Abstract
Peripheral corticotropin-releasing hormone (CRH) is an important regulator of localized inflammatory responses. The aim of this study is to define the pathological signaling pathways in which peripheral CRH receptor-mediated responses reside. We report that PECAM-1-expressing synovial membrane endothelial cells are the principal source of CRH receptor subtype 1α in chronically inflamed synovial tissue (ST). Analysis of ST from an early arthritis patient cohort (n = 9) established that expression of CRH-R1α significantly (P < 0.03) colocalized with PECAM-1 and E-selectin expression in vivo. Freshly excised ST explants released a mediator(s) that acts to promote CRH-R1α mRNA to levels present in inflamed human synovium (n = 8). We tested the ability of conditioned medium and individual inflammatory mediators to modulate CRH-R1α expression. Histamine selectively induced the expression of CRH-R1α, and these effects were mediated through the histamine receptor type 1. Ectopic expression of CRH-R1α in normal human endothelial and synoviocyte cells resulted in the induction of the orphan receptor NR4A2 through the reconstitution of cAMP/protein kinase A/cAMP response element-binding protein signaling and identified a role for CRH in modulating nuclear factor κB transcriptional activity. CRH enhanced the expression of nitric-oxide synthase (NOS III) to promote NO production from CRH-R1α-expressing cells. These data establish a role for CRH receptor-mediated responses in regulating vascular changes associated with chronic synovitis.
Current data support the direct involvement of peripherally produced corticotropin-releasing hormone (CRH) in the modulation of immune responses. The construction of mice lacking CRH confirms that peripheral CRH, in contrast to its direct immunosuppressive effect, is required for the induction of the inflammatory response in vivo.1 Recent findings underscore a direct involvement of CRH in the pathogenesis of human inflammatory joint disease and suggest the potential importance and clinical relevance of this peptide in disease progression. Linkage and association results for CRHprovide evidence that genetic variation of the CRHlocus may be a risk factor for the development of rheumatoid arthritis (RA).2,3 We and others have demonstrated that elevated levels of CRH mRNA and protein are produced locally and regulated by proinflammatory cytokines in synovial cells.4,5 We have also demonstrated the selective up-regulation of the CRH receptor subtype 1α in RA and psoriatic arthritis (PsA) compared with normal synovial tissue (ST).6,7 Recent studies using a rat model of adjuvant-induced arthritis demonstrate that chronic treatment with a CRH receptor type 1 antagonist significantly reduces inflammation-induced degeneration of synovia, cartilage, and bone in arthritic joints.8 Interestingly, in chronically inflamed ST, CRH-R1α expression is restricted to vascular endothelial cells and perivascular mast cell populations.6 In RA, degranulated mast cells release potent mediators, including histamine, prostanoids, and multifunctional cytokines capable of eliciting phenotypic changes to the local environment.9,10 Additional animal studies have established that CRH activates rat skin mast cells, through CRH receptor type 1, leading to changes in vascular permeability.11
The enhanced formation of new blood vessels (angiogenesis) is one of the earliest histopathological findings in RA and PsA and is central to erosive disease resulting in joint destruction.12,13 Until recently, the endothelium lining the blood vessels was thought to be a passive bystander in the inflammatory processes. However, it is now recognized that the endothelium plays an active and pivotal part in the recruitment of leukocytes to the inflamed synovium by responding to proinflammatory cytokines such as tumor necrosis factor-α (TNFα) and interleukin (IL)-1β and vasodilatory mediators including histamine and prostanoids.13,14 The recruitment of leukocytes from the circulation depends on a stepwise series of specific adhesive events mediated by increased amounts of different adhesion receptor/ligand pairs.14 The principal families of adhesion molecules involved are the integrins (αv-β3, -β1, and -β2), the selectins (E-, P-, and L-selectin), and the immunoglobulin superfamily (PE-, V-, and I-CAM).14,15,16 Preclinical animal and human studies suggest that the targeting of cell adhesion molecules may be an effective approach in the control of inflammation in RA.16,17
Using an ex vivo model of RA, we recently established that CRH contributes significantly to synovial tissue production of prostaglandin E2 (PGE2) in a cyclooxygenase (COX)-2-dependent manner.18 Furthermore, CRH can rapidly modulate the nuclear content of transcriptional activators including cAMP response element-binding protein (CREB)/ATF and nuclear receptor NR4A family members in RA synovium.5,18 Inhibition of CREB activity brings about the correction of aberrant synovial cell functions in patients with inflammatory joint disease.19 Members of the NR4A family (NR4A1/NUR77, NR4A2/NURR1, and NR4A3/NOR1) are emerging as critical effector molecules of cytokine, prostanoid, and growth factor action, which exhibit proangiogenic effects in vivo.20,21,22,23,24,25 Taken together, these observations confirm that CRH receptor-mediated signaling may play a role in pathological mechanisms associated with vascular changes that drives chronic synovitis. Nevertheless, the molecular mechanisms of CRH action in the inflammatory lesion remain to be elucidated, and it is clear that further studies are needed to define the pathological signaling pathways in which CRH receptor-mediated responses reside.
In this study, we confirmed that PECAM-1-expressing synovial membrane endothelial cells (SMECs) are a primary site of CRH-R1α expression in inflamed synovium. Quantitative analysis of ST from an early inflammatory arthritis patient cohort (n = 9) establishes that vascular endothelial expression of CRH-R1α significantly (P < 0.03) colocalizes with PECAM-1 and E-selectin expression in vivo. In contrast, αvβ3, a receptor up-regulated on endothelial cells during angiogenesis, localizes to small vessels and shows a highly restricted expression pattern compared with CRH-R1α. We establish that proinflammatory signals contribute to the elevated expression of CRH-R1α observed in vivo by testing the capacity of freshly excised ST explants and monocyte-conditioned medium to modulate endothelial expression of CRH-R1α. Of significance, such inflammatory milieu were capable of up-regulating CRH-R1α transcript levels to levels present in inflamed ST (n = 8). We compared the ability of individual mediators, associated with vascular and inflammatory changes in RA, with modulate CRH-R1α expression. Our data reveal that vasodilatory mediators including histamine, and to a lesser extent PGE2, selectively induce the endothelial expression of CRH-R1α. Importantly, the potent effects of histamine occur in a dose-dependent manner and are mediated through the histamine receptor 1 (HR1). Ectopic expression of CRH-R1α in normal human microvascular endothelial and synoviocyte cells results in the potent and sustained induction of NR4A2 expression through the reconstitution of CREB signaling and identifies a novel role for CRH in modulating nuclear factor κB (NF-κB) transcriptional activity. Finally, CRH enhances the expression of nitric-oxide synthase (NOS III) to promote nitric oxide production from CRH-R1α-expressing cells. Thus, these data identify for the first time the molecular pathways in the inflammatory lesion that control and direct CRH receptor-mediated signaling and further underscore a pathogenic role for CRH in regulating vascular changes associated with chronic synovitis.
Materials and Methods
Patient Details and Tissue Samples
Synovial biopsies were obtained from the knee joint by arthroscopy after informed consent from patients (n = 20) attending the Early Arthritis Clinic at St. Vincent’s University Hospital, Dublin, Ireland. Biopsies were obtained from patients with disease duration of less than 12 months. At the time of biopsy, patients were receiving nonsteroidal anti-inflammatory medication, but none had received disease-modifying agents or prednisolone. Arthroscopic synovial biopsy of the knee was performed on patients under local anesthesia using a 2.7-mm Storz arthroscope and 1.5-mm grasping forceps. Osteoarthritic (OA) synovial tissue (n = 3) was obtained from patients undergoing total knee arthroplasty. Human myometrial tissue (n = 8) expressing CRH-R1α mRNA was acquired by informed consent from nonpregnant premenopausal patients undergoing hysterectomy. Ethical permission was obtained from the Ethics Committee in accordance with the Declaration of Helsinki principles. Biopsy samples were either snap frozen or embedded in TissueTek OCT compound (Sakura, Zoeterwoude, the Netherlands), snap frozen, and stored in liquid nitrogen until used. Cryostat sections (7 μmol/L) were mounted on 3-aminopropyltriethoxysilane-coated glass slides, air dried overnight, wrapped in foil, and stored at −80°C until immunohistochemical analysis was performed.
