Abstract
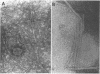
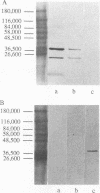
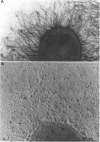
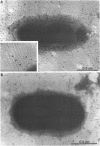
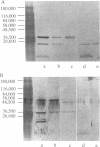
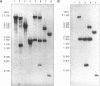
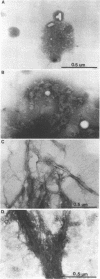
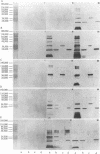
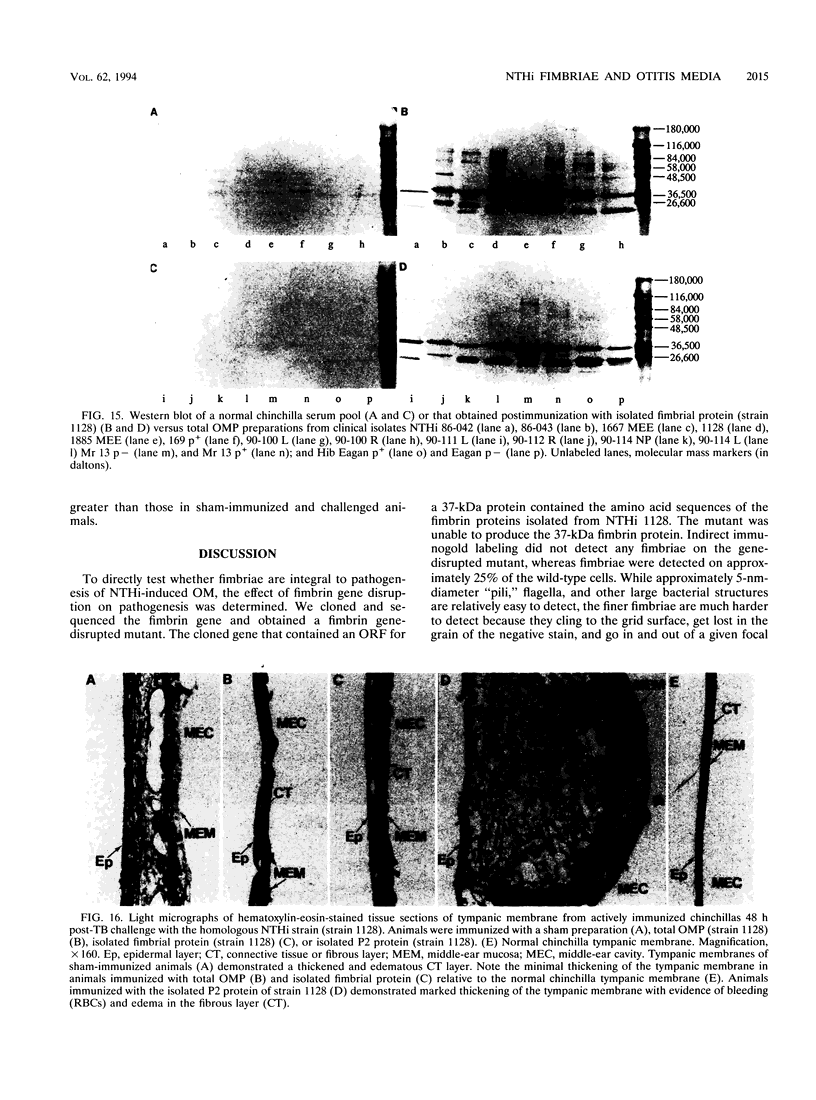
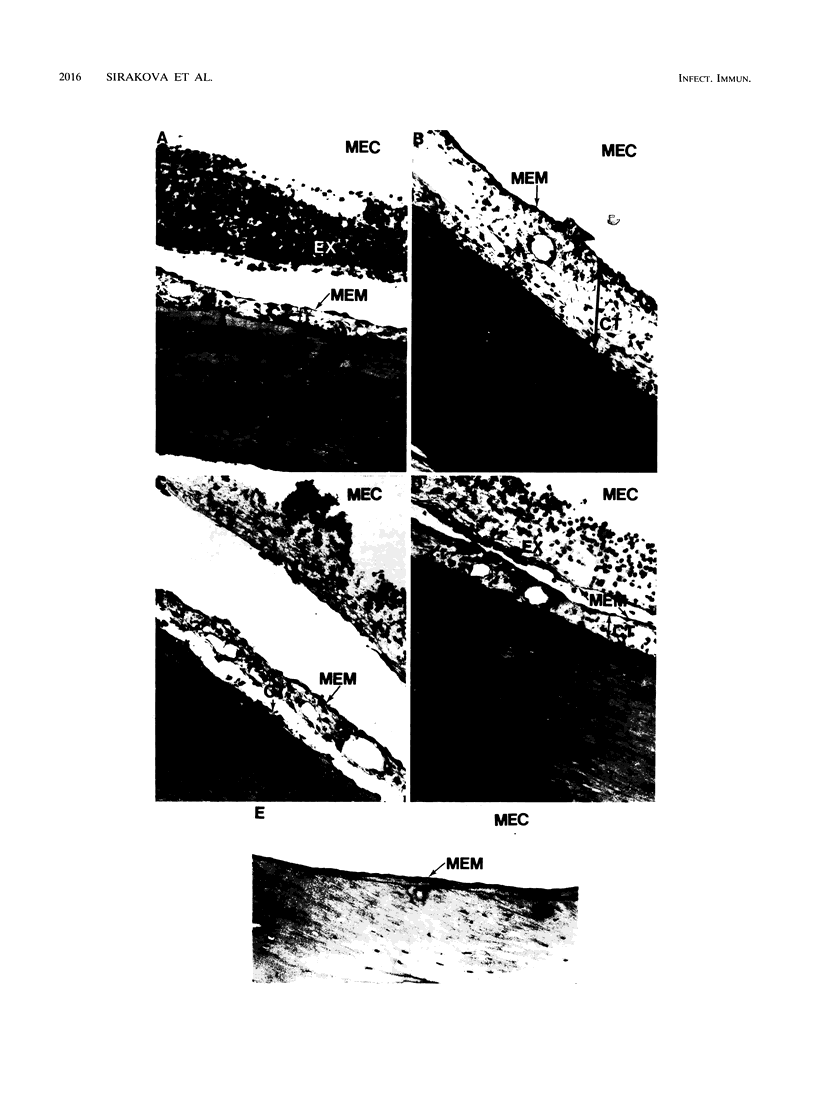
Nontypeable Haemophilus influenzae is a primary pathogen in both acute otitis media (OM) and chronic OM, yet the pathogenesis of this disease is not fully understood. Although fimbriae have been observed on all clinical OM isolates examined to date, their role in pathogenesis remains unclear. Therefore, the gene which codes for the fimbrial subunit protein (fimbrin) in nontypeable H. influenzae 1128 was isolated, cloned, and sequenced. The nucleotide sequence of the fimbrin gene was found to contain an open reading frame of 1,077 bp which would encode a mature fimbrin protein consisting of 338 amino acid with a calculated molecular mass of 36.4 kDa. The translated amino acid sequence was found to be homologous with various OmpA proteins of other gram-negative bacteria, and algorithmic analysis predicted that this protein is organized as a coiled coil. To directly test whether fimbriae are involved in pathogenesis, the fimbrin gene was disrupted, and the biological consequences of disruption were absence of both expression of the fimbrial appendage and the specific immunogold labeling thereof with antisera directed against isolated fimbrial protein, reduced adherence to human oropharyngeal cells in vitro, augmented clearance from the tympanum post-transbullar inoculation, and significantly reduced induction of OM post-intranasal inoculation in a chinchilla model compared with the fimbriated parent strain. We additionally find that either passive immunization or active immunization against isolated fimbrial protein confers partial protection against transbullar challenge. A Western blot (immunoblot) indicated a degree of serological relatedness among fimbrin proteins of 15 nontypeable and type b isolates. These data suggest that fimbrin could be useful as a component of a vaccine to protect against OM.
