Abstract
Macrophages have been suggested to be beneficial for myocardial wound healing. We investigated the role of macrophages in myocardial wound healing by inhibition of macrophage infiltration after myocardial injury. We used a murine cryoinjury model to induce left ventricular damage. Infiltrating macrophages were depleted during the 1st week after cryoinjury by serial intravenous injections of clodronate-containing liposomes. After injury, the presence of macrophages, which secreted high levels of transforming growth factor-β and vascular endothelial growth factor-A, led to rapid removal of cell debris and replacement by granulation tissue containing inflammatory cells and blood vessels, followed by myofibroblast infiltration and collagen deposition. In macrophage-depleted hearts, nonresorbed cell debris was still observed 4 weeks after injury. Secretion of transforming growth factor-β and vascular endothelial growth factor-A as well as neovascularization, myofibroblast infiltration, and collagen deposition decreased. Moreover, macrophage depletion resulted in a high mortality rate accompanied by increased left ventricular dilatation and wall thinning. In conclusion, infiltrating macrophage depletion markedly impairs wound healing and increases remodeling and mortality after myocardial injury, identifying the macrophage as a key player in myocardial wound healing. Based on these findings, we propose that increasing macrophage numbers early after myocardial infarction could be a clinically relevant option to promote myocardial wound healing and subsequently to reduce remodeling and heart failure.
The events that follow tissue damage feature the presence and action of macrophages. Macrophages have been shown to play a central role in wound healing, demonstrating several activities such as phagocytosis of cell debris, induction of apoptosis, recruitment of inflammatory cells and myofibroblasts, regulation of neovascularization, and induction of scar formation.1,2 The importance of macrophages in wound healing has been substantiated by in vivo studies that show that the application of macrophage-activating factors accelerate the wound healing response.3,4 Other studies demonstrated that injection of macrophages into healing cutaneous wounds augments the repair process.5
Although this multifunctional role of macrophages has been well studied, the specific role of macrophages in myocardial wound healing is poorly understood. After myocardial infarction, macrophages are present during the period of replacement of necrotic cell debris by scar tissue and the formation of blood vessels,6,7 which suggests that macrophages play a role in different phases of myocardial wound healing.
Recent studies on the effect of granulocyte-colony-stimulating factor or macrophage-colony-stimulating factor treatment and on reperfusion of ischemic myocardium showed a strong correlation between an increased inflammatory cell infiltration, especially macrophages, into the infarcted area and more effective tissue repair. In addition, a reduction in the degree of left ventricular remodeling and increased recovery or preservation of ventricular function was observed.7,8,9,10,11 These studies suggest that increased macrophage infiltration could favor both wound healing and remodeling.
We hypothesize that macrophages are key players in myocardial wound healing and that inhibition of infiltrating macrophages would therefore lead to an impaired wound healing response. To test this hypothesis, macrophage infiltration was inhibited during the 1st week after myocardial injury by using a liposome-mediated macrophage depletion method. We used a cryoinjury model for myocardial injury of the left ventricle (LV) wall in mice because cryoinjury produces a uniform, highly reproducible area of necrosis, followed by an extensive inflammatory reaction.12
Materials and Methods
Animals
Twelve-week-old male C57BL/6JOlaHsd mice (Harlan Nederland, Horst, The Netherlands) were housed individually in a room with conventional conditions. All procedures performed on mice were approved by the local committee for care and use of laboratory animals and were performed according to strict governmental and international guidelines on animal experimentation.
Operation Procedure
Under general isoflurane (2.5%) anesthesia in combination with a mixture of N2O and O2 (1:1), mice were shaved and disinfected with chlorhexidine. The mice were intubated and ventilated with the anesthetic mixture using a mechanical ventilator (Hugo Sachs Elektronik, March-Hugstetten, Germany). At this point, the analgesic drug buprenorphine (0.03 mg/kg) was given subcutaneously. The heart was exposed through a left lateral thoracotomy. Cryoinjury was inflicted by applying a round 3-mm diameter metal probe cooled to −196°C with liquid nitrogen to the LV wall for 10 seconds. After the frozen myocardium had thawed, the procedure was repeated two times to the same area, as described previously.13 The cryoinjured area was macroscopically identified as a firm white disk-shaped region. The intercostal space and skin were closed with sutures. Mice received 100% oxygen until awakening, after which they were extubated. Sham-operated control mice underwent the same procedure but a nonfrozen probe was used.