Immunohistochemistry Analysis
Synovial tissue sections (7 μmol/L) were fixed in 1% paraformaldehyde, air dried, and incubated for 2 hours with normal blocking serum (Vector Laboratories, Peterborough, UK). The primary antibody for CRH-R1 was a goat polyclonal antibody (C20, 200 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) raised against a peptide mapping at the carboxy terminus of human CRH-R1. C20 was diluted 1:100 in 0.6 mol/L NaCl and incubated on sections at room temperature for 90 minutes. The primary antibody for factor VIII/von Willebrand factor (clone, F8/86, 200 μg/ml; Dako, Glostrup, Denmark), a mouse monoclonal antibody, was diluted 1:100 in normal blocking serum and incubated on sections at room temperature for 1 hour. The primary antibody for αvβ3 (clone, 23C6, 200 μg/ml; Santa Cruz Biotechnology), a mouse monoclonal antibody, was diluted 1:100 in normal blocking serum and incubated on sections at 4°C overnight. The primary antibody for E-selectin (clone, 1.2B6, 200 μg/ml; Novo Castra Laboratories, Newcastle upon Tyne, UK), a mouse monoclonal antibody, was diluted 1:50 in normal blocking serum and incubated on sections at room temperature for 1 hour. The primary antibody for PECAM-1 (clone, JC7OA, 200 μg/ml; Dako), a mouse monoclonal antibody, was diluted 1:100 in normal blocking serum and incubated on sections at room temperature for 1 hour. A biotinylated secondary antibody (1:500; Vector Laboratories) was applied, followed by avidin-biotin-peroxidase complex (Vector ABC kit; Vector Laboratories). For negative controls, all sections were incubated in the corresponding amount of isotype matched nonimmune IgG.
Dual-Labeling Immunofluorescence
Tissue sections were incubated for 1 hour in diluted normal rabbit serum (Vector Laboratories). CRH-R1 polyclonal antibody (C-20), diluted 1:10 in 10% normal human serum, was incubated on the tissue sections for 90 minutes, followed by the addition of biotinylated anti-goat secondary antibody (1:500; Vector Laboratories). Sections were incubated for 1 hour in diluted normal horse serum and then in a 1:20 dilution of the second primary antibody, a mouse monoclonal PECAM-1 antibody (JC7OA), for 1 hour. Fluorescein isothiocyanate-conjugated goat anti-mouse IgG1 antibody (1 mg/ml, diluted 1:50; Southern Biotechnology Associates, Birmingham, AL) was added, followed by the addition of Cy3 fluorochrome conjugated mouse anti-biotin monoclonal antibody (clone, BN-34, diluted 1:100; Sigma-Aldrich, St. Louis, MO). Slides were mounted in fluorescent mounting medium (Dako). Isotype-matched nonimmune IgG was included as a control for each of the primary antibodies, and the primary antibodies were sequentially omitted in selected sections as an additional control to ensure staining was specific for each (data not shown).
Primary Human Synoviocyte and Microvascular Endothelial Cell Culture
SMECs were isolated from total knee synovium (n = 3) obtained at the time of arthroplasty and cultured as previously described.18 Dermal- and lung-derived primary human microvascular endothelial cells (HMVEC-d and HMVEC-L, respectively) were obtained commercially (BioWhittaker, Cambrex Bioscience, Berkshire, UK) and cultured as previously described.18,26 K4 IM synoviocyte cells are an established immortalized human synoviocyte cell line that were derived from a healthy donor and immortalized with SV40 antigen. The K4 IM cell line was provided by Professor H. Eibel (Clinical Research Unit for Rheumatology, University Hospital, Freiburg, Germany) and grown as described previously.18,21
Cell Treatments
HMVECs were cultured in T25-cm2 culture flasks (Becton Dickinson, Franklin Lakes, NJ) and grown to 80% confluency before stimulation. Cells were maintained in serum-free medium for 24 hours. Histamine, PGE2, forskolin, CRH, mepyramine (Sigma-Aldrich), IL-1β, and TNFα (Calbiochem, San Diego, CA) were resuspended according to the manufacturer’s instructions and included at the concentrations indicated. Synovial membrane biopsies were obtained from arthroscopy or arthroplasty, minced asceptically, and coincubated with the HMVECs for 24 hours. Monocyte conditioned medium (diluted 1:20) was coincubated with HMVECs for 24 hours. After stimulation, the medium was removed, the cells were washed and lysed, and RNA extractions using the RNeasy Mini kit (Qiagen, Valencia, CA) were performed. For ectopic expression, studies using pcCRH-R1α, synoviocyte (K4 IM) cells, and HMVEC-L were grown in six-well plates, and 24 hours after nucleofection (Amaxa GmbH, Cologne, Germany), cells were stimulated with CRH or PGE2 for 1 to 3 hours. For the NO assay, cells were cultured in 12-well plates (300,000 cells/well), after nucleofection with pc-CRH-R1α. Twenty-four hours after nucleofection, cells were stimulated as indicated with CRH or a combined cytokine stimulation of interferon-γ (IFNγ) (R&D Systems, Minneapolis, MN), IL-1β, and TNFα for a further 24 hours.
Flow Cytometry
Cell-surface molecule expression was examined on isolated SMECs by flow cytometric analysis. SMECs were adjusted to 1 × 106 cells/ml, and a 100-μl aliquot of this cell suspension was stained with 5 μl of each monoclonal antibody for 10 minutes at room temperature. Stained cells were analyzed on a Becton Dickinson FACS Scan. SMECs were identified using monoclonal antibodies CD 31 (PECAM-1; clone, WM-59), CD 54 (ICAM-1; clone, HA-58), CD 106 (VCAM-1; clone, 51-10C9) and CD 62 E (E-selectin; clone, 68-5H11) (Becton Dickinson). Appropriate isotype control anti-sera IgG1 (fluorescein isothiocyanate) and IgG2a (phycoerthin) were included in each assay.
Synthesis of cDNA and Polymerase Chain Reaction (PCR)
cDNA was prepared by reverse transcription of 1 μg of each total RNA sample as described previously.5 PCR was performed in 50-μl volumes containing 2 μl of cDNA reaction mixture; 1× PCR buffer II; 1.25 mmol/L each of dATP, dCTP, dGTP, and dTTP; 2.5 U of AmpliTaq Gold (Perkin Elmer, Boston, MA); 1.0 mmol/L MgCl2 [for CRH-R1α and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)]; 1.5 mmol/L MgCl2 (for NR4A2 and NOS III); 200 ng of sense and antisense primers (for CRH-R1α); and 100 ng of sense and antisense primers (for NR4A2, NOS III, and GAPDH). The concentrations of MgCl2 and primer, along with annealing temperature, have been previously validated and reported.6, 27 Each PCR sample underwent a 30- to 35-cycle amplification to ensure that the reactions had not reached the plateau phase of amplification. PCR products were electrophoresed on a 1.5% agarose gel and visualized using ImageMaster system (ImageMaster VDS; Amersham Biosciences, Little Chalfont, Buckinghamshire, UK).
The sense 5′-GCCCTGCCCTGCCTTTTTCTA-3′ and antisense 5′-GCTCATGGTTAGCTGGACCA-3′ primer pair was used to amplify CRH-R1α, yielding a 333-bp product. The sense 5′-CGACATTTCTGCCTTCTCC-3′ and antisense 5′-GGTAAAGTGTCCAGGAAAAG-3′ primer pair was used to amplify human NR4A2, yielding a 277-bp product. The sense 5′-CCAGCTAGCCAAAGTCACCAT-3′ and antisense 5′-GTCTCGGAGCCATACAGC-3′ primer pair was used to amplify NOS III, yielding a 353-bp product. The sense 5′-GCCTCAAGATCATCAGCAA-3′ and antisense 5′-CCAGCGTCAAAGGTGGAG-3′ primer pair was used to amplify GAPDH, yielding a 465-bp product. Primers amplifying a 635-bp product for of GAPDH were used where indicated (sense, 5′-CCACCCATGGCAAATTCCATGGCA-3′; antisense, 5′-TCTAGACGGCAGGTCAAGTCCACC-3′). All primer pairs flanked intronic sequences.
Southern and Northern Blot Analysis
Amplified CRH-R1α and GAPDH cDNA PCR products were run on a 1% agarose gel, denatured, neutralized, and transferred overnight onto a nylon membrane via upward capillary transfer. Previous studies from our laboratory confirmed the identity of the CRH-R1α PCR product by subcloning and nucleotide sequencing,6 which corresponds to the human CRH-R1α receptor cDNA.28
Northern blotting was performed according to standard procedures. Equal amounts of RNA (10 μg) were denatured in glyoxal sample buffer (Biowhittaker Molecular Applications, Rockland, ME) and subjected to electrophoresis on a 3-(N-morpholino)propanesulfonic acid (MOPS) buffer-based gel system (Reliant RNA gel system; Biowhittaker Molecular Applications). The RNA was then blotted by capillary transfer onto nylon membranes (Bio-Rad, Hercules, CA). The blots were prehybridized for 4 hours at 42°C in 45% formamide, 0.1% sodium dodecyl sulfate, 4× standard saline citrate, 5× Denhardt’s solution (0.1% Ficoll, 0.1% bovine serum albumin, and 0.1% polyvinylpyrrolidone), 250 μg/ml denatured herring testes DNA, and 0.1 mol/L sodium phosphate buffer, pH 6.5. The full-length E-selectin cDNA was provided by Dr. S. Narumi (Department of Molecular Preventative Medicine, School of Medicine, University of Tokyo, Tokyo, Japan).
