocytes. J Immunol. 1991 Nov 1;147(9):3128–3132. [PubMed] [Google Scholar]
- Champion B. R., Page K. R., Parish N., Rayner D. C., Dawe K., Biswas-Hughes G., Cooke A., Geysen M., Roitt I. M. Identification of a thyroxine-containing self-epitope of thyroglobulin which triggers thyroid autoreactive T cells. J Exp Med. 1991 Aug 1;174(2):363–370. doi: 10.1084/jem.174.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris M. T., Di Tommaso A., Domenighini M., Censini S., Tagliabue A., Oksenberg J. R., Steinman L., Judd A. K., O'Sullivan D., Rappuoli R. Interaction of the pertussis toxin peptide containing residues 30-42 with DR1 and the T-cell receptors of 12 human T-cell clones. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2990–2994. doi: 10.1073/pnas.89.7.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris M. T., Romano M., Bartoloni A., Rappuoli R., Tagliabue A. Human T cell clones define S1 subunit as the most immunogenic moiety of pertussis toxin and determine its epitope map. J Exp Med. 1989 May 1;169(5):1519–1532. doi: 10.1084/jem.169.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris M. T., Romano M., Nuti S., Rappuoli R., Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988 Oct 1;168(4):1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso A., Domenighini M., Bugnoli M., Tagliabue A., Rappuoli R., De Magistris M. T. Identification of subregions of Bordetella pertussis filamentous hemagglutinin that stimulate human T-cell responses. Infect Immun. 1991 Sep;59(9):3313–3315. doi: 10.1128/iai.59.9.3313-3315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighini M., Relman D., Capiau C., Falkow S., Prugnola A., Scarlato V., Rappuoli R. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol Microbiol. 1990 May;4(5):787–800. doi: 10.1111/j.1365-2958.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nencioni L., Volpini G., Peppoloni S., Bugnoli M., De Magistris T., Marsili I., Rappuoli R. Properties of pertussis toxin mutant PT-9K/129G after formaldehyde treatment. Infect Immun. 1991 Feb;59(2):625–630. doi: 10.1128/iai.59.2.625-630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza M., Covacci A., Bartoloni A., Perugini M., Nencioni L., De Magistris M. T., Villa L., Nucci D., Manetti R., Bugnoli M. Mutants of pertussis toxin suitable for vaccine development. Science. 1989 Oct 27;246(4929):497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- Podda A., Nencioni L., De Magistris M. T., Di Tommaso A., Bossù P., Nuti S., Pileri P., Peppoloni S., Bugnoli M., Ruggiero P. Metabolic, humoral, and cellular responses in adult volunteers immunized with the genetically inactivated pertussis toxin mutant PT-9K/129G. J Exp Med. 1990 Sep 1;172(3):861–868. doi: 10.1084/jem.172.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda A., Nencioni L., Marsili I., Peppoloni S., Volpini G., Donati D., Di Tommaso A., De Magistris M. T., Rappuoli R. Phase I clinical trial of an acellular pertussis vaccine composed of genetically detoxified pertussis toxin combined with FHA and 69 kDa. Vaccine. 1991 Oct;9(10):741–745. doi: 10.1016/0264-410x(91)90290-m. [DOI] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Vidard L., Rock K. L., Benacerraf B. Diversity in MHC class II ovalbumin T cell epitopes generated by distinct proteases. J Immunol. 1992 Jul 15;149(2):498–504. [PubMed] [Google Scholar]