Full text
PDF






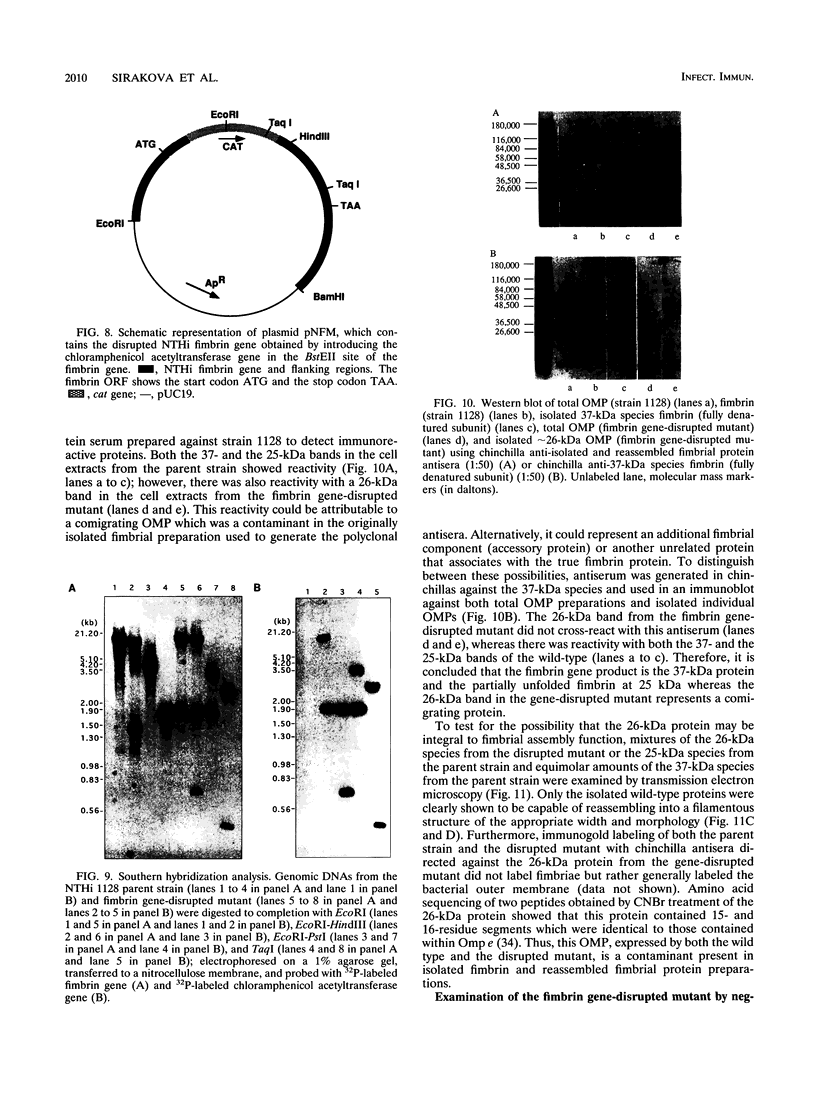
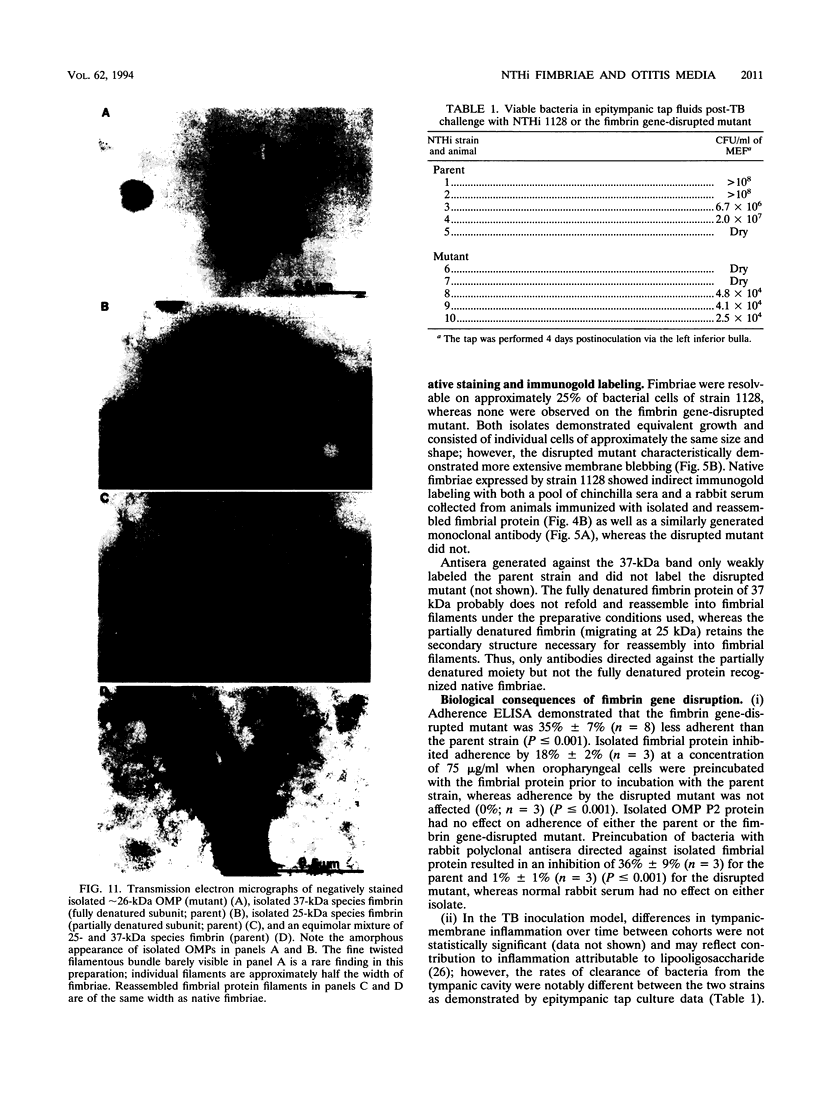
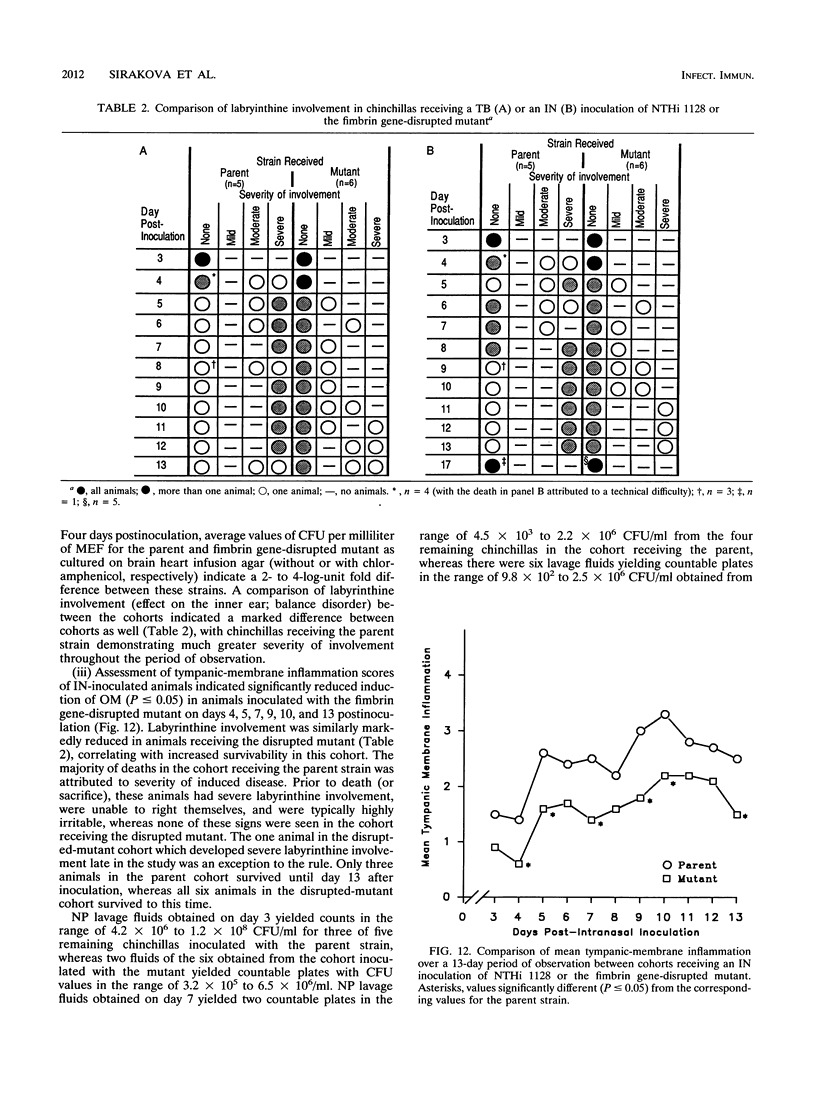
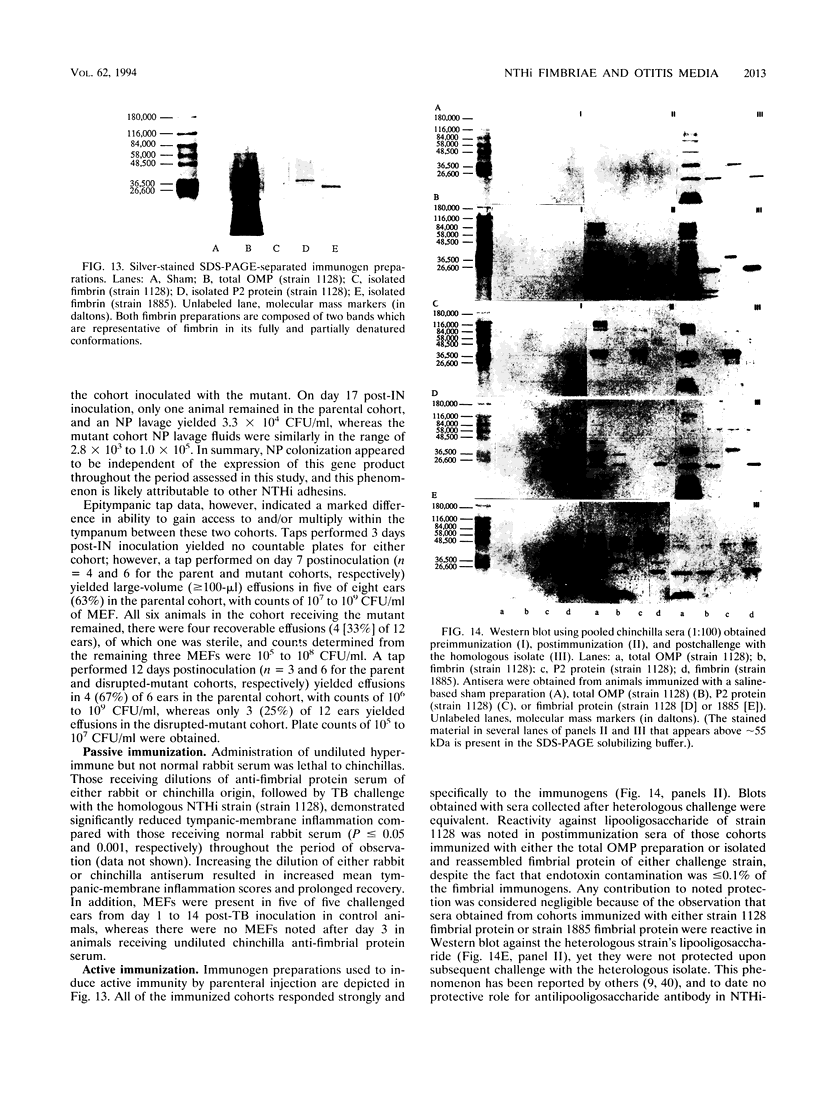





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfa M. J., Degagne P., Hollyer T. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect Immun. 1993 May;61(5):1735–1742. doi: 10.1128/iai.61.5.1735-1742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz L. O., Ahmed M. A., Kolattukudy P. E., Lim D. J., Forney L. J. Cloning and sequence analysis of a pilin-like gene from an otitis media isolate of nontypeable Haemophilus influenzae. J Infect Dis. 1992 Jun;165 (Suppl 1):S201–S203. doi: 10.1093/infdis/165-supplement_1-s201. [DOI] [PubMed] [Google Scholar]
- Bakaletz L. O., Tallan B. M., Andrzejewski W. J., DeMaria T. F., Lim D. J. Immunological responsiveness of chinchillas to outer membrane and isolated fimbrial proteins of nontypeable Haemophilus influenzae. Infect Immun. 1989 Oct;57(10):3226–3229. doi: 10.1128/iai.57.10.3226-3229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz L. O., Tallan B. M., Hoepf T., DeMaria T. F., Birck H. G., Lim D. J. Frequency of fimbriation of nontypable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect Immun. 1988 Feb;56(2):331–335. doi: 10.1128/iai.56.2.331-335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J. Protection by serum antibodies in experimental nontypable Haemophilus influenzae otitis media. Infect Immun. 1986 May;52(2):572–578. doi: 10.1128/iai.52.2.572-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A., Pugsley A. P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980 Aug;143(2):906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Gotschlich E. C. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and the role of protein II. Infect Immun. 1986 Oct;54(1):154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun G., Cole S. T. DNA sequence analysis of the Serratia marcescens ompA gene: implications for the organisation of an enterobacterial outer membrane protein. Mol Gen Genet. 1984;195(1-2):321–328. doi: 10.1007/BF00332766. [DOI] [PubMed] [Google Scholar]