CRH-R1α, E-selectin, or GAPDH cDNAs were radiolabeled to a high specific activity using α-[32P]dCTP and a random primer labeling system (Promega, Southampton, UK). After incubation with a prehybridization solution, the membrane was hybridized with the α-[32P]-labeled cDNA probe at 65°C under high stringency conditions. After hybridization, blots were exposed to film at −80°C using intensifying screens.
Construction of the CRH-R1α Mammalian Expression Vector
The full-length CRH-R1α cDNA, subcloned into pBluescript SK− plasmid (phCRF-R1-BS), was provided by Professor Wylie Vale (Clayton Foundation Laboratories for Peptide Biology, The Salk Institute, La Jolla, CA).28 After transformation of phCRF-R1-BS into competent Escherichia coli cells, phCRF-R1-BS plasmid DNA was isolated using QIAprep miniprep kit (Qiagen). The CRH-R1α cDNA insert was isolated by restriction endonuclease digestion of phCRF-R1-BS plasmid DNA with EcoR1 (Promega) and purified from 1% agarose gel (Agarose gel DNA extraction kit; Boehringer Mannheim GmbH, Ingelheim, Germany). The CRH-R1α cDNA insert was ligated into phosphatase-treated pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA), and after transformation into competent E. coli cells, an endotoxin-free preparation of the pcDNA3.1 vector DNA containing the CRH-R1α cDNA insert (hereafter referred to as pc-CRH-R1α) was made using Endofree plasmid midi kit (Qiagen). The orientation of the CRH-R1α cDNA insert in pc-CRH-R1α was confirmed by sequencing (MWG-Biotech, High Point, NC).
Transient Transfection of Synoviocyte Cells and HMVECs
Transient transfection of cells was achieved using nucleofector technology (Amaxa), permitting DNA delivery directly to the nucleus. Nucleofection of synoviocyte cells and HMVEC-L was performed using the Human Dermal Fibroblast Nucleofector kit (Amaxa) and the HMVEC-L Nucleofector kit (Amaxa), respectively. On reaching 70 to 80% confluency, cells were nucleofected with pc-CRH-R1α, pc-LacZ, CRE-tk-LUC (Promega), or κB-tk-LUC (kindly provided by Dr. Cormac Taylor, University College Dublin, Dublin, Ireland) as indicated. Approximately 1 × 106 synoviocyte cells and 0.6 × 106 HMVEC cells were nucleofected using nucleofector programs U20 and S05, respectively. Twenty-four hours after transfection, media were changed to serum-free media. After treatments, cells were washed with ice-cold phosphate-buffered saline, and total RNA or protein was harvested. Luciferase activity was measured with a luminometer (Turner TD; Turner Biosystems, Sunnyvale, CA) and luciferase assay reagents (Promega).
Detection of CRH-R1α by Immunofluorescence and Confocal Microscopy
After nucleofection, cells were transferred to eight-chamber tissue culture slides (Becton Dickinson), and transfection efficiency was assessed 24 hours later. Cells cultured in eight-chamber tissue culture slides (Becton Dickinson) were air dried for 15 minutes, washed twice with ice-cold phosphate-buffered saline, and fixed in 1% paraformaldehyde. Cells were then incubated in diluted normal rabbit serum (Vector Laboratories) for 2 hours and then in a 1:100 dilution of CRH-R1 polyclonal antibody (C20) (Santa Cruz Biotechnology) in 10% normal human serum for 1 hour. Cells were washed in phosphate-buffered saline and incubated for 30 minutes in biotinylated anti-goat secondary antibody (1:500; Vector Laboratories). A Cy3 fluorochrome-conjugated anti-biotin antibody (BN-34, 1.2 mg/ml, 1:100; Sigma) was added to bind the biotinylated anti-goat added previously. After final washing in phosphate-buffered saline, slides were mounted in fluorescent mounting medium (Dako) or Vectashield fluorescent mounting medium with 4,6-diamidino-2-phenylindole (Vector Laboratories). For negative controls, isotype-matched nonimmune goat IgG was included. Image analysis was performed using fluorescent and confocal microscopy.
Nitric Oxide and Vascular Endothelial Growth Factor (VEGF) Assays
Nitric oxide (NO) production was determined by measuring the release of the nitrite (NO2−) anion into the culture medium using the total NO assay kit (R&D Systems). The assay had sensitivity to detect NO2− concentrations in the micromolar range (≤1.35 μmol/L). Cell culture supernatants were diluted 1:2 as recommended by the kit manufacturers and ultrafiltered through 10,000 molecular weight-cutoff filters (Sigma). In a 96-well plate, NO2− was assayed using the Griess reaction in cell culture supernatants and a range of serial dilutions of a nitrite standard (1000 μmol/L nitrite) by measuring absorbance at 540 nm. Total nitrite production was determined for each sample in duplicate and corrected to a standard curve of nitrite. The Quantikine VEGF immunoassay (R&D Systems) is a solid-phase enzyme-linked immunosorbent assay designed to measure VEGF165 levels in cell culture supernatants. The minimum detectable concentration of VEGF is ≤5.0 pg/ml.
Statistical Analysis
All results, where indicated, are presented as the mean ± SEM. For immunohistochemistry analysis, counting of blood vessels staining positive in synovium obtained from patients diagnosed with RA or PsA (n = 9) was performed in randomly selected high-power fields at ×100 magnification; a minimum of three high-power fields from three separate sections were counted, and the mean count was calculated. Comparisons and correlations of positive blood vessel counts for CRH-R1, PECAM-1, E-selectin, αvβ3, and von Willebrand Factor (factor VIII) were made using Pearson statistical analysis. Comparisons between levels of nitrite production were made using Mann-Whitney U-test. The statistical program used was Statview (SAS Institute, Cary, NC). P values of <0.05 were considered statistically significant.
Results
CRH-R1α Is Abundantly Expressed on Activated Endothelium in Chronically Inflamed Human Synovial Tissue
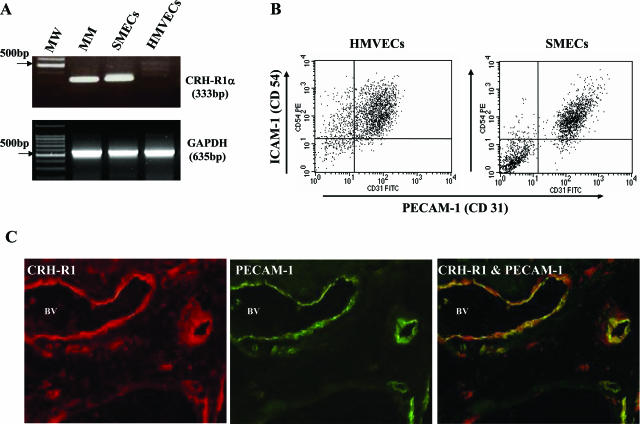
Consistent with our previous observations,6,7 we found a marked increase in CRH-R1α expression by SMECs compared with normal HMVECs (Figure 1A). Reflecting the inflammatory milieu of the arthritic joint, flow cytometric analysis of primary SMECs and normal HMVECs (n = 3) showed increased expression levels of PECAM-1 (Figure 1B), E-selectin, and VCAM-1 (data not shown) on SMECs and high constitutive levels of ICAM-1 on both cell populations (Figure 1B). To confirm further that expression of CRH-R1α colocalizes on endothelial surfaces with markers of endothelial activation, two-color immunofluorescence staining for CRH-R1α and PECAM-1 was performed (Figure 1C). CRH-R1α immunoreactivity showed a homogeneous distribution in the synovial vasculature in all patients with inflammatory arthritis (n = 9) (Figures 1C and 2). Consistent with previous observations in human disease and in animal models of arthritis, the endothelial cells of inflamed synovia studied stained intensely for PECAM-1, with a marked concentration of expression on endothelial intercellular junctions (Figures 1C and 2).29,30 Dual immunofluorescence of CRH-R1α and PECAM-1 revealed significant colocalization of the two proteins to the synovial vasculature (Figure 1C).
Figure 1.
