Abstract
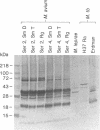
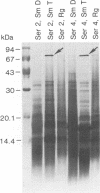
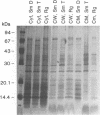
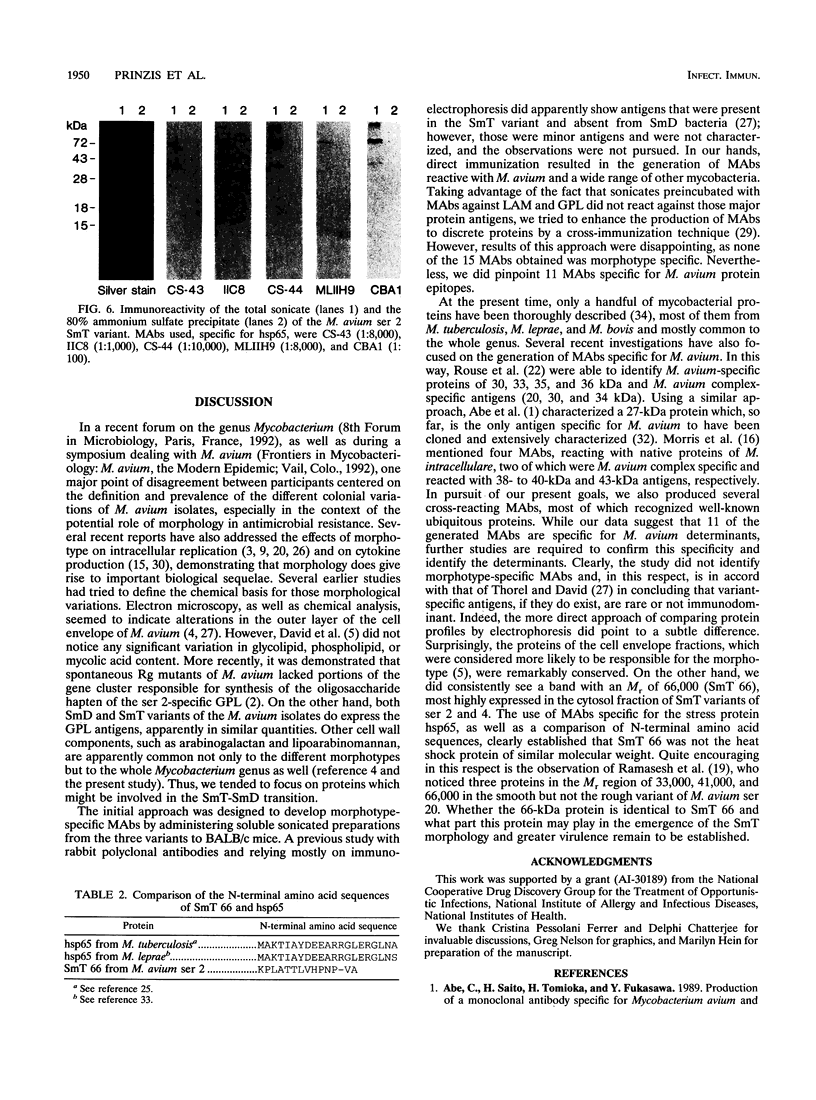
Isolates of Mycobacterium avium exhibit three different colonial variations: smooth domed (SmD), smooth transparent (SmT), and rough (Rg). Because the discrimination between morphotypes is founded on morphological rather than molecular principles and because of the absence of consensus over the relevance of morphology to pathogenesis and drug sensitivity, a comparative study at the protein level was undertaken. By direct immunization of BALB/c mice with the soluble sonicate of one of the morphotypes of M. avium serovar 2, eight monoclonal antibodies (MAbs) were identified, of which one was M. avium specific. Cross immunization of syngeneic mice with serum-absorbed antigens allowed the generation of 15 further MAbs; 11 were M. avium or M. avium complex specific, but none of them was morphotype specific. Subcellular fractions analyzed by electrophoresis showed similar profiles, with the exception of a cytosolic protein with a relative molecular mass of ca. 66 kDa (protein SmT 66), which was most highly expressed in SmT variants of M. avium serotypes 2 and 4. Because a well-known, ubiquitous stress-heat shock protein (hsp65) has a similar molecular mass, protein SmT 66 was compared with hsp65. Western blot (immunoblot) analyses using several cross-reacting MAbs and N-terminal amino acid sequencing established that this protein was not the ubiquitous stress protein. Thus, SmT 66 is the first product to be described which might be associated with the SmT morphotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belisle J. T., Pascopella L., Inamine J. M., Brennan P. J., Jacobs W. R., Jr Isolation and expression of a gene cluster responsible for biosynthesis of the glycopeptidolipid antigens of Mycobacterium avium. J Bacteriol. 1991 Nov;173(21):6991–6997. doi: 10.1128/jb.173.21.6991-6997.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffe M., Brennan P. J., McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J Biol Chem. 1990 Apr 25;265(12):6734–6743. [PubMed] [Google Scholar]
- David H. L., Rastogi N., Clavel-Sérès S., Clément F., Thorel M. F. Structure of the cell envelope of Mycobacterium avium. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Apr;264(1-2):49–66. doi: 10.1016/s0176-6724(87)80124-4. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Goldberger M. J., Parenti D. M. Mycobacterium avium infection and AIDS: a therapeutic dilemma in rapid evolution. J Infect Dis. 1991 Jun;163(6):1326–1335. doi: 10.1093/infdis/163.6.1326. [DOI] [PubMed] [Google Scholar]
- Elsaghier A., Nolan A., Allen B., Ivanyi J. Distinctive western blot antibody patterns induced by infection of mice with individual strains of the Mycobacterium avium complex. Immunology. 1992 Jul;76(3):355–361. [PMC free article] [PubMed] [Google Scholar]
- Furney S. K., Skinner P. S., Roberts A. D., Appelberg R., Orme I. M. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect Immun. 1992 Oct;60(10):4410–4413. doi: 10.1128/iai.60.10.4410-4413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfield G. R., McNeil M., Brennan P. J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990 Feb;172(2):1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Khanolkar-Young S., Kolk A. H., Andersen A. B., Bennedsen J., Brennan P. J., Rivoire B., Kuijper S., McAdam K. P., Abe C., Batra H. V. Results of the third immunology of leprosy/immunology of tuberculosis antimycobacterial monoclonal antibody workshop. Infect Immun. 1992 Sep;60(9):3925–3927. doi: 10.1128/iai.60.9.3925-3927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meylan P. R., Richman D. D., Kornbluth R. S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect Immun. 1990 Aug;58(8):2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini-Norris M. B., Blanchard D. K., Pearson C. A., Djeu J. Y. Differential release of interleukin (IL)-1 alpha, IL-1 beta, and IL-6 from normal human monocytes stimulated with a virulent and an avirulent isogenic variant of Mycobacterium avium-intracellulare complex. J Infect Dis. 1992 Apr;165(4):702–709. doi: 10.1093/infdis/165.4.702. [DOI] [PubMed] [Google Scholar]
