Abstract
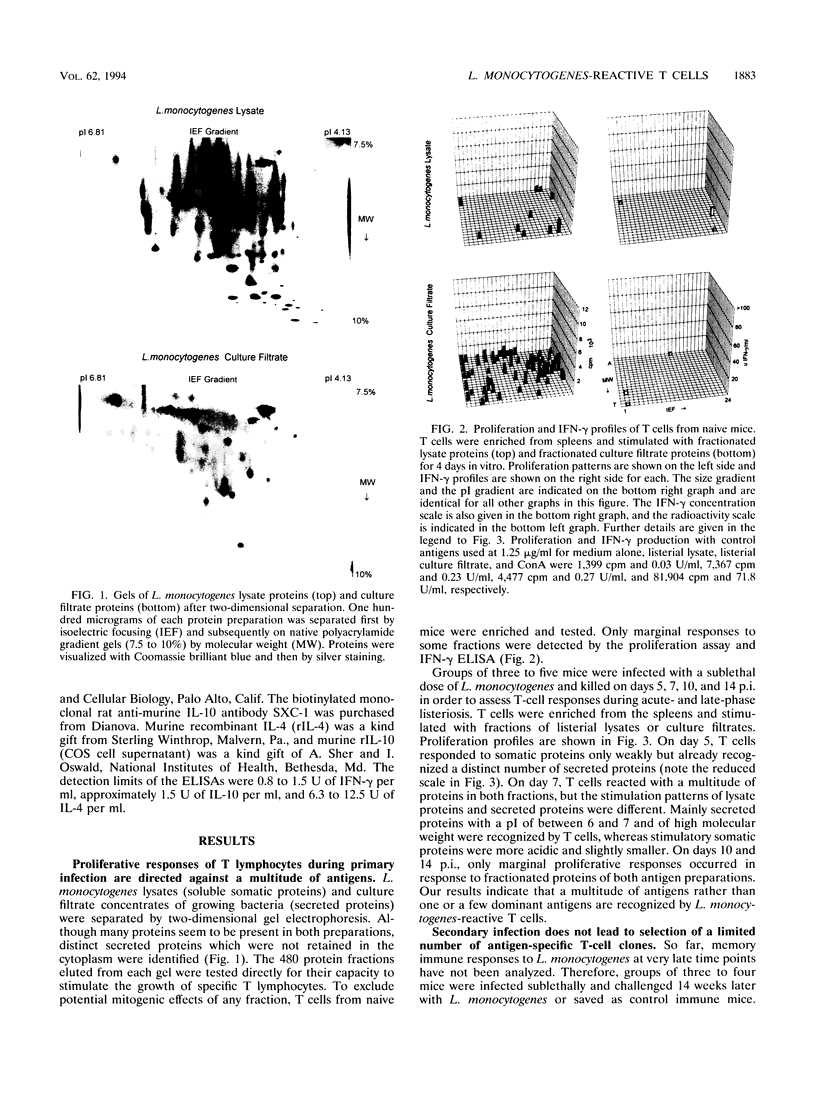
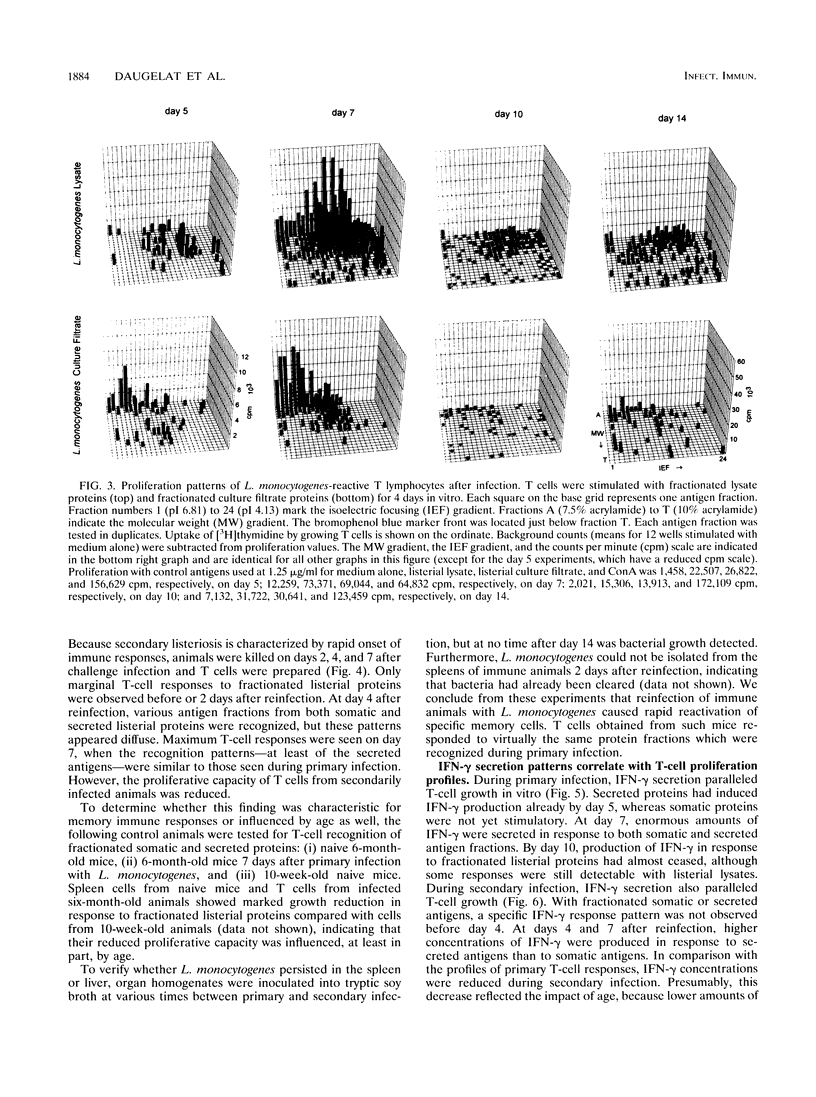
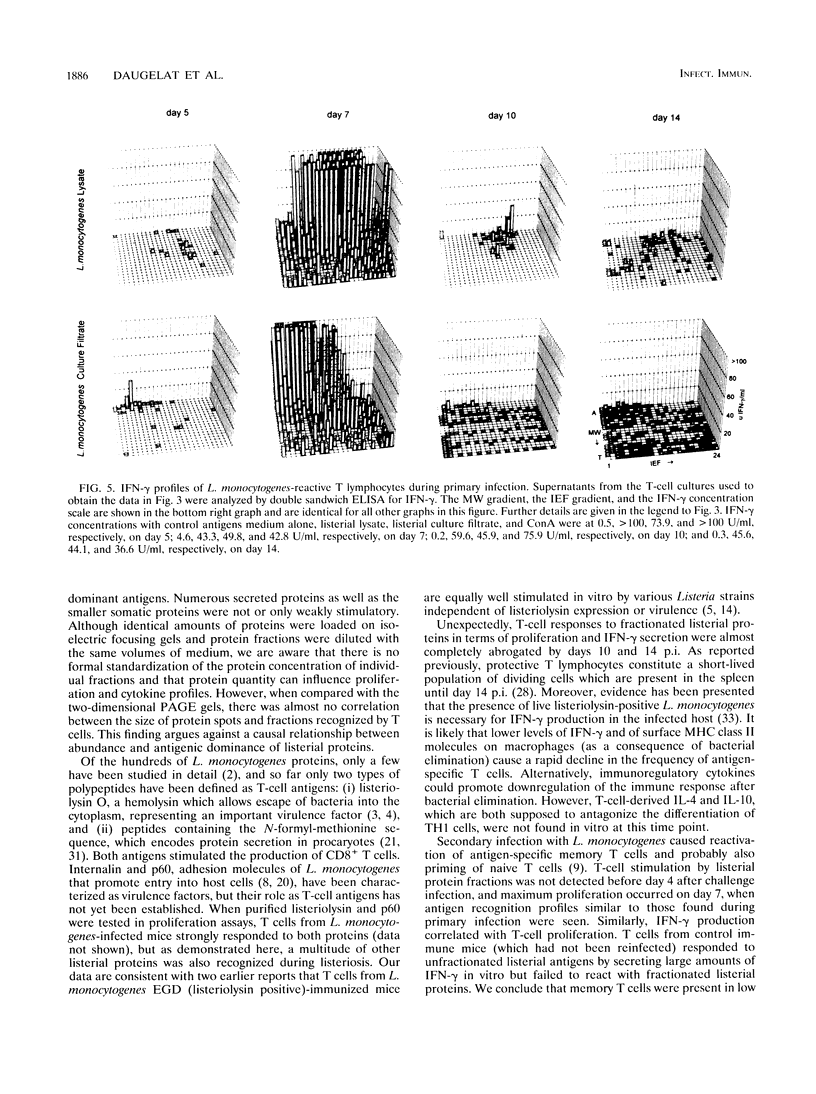
Although murine listeriosis is a widely used experimental model for the analysis of cell-mediated immunity, there is little information about individual T-cell antigens of Listeria monocytogenes which are recognized during primary and secondary infection. To study the antigen responses of L. monocytogenes-reactive T cells, somatic and secreted listerial proteins were separated by two-dimensional gel electrophoresis and subsequently divided into 480 liquid fractions. Antigen-specific T cells isolated from mice at different times of primary and secondary listeriosis were tested for their capacity to proliferate with distinct protein fractions. Supernatants of these cultures were screened for the production of gamma interferon, interleukin-4 (IL-4), and IL-10. Proliferation of antigen-specific T cells correlated with the production of high concentrations of gamma interferon, whereas IL-4 and IL-10 production in response to listerial protein fractions could not be detected. During both primary and secondary listeriosis, T cells recognized a multitude of somatic and secreted proteins rather than one or a few dominant antigens. Secreted proteins were recognized before somatic proteins, and T cells recognized different fractions in secreted and somatic proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993 Aug;5(4):503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- Beattie I. A., Swaminathan B., Ziegler H. K. Cloning and characterization of T-cell-reactive protein antigens from Listeria monocytogenes. Infect Immun. 1990 Sep;58(9):2792–2803. doi: 10.1128/iai.58.9.2792-2803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Geoffroy C., Alouf J. E. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987 Dec 1;139(11):3813–3821. [PubMed] [Google Scholar]