- Braun G., Cole S. T. Molecular characterization of the gene coding for major outer membrane protein OmpA from Enterobacter aerogenes. Eur J Biochem. 1983 Dec 15;137(3):495–500. doi: 10.1111/j.1432-1033.1983.tb07853.x. [DOI] [PubMed] [Google Scholar]
- Braun G., Cole S. T. The nucleotide sequence coding for major outer membrane protein OmpA of Shigella dysenteriae. Nucleic Acids Res. 1982 Apr 10;10(7):2367–2378. doi: 10.1093/nar/10.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Cole S. T., Hindennach I., Henning U., Beck E., Kurz C., Schaller H. Export of a protein into the outer membrane of Escherichia coli K12. Stable incorporation of the OmpA protein requires less than 193 amino-terminal amino-acid residues. Eur J Biochem. 1982 Feb;122(1):223–231. doi: 10.1111/j.1432-1033.1982.tb05870.x. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr, Carter M. J., Derber D. B., Kar S., Kramarik J. A., To A. C., To S. C., Wood S. W. Design and development of pilus vaccines for Haemophilus influenzae diseases. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S54–S61. [PubMed] [Google Scholar]
- Carlone G. M., Thomas M. L., Rumschlag H. S., Sottnek F. O. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986 Sep;24(3):330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandad F., Mouton C. Molecular size variation of the hemagglutinating adhesin HA-Ag2, a common antigen of Bacteroides gingivalis. Can J Microbiol. 1990 Oct;36(10):690–696. doi: 10.1139/m90-117. [DOI] [PubMed] [Google Scholar]