Macrophage Depletion
Administration of clodronate (Cl2MDP)-containing liposomes is a commonly used method to deplete macrophages. The method is specific for depletion of monocytes and macrophages, which undergo apoptosis on phagocytosis of Cl2MDP liposomes.14,15 Cl2MDP or the liposomes are not toxic by themselves. Cl2MDP, 0.6 mol/L (250 mg/ml; a gift of Roche Diagnostics GmbH, Mannheim, Germany), and phosphate-buffered saline (PBS) were encapsulated in liposomes composed of phosphatidylcholine (100 mg/ml) and cholesterol (0.8 mg/ml) as described previously.16 Approximately 1% of the Cl2MDP was encapsulated in the liposomes. The nonencapsulated Cl2MDP was removed by centrifugation of the liposomes and careful removal of the white band of liposomes using a pipette. After washing the Cl2MDP liposomes using sterilized PBS, the Cl2MDP liposomes were resuspended in sterilized PBS. The final Cl2MDP liposome suspension contained 5 mg of Cl2MDP per ml. Previous studies optimized the dose and time course of Cl2MDP liposome administration used in this study as effective for macrophage depletion in the mouse.16,17 Therefore, cryoinjured animals received under anesthesia serial intravenous injections of 0.2 ml of Cl2MDP liposomes (n = 47), PBS liposomes (n = 12), or phosphate-buffered saline (PBS, n = 32) 4 hours before infliction of cryoinjury and 1, 3, and 6 days after injury. Part of the sham-operated mice (n = 8) received intravenous injections of Cl2MDP liposomes using the same injection protocol to exclude the possibility of observations not being due to depletion of infiltrating macrophages after cryoinjury but due to a toxic effect or a too-high dose of the Cl2MDP liposomes. The other sham-operated animals were not injected (n = 12). A small group of cryoinjured mice (n = 8) were not injected to exclude the influence of serial injections on wound healing. The efficacy of macrophage depletion was evaluated by immunohistochemistry as described below.
White Blood Cell Counts
At each termination point, blood samples were taken (n = 3 or 4 per time point). Total and differential white blood cell counts were measured by a hematology-automated analyzer (Sysmex-2100-2; Sysmex Corporation, Kobe, Japan) at the Hematology Laboratory of the University Medical Center Groningen. These results were verified by manual differential counts on blood smears that were stained with May Grünwald Giemsa at the Hematology Laboratory of the University Medical Center Groningen, and at least 200 cells were counted. Relative monocyte, neutrophil, and lymphocyte percentages were calculated. For baseline levels, blood samples were taken from untreated, unoperated C57BL/6JOlaHsd mice (n = 3).
Tissue Preparation
Four, 7, 14, and 28 days after surgery, mice were sacrificed, and the hearts were excised immediately. Freshly dissected hearts were soaked in ice-cold 30 mmol/L KCl in PBS to induce diastolic arrest. Subsequently, hearts were snap-frozen in liquid nitrogen and stored at −80°C (n = 6–9 per group per time point) or fixed in 2% glutaraldehyde (GA; Merck, Darmstadt, Germany) for at least 24 hours (n = 2–3 per group per time point). Hearts fixed in GA were embedded in Technovit 7100 (T-7100; Heraeus Kulzer, Wehrheim, Germany), according to the manufacturer’s instructions.
Histological Staining
Sections (2 μm) of the ventricles of T-7100 embedded hearts, cut transversally in the middle third between base and apex, were stained with toluidine blue (Fluka Chemie, Buchs, Switzerland) and mounted in Permount (Fisher Scientific, Fair Lawn, NJ). Masson’s trichrome, which stains collagen blue and myocardial cells red, was performed according to a standard protocol on cryosections of 5 to 7 μm, fixed in 4% formaldehyde at room temperature for 1 hour, followed by Bruin’s fixative at 60°C for 5 minutes.