PECAM-1 (CD31)-expressing endothelia are a major source of CRH-R1α in human inflammatory arthritis. A: Representative reverse transcriptase (RT)-PCR products generated using CRH-R1α and GAPDH-specific primers after amplification of mRNA from human myometrium (MM), primary SMECs, and primary HMVECs; MW, 100-bp DNA molecular weight markers. B: Flow cytometric analysis of cultured primary SMECs and HMVECs using antibodies directed against PECAM-1 (CD31) and ICAM-1 (CD54). C: Representative immunostaining for CRH-R1 and PECAM-1 in synovial tissue from a patient with early PsA. Synovial tissue sections were incubated with a Cy3-labeled CRH-R1 antibody. CRH-R1 immunofluorescence is indicated by intense red staining on the vascular endothelium and discrete perivascular cells. Subsequently, the same section was incubated with fluorescein isothiocyanate-labeled immune serum directed against PECAM-1, as indicated by the green fluorescence. Superimposition results in distinct yellow fluorescence, confirming the colocalization of CRH-R1 with PECAM-1-expressing endothelium. BV, blood vessel. Original magnification, ×200.
Figure 2.
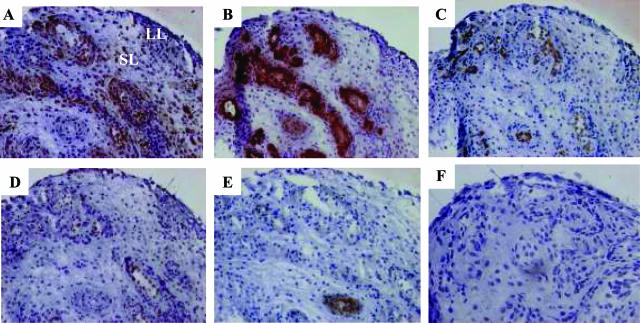
Coexpression of CRH-R1α with activation and angiogenic molecules on endothelial cells in early inflamed human synovial tissue. Representative serial PsA synovial tissue cryosections were stained with immune serum directed against CRH-R1 (A), von Willebrand factor (factor VIII) (B), PECAM-1 (CD31) (C), E-selectin (D), αvβ3 (E), or isotype-matched nonimmune IgG (F). Positive cells are indicated by dark brown staining. Nuclei are counterstained with hematoxylin. LL, lining layer; SL, sublining layer. Original magnification for each stain, ×100 (A–F).
Using ST from an early arthritis patient cohort (n = 9), a more detailed immunohistochemical analysis was undertaken to evaluate the synovial distribution of CRH-R1α. Serial ST sections for each patient were treated with antibodies to endothelial markers including von Willebrand factor, PECAM-1, E-selectin, and αvβ3, and the pattern of staining was compared with CRH-R1α. Representative ST analysis from our arthritis patient cohort (n = 9) is shown in Figure 2. The highly specific and ubiquitous endothelial cell marker, von Willebrand factor, established that the inflamed synovium contained an increased number of large and small vessels (Figure 2B). Consistent with vessels at sites of inflammation, both large and small vessels expressed the endothelial cell activation molecules PECAM-1 and E-selectin (Figure 2, C and D).15 Homogenous PECAM-1 staining was seen on blood vessels present in synovium, whereas E-selectin was seen in a subset of PECAM-1-positive vessels. Endothelial staining for CRH-R1α was extensive throughout the synovium with a distribution of expression similar to von Willebrand factor (Figure 2, A and B). Endothelial expression of CRH-R1α correlated significantly with PECAM-1 (n = 9; r = 0.79; P < 0.03) and E-selectin (n = 9; r = 0.64; P < 0.03) expression (Figure 2, C and D). In contrast, αvβ3, a receptor transiently up-regulated on endothelial cells during angiogenesis,31 was localized to small vessels throughout the synovium and expressed on only a subset of endothelial cells (Figure 2E). Although αvβ3-expressing endothelia were CRH-R1α positive, CRH-R1α expression was extensively more widespread throughout the synovial vasculature in all of the patient tissue studied (n = 9). Using species- and isotype-matched immunoglobulins instead of primary antibodies, staining was absent in all sections of synovium (Figure 2F; data not shown).
Histamine, through the Histamine Receptor 1, Promotes Endothelial CRH-R1α Expression
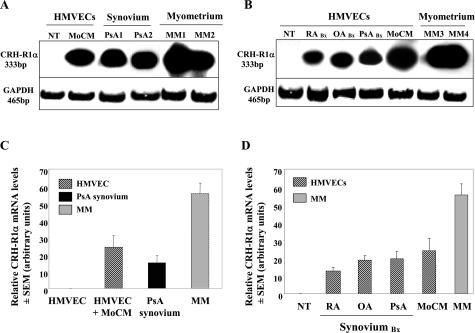
Consistent with our ST immunolocalization findings, high levels of CRH-R1α mRNA were present in primary SMECs but were undetectable in normal HMVECs (Figures 1 and 3). To determine the regulatory signals capable of modulating endothelial CRH-R1α expression, we examined the ability of proinflammatory agonists to regulate steady-state CRH-R1α mRNA levels (Figure 3). HMVECs stimulated with monocyte-conditioned medium (MoCM), a complex combination of inflammatory mediators reported to accurately reflect in vivo cytokine concentrations, strongly increased CRH-R1α mRNA expression over 24 hours to levels equivalent to those present in ST obtained from patients with PsA (n = 8) (Figure 3).
Figure 3.
CRH-R1α mRNA can be induced in normal HMVECs to equivalent transcript levels measured in human inflamed synovial tissue and human myometrium. A: RT-PCR analysis was performed using primers for CRH-R1α and GAPDH with total RNA extracted from HMVECs left untreated (NT) or coincubated for 24 hours with MoCM, PsA synovial tissue (PsA1 and PsA2), and human myometrium (MM1 and MM2). B: HMVECs left untreated (NT) or coincubated for 24 hours with synovial biopsies (RA, PsA, and OA Bx) or MoCM and human myometrium (MM3 and MM4). C and D: Densitometric analysis representing CRH-R1α mRNA levels in HMVECs. Values are the means ± SEM. For HMVECs, each value is representative of three separate experiments. For human synovial tissue, n = 8 and myometrium, n = 8.
Next, we sought to verify the ability of mediators produced and released locally from ST to alter endothelial CRH-R1α expression. Using a coculture system, freshly explanted ST from patients diagnosed with RA, PsA, or OA were individually cocultured with HMVEC monolayers. After 24 hours, total RNA from HMVEC monolayers was extracted, and CRH-R1α mRNA levels were measured. The induction of CRH-R1α mRNA levels by mediators released from ST explants were comparable with transcript levels induced by MoCM and those present in inflamed ST (Figure 3, A–D).
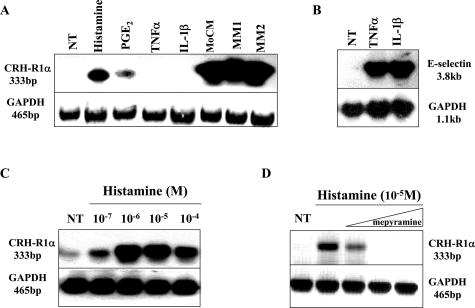
We extended this analysis further to investigate the effects of individual proinflammatory agonists, associated with ST vascular changes, to regulate CRH-R1α mRNA expression (Figure 4). Histamine, and to a lesser extent PGE2, consistently up-regulated CRH-R1α mRNA over 24 hours; however, although IL-1β and TNFα had no effect on CRH-R1α mRNA (Figure 4A), both mediators robustly induced the expression of E-selectin mRNA in the same cells (Figure 4B). The regulation of pituitary CRH-R1αgene expression by CRH through activation of the adenylate cyclase/protein kinase A signaling pathway has previously been established. We therefore tested the ability of CRH and forskolin, an adenylate cyclase-activating agent, to stimulate CRH-R1α in HMVECs. Neither CRH nor forskolin showed any effect on CRH-R1α mRNA levels in these cells at any of the concentrations tested (data not shown).
Figure 4.
Modulation of CRH-R1α mRNA levels in HMVECs by the vasoactive mediator histamine. A: Expression of CRH-R1α, E-selectin, and GAPDH mRNA was analyzed using total RNA isolated from HMVECs left untreated (NT), treated for 24 hours with histamine (10−2 mol/L), PGE2 (10−6 mol/L), TNFα (10 ng/ml), IL-1β (10 ng/ml), or MoCM and human myometrium (MM1 and MM2). B: HMVECs left untreated (NT) or treated for 4 hours with TNFα (10 ng/ml) or IL-1β (10 ng/ml). C: HMVECs left untreated (NT) or treated for 24 hours with increasing concentrations of histamine (10−7 to 10−4 mol/L). D: HMVECs left untreated (NT) or treated for 24 hours with histamine (10−5 mol/L) in the absence or presence of increasing concentrations of mepyramine (0.1, 1.0, and 10.0 μmol/L).