- Chanyangam M., Smith A. L., Moseley S. L., Kuehn M., Jenny P. Contribution of a 28-kilodalton membrane protein to the virulence of Haemophilus influenzae. Infect Immun. 1991 Feb;59(2):600–608. doi: 10.1128/iai.59.2.600-608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria T. F., Yamaguchi T., Bakaletz L. O., Lim D. J. Serum and middle ear antibody response in the chinchilla during otitis media with effusion induced by nonviable nontypeable Haemophilus influenzae. J Infect Dis. 1992 Jun;165 (Suppl 1):S196–S197. doi: 10.1093/infdis/165-supplement_1-s196. [DOI] [PubMed] [Google Scholar]
- DeMaria T. F., Yamaguchi T., Lim D. J. Quantitative cytologic and histologic changes in the middle ear after the injection of nontypable Hemophilus influenzae endotoxin. Am J Otolaryngol. 1989 Jul-Aug;10(4):261–266. doi: 10.1016/0196-0709(89)90006-9. [DOI] [PubMed] [Google Scholar]
- Deslauriers M., Mouton C. Epitope mapping of hemagglutinating adhesion HA-Ag2 of Bacteroides (Porphyromonas) gingivalis. Infect Immun. 1992 Jul;60(7):2791–2799. doi: 10.1128/iai.60.7.2791-2799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Gilsdorf J. R., Wong D. C. Effect of pili-specific antibodies on the adherence of Haemophilus influenzae type b to human buccal cells. J Infect Dis. 1992 Mar;165(3):464–470. doi: 10.1093/infdis/165.3.464. [DOI] [PubMed] [Google Scholar]
- Freudl R., Cole S. T. Cloning and molecular characterization of the ompA gene from Salmonella typhimurium. Eur J Biochem. 1983 Aug 15;134(3):497–502. doi: 10.1111/j.1432-1033.1983.tb07594.x. [DOI] [PubMed] [Google Scholar]
- Gentry-Weeks C. R., Hultsch A. L., Kelly S. M., Keith J. M., Curtiss R., 3rd Cloning and sequencing of a gene encoding a 21-kilodalton outer membrane protein from Bordetella avium and expression of the gene in Salmonella typhimurium. J Bacteriol. 1992 Dec;174(23):7729–7742. doi: 10.1128/jb.174.23.7729-7742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., Chang H. Y., McCrea K. W., Bakaletz L. O. Comparison of hemagglutinating pili of Haemophilus influenzae type b with similar structures of nontypeable H. influenzae. Infect Immun. 1992 Feb;60(2):374–379. doi: 10.1128/iai.60.2.374-379.