- Bouwer H. G., Nelson C. S., Gibbins B. L., Portnoy D. A., Hinrichs D. J. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J Exp Med. 1992 Jun 1;175(6):1467–1471. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke S., Chakraborty T., Mohasseb I., Reichert H., Lombardi O., Hahn H., Mielke M. Protective immunity and granulomatous inflammation is mediated in vivo by T cells reactive to epitopes common to avirulent and listeriolysin-negative mutants of Listeria monocytogenes. Cell Immunol. 1992 Mar;140(1):42–53. doi: 10.1016/0008-8749(92)90175-o. [DOI] [PubMed] [Google Scholar]
- Daugelat S., Gulle H., Schoel B., Kaufmann S. H. Secreted antigens of Mycobacterium tuberculosis: characterization with T lymphocytes from patients and contacts after two-dimensional separation. J Infect Dis. 1992 Jul;166(1):186–190. doi: 10.1093/infdis/166.1.186. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Mielke M. E., Blankenstein T., Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992 Nov 1;149(9):3016–3022. [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Frehel C., Gouin E., Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991 Jun 28;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Gray D. Immunological memory: a function of antigen persistence. Trends Microbiol. 1993 May;1(2):39–42. doi: 10.1016/0966-842x(93)90026-n. [DOI] [PubMed] [Google Scholar]
- Gulle H., Schoel B., Chiplunkar S., Gangal S., Deo M. G., Kaufmann S. H. T-cell responses of leprosy patients and healthy contacts toward separated protein antigens of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1992 Mar;60(1):44–53. [PubMed] [Google Scholar]