- Morris S. L., Rouse D. A., Hussong D., Chaparas S. D. Isolation and characterization of a recombinant lambda gt11 bacteriophage which expresses an immunoreactive Mycobacterium intracellulare protein in Escherichia coli. Infect Immun. 1988 Dec;56(12):3026–3031. doi: 10.1128/iai.56.12.3026-3031.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S. D., Byrd L. T., Southern P. M., Jockusch J. D., Cal S. X., Wynne B. A. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992 Jun;165(6):1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ramasesh N., Wright E. L., Barrow W. W. Cell-free system responsible for internal radiolabeling of glycopeptidolipids of the Mycobacterium avium complex. Infect Immun. 1992 Jan;60(1):308–311. doi: 10.1128/iai.60.1.308-311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Levy-Frebault V., Blom-Potar M. C., David H. L. Ability of smooth and rough variants of Mycobacterium avium and M. intracellulare to multiply and survive intracellularly: role of C-mycosides. Zentralbl Bakteriol Mikrobiol Hyg A. 1989 Jan;270(3):345–360. doi: 10.1016/s0176-6724(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Rivoire B., Ranchoff B. J., Chatterjee D., Gaylord H., Tsang A. Y., Kolk A. H., Aspinall G. O., Brennan P. J. Generation of monoclonal antibodies to the specific sugar epitopes of Mycobacterium avium complex serovars. Infect Immun. 1989 Oct;57(10):3147–3158. doi: 10.1128/iai.57.10.3147-3158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D. A., Morris S. L., Karpas A. B., Probst P. G., Chaparas S. D. Production, characterization, and species specificity of monoclonal antibodies to Mycobacterium avium complex protein antigens. Infect Immun. 1990 May;58(5):1445–1449. doi: 10.1128/iai.58.5.1445-1449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Sato K., Tasaka H., Dawson D. J. Identification of various serovar strains of Mycobacterium avium complex by using DNA probes specific for Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol. 1990 Aug;28(8):1694–1697. doi: 10.1128/jcm.28.8.1694-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Toba H., Ellner J. J. Strain- and donor-related differences in the interaction of Mycobacterium avium with human monocytes and its modulation by interferon-gamma. J Infect Dis. 1990 Oct;162(4):932–938. doi: 10.1093/infdis/162.4.932. [DOI] [PubMed] [Google Scholar]
- Thorel M. F., David H. L. Specific surface antigens of SmT variants of Mycobacterium avium. Infect Immun. 1984 Jan;43(1):438–439. doi: 10.1128/iai.43.1.438-439.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba H., Crawford J. T., Ellner J. J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of gamma interferon. Infect Immun. 1989 Jan;57(1):239–244. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Ellner J. J., Shiratsuchi H. Macrophages, mycobacteria and HIV: the role of cytokines in determining mycobacterial virulence and regulating viral replication. Res Microbiol. 1992 May;143(4):398–405. doi: 10.1016/0923-2508(92)90053-q. [DOI] [PubMed] [Google Scholar]
- Williams C. V., Stechmann C. L., McLoon S. C. Subtractive immunization techniques for the production of monoclonal antibodies to rare antigens. Biotechniques. 1992 Jun;12(6):842–847. [PubMed] [Google Scholar]
- Yajko D. M. In vitro activity of antimicrobial agents against the Mycobacterium avium complex inside macrophages from HIV1-infected individuals: the link to clinical response to treatment? Res Microbiol. 1992 May;143(4):411–419. doi: 10.1016/0923-2508(92)90055-s. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Takahashi M., Fukasawa Y., Wada M., Abe C. Cloning and expression of the gene for the Avi-3 antigen of Mycobacterium avium and mapping of its epitopes. Infect Immun. 1992 Mar;60(3):1210–1216. doi: 10.1128/iai.60.3.1210-1216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]