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorby G. L., Schaefer G. B. Effect of attachment factors (pili plus Opa) on Neisseria gonorrhoeae invasion of human fallopian tube tissue in vitro: quantitation by computerized image analysis. Microb Pathog. 1992 Aug;13(2):93–108. doi: 10.1016/0882-4010(92)90070-5. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C. Conserved gonococcal surface antigens. Ann Sclavo Collana Monogr. 1986;3(1-2):415–426. [PubMed] [Google Scholar]
- Green B. A., Farley J. E., Quinn-Dey T., Deich R. A., Zlotnick G. W. The e (P4) outer membrane protein of Haemophilus influenzae: biologic activity of anti-e serum and cloning and sequencing of the structural gene. Infect Immun. 1991 Sep;59(9):3191–3198. doi: 10.1128/iai.59.9.3191-3198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes L. M., Hoekstra W. P. Characterization of an Escherichia coli K-12 F-Con-mutant. J Bacteriol. 1976 May;126(2):593–600. doi: 10.1128/jb.126.2.593-600.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Cole S. T., Bremer E., Hindennach I., Schaller H. Gene fusions using the ompA gene coding for a major outer-membrane protein of Escherichia coli K12. Eur J Biochem. 1983 Nov 2;136(2):233–240. doi: 10.1111/j.1432-1033.1983.tb07732.x. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. A highly conserved region present in transcripts encoding heterologous M proteins of group A streptococci. Infect Immun. 1987 Dec;55(12):3237–3239. doi: 10.1128/iai.55.12.3237-3239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Karasic R. B., Trumpp C. E., Gnehm H. E., Rice P. A., Pelton S. I. Modification of otitis media in chinchillas rechallenged with nontypable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis. 1985 Feb;151(2):273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- Kuehn M., Lent K., Haas J., Hagenzieker J., Cervin M., Smith A. L. Fimbriation of Pseudomonas cepacia. Infect Immun. 1992 May;60(5):2002–2007. doi: 10.1128/iai.60.5.2002-2007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. G., Ochman H., Hartl D. L. Molecular and evolutionary relationships among enteric bacteria. J Gen Microbiol. 