- Gulle H., Schoel B., Kaufmann S. H. Direct blotting with viable cells of protein mixtures separated by two-dimensional gel electrophoresis. J Immunol Methods. 1990 Oct 19;133(2):253–261. doi: 10.1016/0022-1759(90)90366-4. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hess J., Kaufmann S. H. Vaccination strategies against intracellular microbes. FEMS Immunol Med Microbiol. 1993 Aug;7(2):95–103. doi: 10.1111/j.1574-695X.1993.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Acquired resistance to facultative intracellular bacteria: relationship between persistence, cross-reactivity at the T-cell level, and capacity to stimulate cellular immunity of different Listeria strains. Infect Immun. 1984 Jul;45(1):234–241. doi: 10.1128/iai.45.1.234-241.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Enumeration of Listeria monocytogenes-reactive L3T4+ T cells activated during infection. Microb Pathog. 1986 Jun;1(3):249–260. doi: 10.1016/0882-4010(86)90049-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Berger R., Kirchner H. Interferon-gamma production by Listeria monocytogenes-specific T cells active in cellular antibacterial immunity. Eur J Immunol. 1983 Mar;13(3):265–268. doi: 10.1002/eji.1830130318. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Regulatory interactions between macrophages and T-cell subsets in Listeria monocytogenes-specific T-cell activation. Infect Immun. 1982 Dec;38(3):907–913. doi: 10.1128/iai.38.3.907-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongshavn P. A. Genetic control of resistance to Listeria infection. Curr Top Microbiol Immunol. 1986;124:67–85. doi: 10.1007/978-3-642-70986-9_5. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989 Jan;57(1):55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlander R. J., Shawar S. M., Brown M. L., Rich R. R. Specialized role for a murine class I-b MHC molecule in prokaryotic host defenses. Science. 1992 Jul 31;257(5070):678–679. doi: 10.1126/science.1496381. [DOI] [PubMed] [Google Scholar]