To establish the most effective histamine concentration for maximal induction of CRH-R1α expression, endothelial cells were treated with a range of histamine concentrations (10−7 to 10−4 mol/L) for 24 hours. A representative example of three individual experiments illustrates that histamine regulation of CRH-R1α mRNA levels occurs in a concentration-dependent manner (Figure 4C). Histamine at all concentrations tested [10−7 mol/L (2.2 ± 0.3-fold), 10−6 mol/L (3.8 ± 0.4-fold), 10−5 mol/L (4.6 ± 1.8-fold), and 10−4 mol/L (3.2 ± 0.6-fold)] significantly (P < 0.01) increased CRH-R1α mRNA levels with maximal induction occurring at 10−5 mol/L histamine. To investigate further the mechanism of histamine modulation of CRH-R1α expression, we examined the effects of mepyramine, a selective histamine receptor 1 antagonist, in our endothelial cell system. As shown in Figure 4D, pretreatment with mepyramine (0.1, 1.0, and 10.0 μmol/L) for 2 hours prevented the maximal histamine (10−5 mol/L)-dependent effects on CRH-R1α mRNA levels in a concentration-dependent manner.
NR4A2 Is a Transcriptionally Regulated Target Gene Downstream of CRH-R1α Signaling
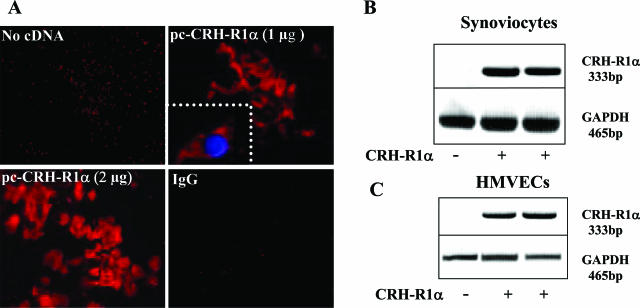
To explore further the biological effect(s) of CRH receptor-mediated responses in chronically inflamed synovium, we transiently transfected normal human synoviocyte and HMVECs with 1 to 2 μg of a eukaryotic expression vector containing the full-length human CRH-R1α cDNA. Consistent with our earlier observations, there was a clear absence of CRH-R1α expression by normal human synoviocyte cells and HMVECs; however, after vector transfection, CRH-R1α mRNA and protein levels were markedly increased and maintained over 24 hours (Figure 5, A–C) and 48 hours (data not shown). Confocal microscopic analysis confirmed cytoplasmic localization and membrane distribution of ectopically expressed CRH-R1α (Figure 5A, inset).
Figure 5.
Ectopic expression of CRH-R1α in normal human synoviocytes and HMVECs. A: Synoviocytes nucleofected with 1.0 or 2.0 μg of pc-CRH-R1α for 24 hours were stained with a Cy3-labeled CRH-R1 antibody (C20) or isotype-matched nonimmune IgG. CRH-R1α expression is indicated by intense red fluorescence. Original magnification, ×400. Inset: 4,6-diamidino-2-phenylindole staining, visible as blue fluorescence, distinguishes the nucleus and localizes CRH-R1α to the cytoplasmic membrane. Original magnification, ×600. B and C: RT-PCR analysis of CRH-R1α and GAPDH mRNA expression in synoviocytes and HMVECs after nucleofection with or without the CRH-R1α cDNA.
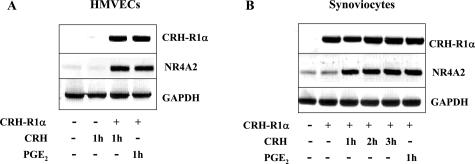
We have previously shown the orphan nuclear receptor NR4A2 to be the principal member of the NR4A subfamily to contribute to CRH signaling in the anterior pituitary and in the pathological context in RA and PsA synovial tissue explants.5,32 In this study, in the absence of CRH-R1α expression, CRH (10−8 mol/L) had no effect on NR4A2 mRNA levels in HMVECs (Figure 6) or synoviocytes (data not shown). Proinflammatory mediators, including PGE2, markedly enhance NR4A2 mRNA and protein levels within 1 to 4 hours.20 After transfection of CRH-R1α cDNA, the temporal induction of NR4A2 gene expression by CRH (10−8 mol/L) was equivalent to the effects of PGE2 (10−6 mol/L) in both cell types (Figure 6, A and B).
Figure 6.
CRH-induced NR4A2 mRNA expression is dependent on CRH-R1α. HMVECs (A) and synoviocytes (B) were nucleofected with or without 2.0 μg of pc-CRH-R1α for 24 hours, left untreated, or treated with CRH (10−8 mol/L) or PGE2 (10−6 mol/L) for a further 1 to 3 hours. Total RNA was extracted, and RT-PCR was performed using primers specific for CRH-R1α, NR4A2, and GAPDH.
Activation of CREB and NF-κB Signaling by CRH in CRH-R1α-Expressing Cells
The stimulatory effect of CRH on gene transcription has been shown to be mediated through cAMP/protein kinase A (PKA) activation.7,33,34 Increased cytosolic levels of cAMP leads to the activation of intracellular kinases including PKA, which phosphorylates CREB. This phosphorylated and activated form CREB binds to the cAMP response element (CRE) consensus sequence to regulate gene transcription.
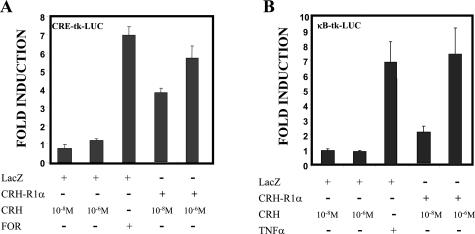
We confirm the ability of CRH to modulate CREB signaling in CRH-R1α-expressing cells (Figure 7A). Expression vectors containing either LacZ or CRH-R1α were cotransfected with the reporter CRE-tk-luciferase, which contains three copies of the CRE fused with the minimal tk promoter. Treatment of LacZ-expressing cells with forskolin (25 μmol/L) strongly activated the transcription of the reporter in contrast to negligible effects with increasing CRH concentrations (10−8 to 10−6 mol/L). However, in CRH-R1α-expressing cells, CRH strongly activated transcription of the CRE reporter, similar to the effects of forskolin, in a concentration-dependent manner (Figure 7A).
Figure 7.
CRH signaling, via CRH-R1α, can modulate CREB and NF-κB transcriptional activity. Synoviocytes were nucleofected with 2.0 μg of pc-LacZ or 2.0 μg of pc-CRH-R1α together with 500 ng of a CRE-tk-LUC reporter plasmid (A) or 500 ng of a κB-tk-LUC reporter plasmid (B). After nucleofection, cells were treated with increasing concentrations of CRH (10−8 to 10−6 mol/L), forskolin (FOR, 25 μmol/L), or TNFα (10 ng/ml) for 24 hours. Values are presented as fold induction after normalization. All transfection experiments were performed in triplicate dishes. The mean ± SEM of four individual experiments is shown.
To explore novel proinflammatory pathways downstream of CRH, we tested the effect of CRH on the activation of NF-κB signaling using CRH-R1α-expressing cells (Figure 7B). The reporter κB-tk-luciferase containing three copies of the κB consensus sequence was cotransfected with either LacZ or CRH-R1α expression vectors. Treatment of LacZ-expressing cells with CRH showed no effect on NF-κB activation at any of the concentrations tested (Figure 7B). However, with CRH-R1α-expressing cells, CRH in a concentration-dependent manner strongly activated transcription of the κB reporter equivalent to the effects of TNFα (10 ng/ml).
CRH Enhances the Expression of NOS III to Promote NO Production in CRH-R1α-Expressing Cells
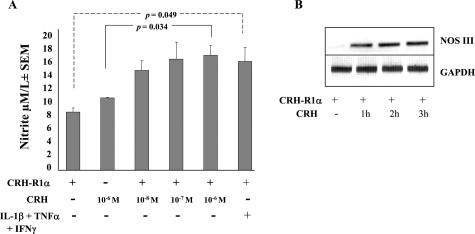
Our in vivo and in vitro data suggest that the vascular endothelium is a major target of CRH action in chronically inflamed ST. Therefore, we investigated whether CRH had any effects on the endothelial production of mediators associated with vascular permeability and angiogenesis. Consistent with published observations,35,36 we found that proinflammatory cytokines can significantly (P = 0.049) increase the endothelial production of NO (Figure 8A) and VEGF (data not shown). We next explored the ability of CRH to modulate NO and VEGF production by CRH-R1α-expressing endothelium. No significant effects of CRH (10−6 mol/L) on NO production were observed in endothelial cells lacking CRH-R1α. In contrast, CRH in a concentration-dependent manner significantly (P = 0.034) increased NO production in CRH-R1α-bearing cells (Figure 8A). A combination of TNFα, IL1-β, and lipopolysaccharide have been shown to induce NO from rheumatoid synoviocytes by 2.5-fold. The increased production of NO by CRH was equivalent to the magnitude of such cytokine effects (Figure 8A). Using the same transfection system, CRH had no measurable effects on the endothelial production of VEGF in the presence or absence of ectopically expressed CRH-R1α (data not shown).