1991 Aug;137(8):1911–1921. doi: 10.1099/00221287-137-8-1911. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Sojar H. T., Bedi G. S., Genco R. J. Porphyromonas (Bacteroides) gingivalis fimbrillin: size, amino-terminal sequence, and antigenic heterogeneity. Infect Immun. 1991 Jan;59(1):383–389. doi: 10.1128/iai.59.1.383-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Sojar H. T., Bedi G. S., Genco R. J. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992 Apr;60(4):1662–1670. doi: 10.1128/iai.60.4.1662-1670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mouton C., Bouchard D., Deslauriers M., Lamonde L. Immunochemical identification and preliminary characterization of a nonfimbrial hemagglutinating adhesin of Bacteroides gingivalis. Infect Immun. 1989 Feb;57(2):566–573. doi: 10.1128/iai.57.2.566-573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Ni Eidhin D., Deslauriers M., Lamy L. The hemagglutinating adhesin HA-Ag2 of Bacteroides gingivalis is distinct from fimbrilin. Oral Microbiol Immunol. 1991 Feb;6(1):6–11. doi: 10.1111/j.1399-302x.1991.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Grass S., West R. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect Immun. 1993 Sep;61(9):4017–4020. doi: 10.1128/iai.61.9.4017-4020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H., Jaffé A., Imamura R., Ogura T., Hiraga S. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991 Jan;10(1):183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Courtney H. S., Schifferli D. M., Beachey E. H. Enzyme-linked immunosorbent assay for adherence of bacteria to animal cells. J Clin Microbiol. 1986 Oct;24(4):512–516. doi: 10.1128/jcm.24.4.512-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1977 Mar;129(3):1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola S. M., Castellazzo A., Shero M., Apicella M. A. Characterization of pili expressed by Haemophilus ducreyi. Microb Pathog. 1990 Dec;9(6):417–426. doi: 10.1016/0882-4010(90)90060-4. [DOI] [PubMed] [Google Scholar]