- Locksley R. M. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Niedobitek G., Stein H., Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989 Aug 1;170(2):589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Arnoldi J., Russ F., Tonegawa S., Kaufmann S. H. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993 Sep 2;365(6441):53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kohanawa M., Chen Y., Sato H., Moriyama M., Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989 Nov;57(11):3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Berche P. A., Newborg M. F. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J Immunol. 1981 Jul;127(1):342–346. [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Umland S., De France T., Christiansen J. 'B-cell factors' are pleiotropic. Immunol Today. 1988 Feb;9(2):45–54. doi: 10.1016/0167-5699(88)91259-5. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Andersen P., Boom W. H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993 Jun;167(6):1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- Pamer E. G., Wang C. R., Flaherty L., Lindahl K. F., Bevan M. J. H-2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992 Jul 24;70(2):215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A. Innate immunity to a facultative intracellular bacterial pathogen. Curr Opin Immunol. 1992 Feb;4(1):20–24. doi: 10.1016/0952-7915(92)90118-x. [DOI] [PubMed] [Google Scholar]
- Poston R. M., Kurlander R. J. Cytokine expression in vivo during murine listeriosis. Infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J Immunol. 1992 Nov 1;149(9):3040–3044. [PubMed] [Google Scholar]
- Prat M., Gribaudo G., Comoglio P. M., Cavallo G., Landolfo S. Monoclonal antibodies against murine gamma interferon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoel B., Gulle H., Kaufmann S. H. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts to Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect Immun. 1992 Apr;60(4):1717–1720. doi: 10.1128/iai.60.4.1717-1720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]