Figure 8.
CRH promotes nitric oxide production from CRH-R1α-expressing cells. A: HMVECs were nucleofected with or without pc-CRH-R1α and seeded into 12-well plates (300,000 cells/well). Cells were left unstimulated or stimulated with increasing concentrations of CRH (10−8 to 10−6 mol/L) or a combination of IL-1β (25 ng/ml), TNFα (25 ng/ml), and interferon-γ (1000 U/ml) for 24 hours. Cell culture medium was harvested, and nitric oxide production was determined by measuring nitrite production using the Griess reaction. Values are the means ± SEM from three separate experiments. B: Synoviocytes transfected with pc-CRH-R1α for 24 hours were treated with CRH (10−8 mol/L) for a further 1 to 3 hours. Expression of NOS III and GAPDH mRNA was analyzed using total RNA. Each gel is representative of three separate experiments.
NO is produced by the nitric-oxide synthase (NOS) family of enzymes in which three forms of NOS exist: constitutive (NOS I), inducible (NOS II), and endothelial (NOS III). Previous studies using human myometrial cells and placental explants show a selective role for CRH in modulating NO production through increased expression of NOS III protein levels within 8 hours.37,38 Significantly, in these published studies, CRH showed no effect on the levels of NOS II expression. Thus, to elucidate the mechanism of action of CRH in regulating NO production in our CRH-R1α transfectants, we measured the effects of CRH on the expression levels of NOS III. In CRH-R1α-expressing synoviocyte cells, CRH induced a rapid increase in NOS III mRNA levels, which peaked within 3 hours (Figure 8B). High constitutive levels of NOS III mRNA were measured in endothelial cells, and treatment of CRH-R1α transfectants with CRH (10−8 mol/L) showed no discernible effect on the transcription of NOS III above the high steady-state levels observed (data not shown).
Discussion
Modulation of locally produced CRH is an important component of the cytokine network in human and animal models of inflammatory arthritis.4,5,6,8,18,39 In this study, analysis of ST from patients with recent-onset RA and PsA confirms increased endothelial expression of CRH-R1α mRNA and protein. Our results demonstrate significant colocalization of CRH-R1α with endothelial adhesion molecules revealing that activated endothelia are a principal target of peripheral CRH action in chronically inflamed synovium. To elucidate the regulatory mechanisms underlying peripheral CRH-R1α gene expression, we established the ability of proinflammatory pathways associated with vascular changes to stimulate CRH-R1α expression. We demonstrate for the first time that CRH-R1α mRNA expression in primary endothelial cells is increased by mediator(s) released from freshly explanted ST. Our studies reveal the ability of monocyte-conditioned medium and vasoactive mediators, including histamine, to modulate endothelial CRH-R1α transcription to levels present in vivo in inflamed ST. Transient overexpression of CRH-R1α in normal endothelial and synoviocyte cells facilitates the reconstitution of CRH-dependent CREB signaling and identifies a role for NR4A2 and NF-κB as effector molecules of CRH action. Finally, in cells ectopically expressing CRH-R1α, CRH enhances the expression of nitric-oxide synthase III and promotes NO production. Thus, this study provides substantial and novel evidence to support the conclusion that modulation of CRH-R1α expression by chronically inflamed synovium contributes to CRH-induced vascular changes in human joint disease.
The ingress of leukocytes into sites of inflammation is crucial for the pathogenesis of arthritis and other inflammatory conditions.14 Increased angiogenesis and cytokine-dependent induction of adhesion molecule expression on synovial membrane endothelial cells are the earliest histopathological features of RA and PsA.12,40 Several adhesion molecules, termed integrins, selections, and immunoglobulins, act in concert to regulate angiogenesis and leukocyte extravasation. The integrin αvβ3 has been shown to be both a marker and critical effector for blood vessels during synovial angiogenesis.31 In animal models, CRH has been implicated in the enhancement of local angiogenesis and epithelial tumor growth in the skin.41 Consistent with this observation, our analysis demonstrates that, in RA and PsA synovium, αvβ3-expressing endothelia are CRH-R1α-positive. Interestingly, compared with the restricted expression of αvβ3, the expression of CRH-R1α is extensively more widespread throughout the synovial vasculature. In RA synovium, E-selectin acts to promote leukocyte tethering and rolling and has been shown to be a marker of endothelial activation in leukocyte-rich regions.14,42 Notably, E-selectin shows a highly endothelial-selective distribution and is expressed on activated endothelial cells within hours after exposure to inflammatory mediators such as IL-1β, TNFα, and interferon-γ.15,43 PECAM-1, an immunoglobulin-related molecule, is expressed on leukocytes as well as endothelial cell junctions where it is a key participant in the adhesion cascade leading to transendothelial migration.30 Blockage of PECAM-1 on the endothelium inhibits leukocytes from transendothelial migration.44 In this study, our analysis indicates that activated synovial membrane endothelial cells are a major site of CRH-R1α expression. The significant colocalization of CRH-R1α with PECAM-1 and E-selectin expression in vivo highlights the clinical significance of enhanced endothelial CRH-R1α expression in chronically inflamed tissue. The relevance of these observations in the pathogenesis of human inflammatory joint disease is further underscored by the observation that in rat adjuvant-induced arthritis, administration of antalarmin, a CRH-R1 antagonist, significantly attenuates the progressive inflammatory-induced degeneration of synovium, cartilage, and bone in arthritic joints.8
Previous work has established that CRH expression in RA synovium correlates significantly (P < 0.0005) with the extent and intensity of mononuclear cell infiltration.4 Consistent with these in vivo observations, we have established that proinflammatory mediators released by explanted ST and activated mononuclear cells may also contribute to synovial CRH signaling through the increased expression of endothelial CRH-R1α. Inflammatory cytokines including IL-1β and TNFα have been shown to modulate steady-state CRH mRNA expression in primary synoviocytes, and importantly, the human CRH promoter also responds to these mediators in a similar manner.5 To elucidate the regulatory mechanisms underlying endothelial CRH-R1αgene expression, we tested the ability of the same immunological stimuli to stimulate receptor synthesis. The effects of histamine and PGE2 were also incorporated into this study because CRH receptor-mediated responses have been shown to contribute significantly to PGE2 levels in RA synovial and histamine release through mast cell degranulation.11,18 In contrast to the regulation of CRH mRNA by diverse stimuli, the expression of CRH-R1α is primarily induced by histamine and to a lesser extent by PGE2. Histamine binds to four different G protein-coupled receptors (HR1–4) that transduce signals to cells through distinct pathways.45 Endothelial cells express functional HR1 and HR2, and the engagement of H1 receptors is central to histamine effects on leukocyte adhesion and vascular leakage.45,46 As evidenced by associated signal transduction elements, HR1 acts to potentiate a proinflammatory response, whereas HR2 suppresses inflammatory functions.45 Our data demonstrate the involvement of the HR1 receptor subtype in mediating histamine-induced changes in endothelial CRH-R1α gene transcription. Down-regulation of NF-κB, which acts as a critical transcription factor in mediating inflammatory responses, represents a mechanism for HR1-antihistamines to inhibit inflammatory cell accumulation.47 Interestingly, the recent isolation of the 5′ region of the human CRH-R1gene reveals a potential NF-κB consensus binding site located within the −53 and −43-bp region of the proximal promoter.48 Taken together, these data suggest that synovial CRH may signal in a paracrine manner through the enhanced release of histamine and PGE2, which, in turn, act to amplify local CRH effects through the increased expression of endothelial CRH-R1α.