- Spinola S. M., Griffiths G. E., Shanks K. L., Blake M. S. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect Immun. 1993 Apr;61(4):1346–1351. doi: 10.1128/iai.61.4.1346-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990 Dec;58(12):4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Sugawara E., Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992 Feb 5;267(4):2507–2511. [PubMed] [Google Scholar]
- Swanson J., Bergström S., Barrera O., Robbins K., Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985 Aug 1;162(2):729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Gonococcal adherence: selected topics. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S678–S684. doi: 10.1093/clinids/5.supplement_4.s678. [DOI] [PubMed] [Google Scholar]
- Tagawa Y., Haritani M., Ishikawa H., Yuasa N. Characterization of a heat-modifiable outer membrane protein of Haemophilus somnus. Infect Immun. 1993 May;61(5):1750–1755. doi: 10.1128/iai.61.5.1750-1755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Virji M., Alexandrescu C., Ferguson D. J., Saunders J. R., Moxon E. R. Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol Microbiol. 1992 May;6(10):1271–1279. doi: 10.1111/j.1365-2958.1992.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Virji M., Makepeace K., Ferguson D. J., Achtman M., Sarkari J., Moxon E. R. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992 Oct;6(19):2785–2795. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Gotschlich E. C. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991 Jul;59(7):2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. E. The heat-modifiable outer membrane protein of Actinobacillus actinomycetemcomitans: relationship to OmpA proteins. Infect Immun. 1991 Jul;59(7):2505–2507. doi: 10.1128/iai.59.7.2505-2507.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]