The NR4A orphan nuclear receptors are emerging as key regulators of cytokine and growth factor action in chronic inflammatory diseases including arthritis, atherosclerosis, and cancer.20,21,22,23,24,49 Members of the NR4A subfamily are necessary and sufficient to regulate VEGF-mediated angiogenesis in vitro and in vivo.25 As ligand-independent nuclear receptors, activity of these transcription factors is tightly controlled at the level of protein expression. Aberrant NR4A2 expression in the synovial lining layer, subsynovial synoviocytes, and vascular endothelial cells in inflammatory arthritides confirms that NR4A2 is expressed in cells believed to be at the leading edge of invading pannus.5,20 In this study, we have confirmed that NR4A2 is a downstream effector molecule in the modulation of endothelial function by CRH. Using primary human endothelial cells, stimulation of CRH-R1α-expressing transfectants results in a rapid CRH-dependent induction of NR4A2 mRNA. In human synoviocyte cells, a major site for cytokine-induced NR4A2 expression in RA,20 comparable CRH-receptor-mediated effects on NR4A2 expression are achieved. In the absence of CRH-R1α, CRH had no effect on the expression of NR4A2 in both cell types, thus confirming our previous observation of the absence of CRH-R1α expression on normal endothelial cells and primary RA synoviocytes.
Studies in RA synovial tissue have shown simultaneous expression of transcription factors including NF-κB, cAMP response element-binding protein (CREB), cJun, and Oct-1, suggesting that the coordinate expression of such transcriptional mediators results in enhanced angiogenesis, inflammatory cell influx, synoviocyte hyperactivity, and tissue outgrowth.19,50,51,52 CRH signals through PKA and CREB/activating transcription factor (ATF) factors in RA synovium and rapidly contributes to CREB-1 and ATF-2 binding activity in primary synovial membrane endothelial cells.18 In the present study, we broaden these observations to verify that synovial CRH-R1α signaling leads to significant transcriptional activation through the CRE consensus sequence. In addition, our data reveal a role for CRH in mediating NF-κB transcriptional activity through the κB DNA binding motif. Activation of NF-κB is a central event in inflammatory diseases, and the construction of NF-κB/p50-deficient mice has established that the p50 subunit of NF-κB is essential for the development of local joint inflammation and destruction in models of collagen-induced arthritis.53,54 Interestingly, the transcriptional regulation of NR4A2 by proinflammatory mediators requires proximal promoter regions that contain CRE and κB consensus sequences.20 High DNA-binding activity of CREB-1 and NF-κB/p50 homodimer subunits to the human NR4A2 promoter has been measured in RA synovium.20 Consequently, the promotion of CREB and NF-κB transcriptional activities by CRH confirms that, in CRH-R1α-bearing cells, NR4A2 is a molecular target downstream of CRH signaling.
In animal models, CRH acts as a potent vasodilator by increasing vascular permeability in a CRH-R1-dependent manner.11,55 It has been established that CRH-induced vasodilation in human skin occurs via mast cell degranulation and is principally mediated by histamine and, to a lesser extent, by prostacyclin and NO.56 A number of studies, although not in the microvasculature, suggest that CRH-induced dilation is mediated through NO.57,58 Jain et al59 demonstrated that NO mediates the relaxation responses to CRH in rat aorta. CRH acting via type 1 CRH receptors in human myometrial cells exerts stimulatory effects on NO production via PKA-dependent and -independent mechanisms.37 We have confirmed that NO production can be significantly up-regulated by CRH in CRH-R1α-expressing endothelial cells. Thus, these findings, taken together with those from previous studies, suggest that histamine-and NO-dependent vasodilation may be mediated, at least in part, through CRH and downstream of CRH-R1α signaling. Notably, CRH-R1α signaling does not seem to increase endothelial VEGF production despite the recent observation that CRH induces the release of mast cell-derived VEGF through CHR-R1-dependent activation of PKA.60 These data suggest that the target genes downstream of CRH-R1 signaling are likely to be tissue and cell type dependent.
In summary, the results from this study confirm that, during chronic inflammation, activated endothelial cells are a major target of CRH action. Engagement of type 1α receptors by CRH activates distinct intracellular signals leading to molecular and functional changes in endothelial cells. An understanding of these molecular and functional changes may lead to the development of endothelium-targeted therapies for RA and other chronic inflammatory conditions.
Acknowledgments
We thank Dr. Eithne Murphy, Dr. David Kane, and the late Dr. Leanne Stafford for synovial biopsy collection and Martina Gogarty for tissue sectioning. We also thank all members of the Murphy lab for helpful discussions.
Footnotes
Address reprint requests to Evelyn P. Murphy, Veterinary Sciences Centre, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: evelyn.murphy@ucd.ie.
Supported by grants from the Health Research Board of Ireland and Science Foundation Ireland (to E.P.M.).
References
- Karalis KP, Kontopoulos E, Muglia LJ, Majzoub JA. Corticotropin-releasing hormone deficiency unmasks the proinflammatory effect of epinephrine. Proc Natl Acad Sci USA. 1999;96:7093–7097. doi: 10.1073/pnas.96.12.7093. [erratum 2000, 97:3782] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife MS, Fisher SA, John S, Worthington J, Shah CJ, Ollier WE, Panayi GS, Lewis CM, Lanchbury JS. Multipoint linkage analysis of a candidate gene locus in rheumatoid arthritis demonstrates significant evidence of linkage and association with the corticotropin-releasing hormone genomic region. Arthritis Rheum. 2000;43:1673–1678. doi: 10.1002/1529-0131(200008)43:8<1673::AID-ANR2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Fife M, Steer S, Fisher S, Newton J, McKay K, Worthington J, Shah C, Polley A, Rosenthal A, Ollier W, Lewis C, Wordsworth P, Lanchbury J. Association of familial and sporadic rheumatoid arthritis with a single corticotropin-releasing hormone genomic region (8q12.3) haplotype. Arthritis Rheum. 2002;46:75–82. doi: 10.1002/1529-0131(200201)46:1<75::AID-ART10034>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Sano H, Karalis K, Friedman TC, Epps HR, Remmers EF, Mathern P, Chrousos GP, Wilder RL. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol. 1993;151:1587–1596. [PubMed] [Google Scholar]
- Murphy EP, McEvoy A, Conneely OM, Bresnihan B, FitzGerald O. Involvement of the nuclear orphan receptor NURR1 in the regulation of corticotropin-releasing hormone expression and actions in human inflammatory arthritis. Arthritis Rheum. 2001;44:782–793. doi: 10.1002/1529-0131(200104)44:4<782::AID-ANR134>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- McEvoy A, Bresnihan B, FitzGerald O, Murphy E. Corticotropin releasing hormone (CRH) signaling in synovial tissue from patients with early inflammatory arthritis is mediated by the type 1α CRH receptor. Arthritis Rheum. 2001;44:1761–1767. doi: 10.1002/1529-0131(200108)44:8<1761::AID-ART311>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- McEvoy AN, Bresnihan B, Fitzgerald O, Murphy EP. Corticotropin-releasing hormone signaling in synovial tissue vascular endothelium is mediated through the cAMP/CREB pathway. Ann NY Acad Sci. 2002;966:119–130. doi: 10.1111/j.1749-6632.2002.tb04209.x. [DOI] [PubMed] [Google Scholar]
- Webster E, Barrientos RM, Contoreggi C, Isaac MG, Ligier S, Gabry KE, Chrousos GP, McCarthy EF, Rice KC, Gold PW, Sternberg EM. Corticotropin-releasing hormone (CRH) antagonist attenuates adjuvant induced arthritis: role of CRH in peripheral inflammation. J Rheumatol. 2002;29:1252–1261. [PubMed] [Google Scholar]
- Tetlow LC, Harper N, Dunningham T, Morris MA, Bertfield H, Woolley DE. Effects of induced mast cell activation on prostaglandin E and metalloproteinase production by rheumatoid synovial tissue in vitro. Ann Rheum Dis. 1998;57:25–32. doi: 10.1136/ard.57.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DE, Tetlow LC. Mast cell activation and its relation to proinflammatory cytokine production in the rheumatoid lesion. Arthritis Res. 2000;2:65–74. doi: 10.1186/ar70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- Koch AE. Angiogenesis: implications for rheumatoid arthritis (Review). Arthritis Rheum. 1998;41:951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Szekanecz ZKA. Cell-cell interactions in synovitis: endothelial cells and immune cell migration. Arthritis Res. 2000;2:368–373. doi: 10.1186/ar114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant TK, Patel DD. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology. 2006;13:1–14. doi: 10.1016/j.pathophys.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Middleton J, Americh L, Gayon R, Julien D, Aguilar L, Amalric F, Girard JP. Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res Ther. 2004;6:60–72. doi: 10.1186/ar1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z, Koch AE. Therapeutic inhibition of leukocyte recruitment in inflammatory diseases. Curr Opin Pharmacol. 2004;4:423–428. doi: 10.1016/j.coph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis Rheum. 2005;52:710–721. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Cyclooxygenase 2-derived prostaglandin E2 production by corticotropin-releasing hormone contributes to the activated cAMP response element binding protein content in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2004;50:1132–1145. doi: 10.1002/art.20157. [DOI] [PubMed] [Google Scholar]
- Takeba Y, Suzuki N, Wakisaka S, Takeno M, Kaneko A, Asai T, Sakane T. Involvement of cAMP responsive element binding protein (CREB) in the synovial cell hyperfunction in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:47–55. [PubMed] [Google Scholar]
- McEvoy AN, Murphy EA, Ponnio T, Conneely OM, Bresnihan B, FitzGerald O, Murphy EP. Activation of nuclear orphan receptor NURR1 transcription by NF-kappa B and cyclic adenosine 5′-monophosphate response element-binding protein in rheumatoid arthritis synovial tissue. J Immunol. 2002;168:2979–2987. doi: 10.4049/jimmunol.168.6.2979. [DOI] [PubMed] [Google Scholar]
- Ralph JA, McEvoy AN, Kane D, Bresnihan B, FitzGerald O, Murphy EP. Modulation of orphan nuclear receptor NURR1 expression by methotrexate in human inflammatory joint disease involves adenosine A2A receptor-mediated responses. J Immunol. 2005;175:555–565. doi: 10.4049/jimmunol.175.1.555. [DOI] [PubMed] [Google Scholar]
- Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- Pei L, Castrillo A, Tontonoz P. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol. 2006;20:786–794. doi: 10.1210/me.2005-0331. [DOI] [PubMed] [Google Scholar]
- Holla VR, Mann JR, Shi Q, DuBois RN. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem. 2006;281:2676–2682. doi: 10.1074/jbc.M507752200. [DOI] [PubMed] [Google Scholar]
- Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203:719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Swerlick RA, Sepp N, Bosse D, Ades EW, Lawley TJ. Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1). J Invest Dermatol. 1994;102:833–837. doi: 10.1111/1523-1747.ep12382086. [DOI] [PubMed] [Google Scholar]
- Lammi J, Huppunen J, Aarnisalo P. Regulation of the osteopontin gene by the orphan nuclear receptor Nurr1 in osteoblasts. Mol Endocrinol. 2004;18:1546–1557. doi: 10.1210/me.2003-0247. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volin MV, Szekanecz Z, Halloran MM, Woods JM, Magua J, Damergis JA, Jr, Haines KG, III, Crocker PR, Koch AE. PECAM-1 and leukosialin (CD43) expression correlate with heightened inflammation in rat adjuvant-induced arthritis. Exp Mol Pathol. 1999;66:211–219. doi: 10.1006/exmp.1999.2261. [DOI] [PubMed] [Google Scholar]
- Elewaut D, De Keyser F, De Wever N, Baeten D, Van Damme N, Verbruggen G, Cuvelier C, Veys EM. A comparative phenotypical analysis of rheumatoid nodules and rheumatoid synovium with special reference to adhesion molecules and activation markers. Ann Rheum Dis. 1998;57:480–486. doi: 10.1136/ard.57.8.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgard C, Stupack D, Jonczyk A, Cheresh D. Decreased angiogenesis and arthritic disease in rabbits treated with avb3 antagonist. J Clin Invest. 1999;103:47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol. 1997;11:39–47. doi: 10.1210/mend.11.1.9874. [DOI] [PubMed] [Google Scholar]
- Rossant CJ, Pinnock RD, Hughes J, Hall MD, McNulty S. Corticotropin-releasing factor type-I and type-2a receptors regulate phosphorylation of calcium/cyclic adenosine 3′, 5′-monophosphate response element-binding protein and activation of p42/44 mitogen-activated protein kinase. Endocrinology. 1999;140:1525–1536. doi: 10.1210/endo.140.4.6656. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, Kanno K, Saito I, Miyasaka N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357–2363. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleolog EM, Young S, Stark AC, McCloskey RV, Feldmann M, Maini RN. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998;41:1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Aggelidou E, Hillhouse EW, Grammatopoulos DK. Up-regulation of nitric oxide synthase and modulation of the guanylate cyclase activity by corticotropin-releasing hormone but not urocortin II or urocortin III in cultured human pregnant myometrial cells. Proc Natl Acad Sci USA. 2002;99:3300–3305. doi: 10.1073/pnas.052296399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Vatish M, Hillhouse EW, Grammatopoulos DK. Preeclampsia is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. J Clin Endocrinol Metab. 2005;90:3680–3687. doi: 10.1210/jc.2004-2210. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Sano H, Karalis K, Webster EL, Goldmuntz EA, Chrousos GP, Wilder RL. Local secretion of corticotropin-releasing hormone in the joints of Lewis rats with inflammatory arthritis. J Clin Invest. 1992;90:2555–2564. doi: 10.1172/JCI116150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak P, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis. Arthritis Rheum. 2000;43:2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Karalis K, Viswanathan A, Koike C, Anand-Apte B, Flynn E, Zetter B, Majzoub JA. Corticotropin-releasing hormone stimulates angiogenesis and epithelial tumor growth in the skin. J Invest Dermatol. 1999;113:838–842. doi: 10.1046/j.1523-1747.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- Kriegsmann J, Keyszer GM, Geiler T, Lagoo AS, Lagoo-Deenadayalan S, Gay RE, Gay S. Expression of E-selectin messenger RNA and protein in rheumatoid arthritis. Arthritis Rheum. 1995;38:750–754. doi: 10.1002/art.1780380606. [DOI] [PubMed] [Google Scholar]
- Proudman SM, Cleland LG, Mayrhofer G. Effects of tumor necrosis factor-alpha, interleukin 1beta, and activated peripheral blood mononuclear cells on the expression of adhesion molecules and recruitment of leukocytes in rheumatoid synovial xenografts in SCID mice. J Rheumatol. 1999;26:1877–1889. [PubMed] [Google Scholar]
- Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- Akdis CA, Blaser K. Histamine in the immune regulation of allergic inflammation. J Allergy Clin Immunol. 2003;112:15–22. doi: 10.1067/mai.2003.1585. [DOI] [PubMed] [Google Scholar]
- Asako H, Kurose I, Wolf R, DeFrees S, Zheng ZL, Phillips ML, Paulson JC, Granger DN. Role of H1 receptors and P-selectin in histamine-induced leukocyte rolling and adhesion in postcapillary venules. J Clin Invest. 1994;93:1508–1515. doi: 10.1172/JCI117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RA, Schoonus SB, Smit MJ, Timmerman H, Leurs R. Histamine H(1)-receptor activation of nuclear factor-kappa B: roles for G beta gamma- and G alpha(q/11)-subunits in constitutive and agonist-mediated signaling. Mol Pharmacol. 2001;60:1133–1142. doi: 10.1124/mol.60.5.1133. [DOI] [PubMed] [Google Scholar]
- Parham KL, Zervou S, Karteris E, Catalano RD, Old RW, Hillhouse EW. Promoter analysis of human corticotropin-releasing factor (CRF) type 1 receptor and regulation by CRF and urocortin. Endocrinology. 2004;145:3971–3983. doi: 10.1210/en.2004-0194. [DOI] [PubMed] [Google Scholar]
- Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear Receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Suzuki N, Takeba Y, Nagafuchi H, Saito N, Hashimoto H, Tomita T, Ochi T, Sakane T. Involvement of simultaneous multiple transcription factor expression, including cAMP responsive element binding protein and OCT-1 for synovial cell outgrowth in patients with rheumatoid arthritis. Ann Rheum Dis. 1998;57:487–494. doi: 10.1136/ard.57.8.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS, Manning AM. Signal transduction and transcription factors in rheumatic disease. Arthritis Rheum. 1999;42:609–621. doi: 10.1002/1529-0131(199904)42:4<609::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Gerondakis S, O’Donnell K, Wicks IP. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest. 2000;105:1799–1806. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Dobner P, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Read MA, Leitch IM, Boura AL, Robinson PJ, Smith R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal placental circulation. J Clin Endocrinol Metab. 1994;79:666–669. doi: 10.1210/jcem.79.2.8045990. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Read MA, Leitch IM, Giles WB, Boura AL, Robinson PJ, Smith R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal-placental circulation: involvement of the nitric oxide-cyclic guanosine 3′,5′-monophosphate-mediated pathway. J Clin Endocrinol Metab. 1995;80:2888–2893. doi: 10.1210/jcem.80.10.7559870. [DOI] [PubMed] [Google Scholar]
- Jain V, Vedernikov YP, Saade GR, Chwalisz K, Garfield RE. The relaxation responses to corticotropin-releasing factor in rat aorta are endothelium dependent and gestationally regulated. Am J Obstet Gynecol. 1997;176:234–240. doi: 10.1016/s0002-9378(97)80042-7. [DOI] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]