Immunohistochemistry
Cryosections (5 μm) from snap-frozen hearts, cut transversally in the middle third between base and apex, were stained by immunohistochemistry for neutrophils (monoclonal rat anti-mouse neutrophils; Serotec Ltd., Oxford, UK), macrophages (monoclonal rat anti-mouse F4/80; Serotec Ltd.), α-smooth muscle actin (α-SMA) (monoclonal mouse anti-human α-SMA; DakoCytomation, Glostrup, Denmark), collagen type I (polyclonal rabbit anti-mouse collagen 1; Abcam, Cambridge, UK), transforming growth factor-β1 (TGF-β1) (polyclonal goat anti-human TGF-β1; Santa Cruz Biotechnology, Santa Cruz, CA), and vascular endothelial growth factor-A (VEGF-A) (polyclonal rabbit anti-human VEGF-A-20; Santa Cruz Biotechnology). α-SMA staining was performed using a Mouse on Mouse Peroxidase kit according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA). Endogenous biotin and avidin were blocked with avidin/biotin blocking kit according to the manufacturer’s instructions (Vector Laboratories). Endogenous peroxidase was blocked by incubation with 0.1% H2O2 in PBS for 10 minutes. Color development in immunoperoxidase staining was performed with 3-amino-9-ethyl-carbazole (AEC; Sigma-Aldrich, Steinheim, Germany), and sections were counterstained using Mayer’s hematoxylin (Fluka Chemie, Buchs, Switzerland). For staining of α-SMA, α-SMA double staining with TGF-β1, and VEGF-A and TGF-β1 double staining with F4/80, peroxidase-conjugated antibodies were replaced by antibodies conjugated to Cy3 (Zymed Laboratories Inc., San Francisco, CA) or fluorescein isothiocyanate (Southern Biotechnology Associates, ITK Diagnostics, Uithoorn, The Netherlands). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, Zwijndrecht, The Netherlands).
Quantitative Analyses
All evaluations were performed independently by at least two investigators on blinded samples. LV lumen area and diameter of the LV wall at the thinnest point were measured in images made with 1.25× objective of Masson’s trichrome-stained heart sections of mice (n = 3 to 6 mice/time point) and time point (four time points) with customized image processing software (Leica Qwin 4.0; Leica Microsystems, Bannockburn, IL) for area and length measurement.
Percentage of collagen type I and α-SMA in the cryolesion was measured using computerized morphometry (Leica Qwin 4.0 software) on heart sections of each mouse immunostained for collagen type I without counterstaining and α-SMA without DAPI staining. Proportional areas of staining in six equally selected images were measured. Vascular expression of collagen type I and α-SMA was excluded from measurements.
Macrophages and neutrophils in the cryolesion were quantified by counting the number of red-stained cells (AEC) with blue-stained nuclei (hematoxylin) in digital micrographs (×400) taken equally distributed over six areas in the cryolesion. Average numbers were calculated and expressed as number per mm2.
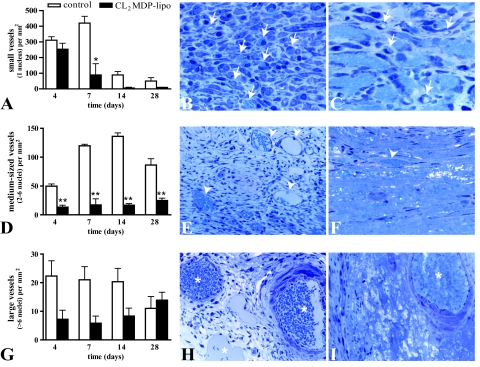
Small (one endothelial cell nucleus), medium-sized (two to six endothelial cell nuclei), and large (>6 endothelial cell nuclei) blood vessels were quantified on toluidine blue-stained T-7100 sections by counting their number in micrographs (×400) taken equally distributed over six areas of the cryolesion. Average numbers were calculated and expressed as number per mm2.
Statistical Analyses
Data are expressed as mean ± SEM. The data were analyzed using statistical software (GraphPad Prism, version 3.00; GraphPad Software, San Diego, CA). Interobserver agreement was evaluated by paired samples t-testing. A survival analysis was performed by the Kaplan-Meier method, and between-group difference in survival was tested by the log-rank test. Comparison of the means within groups was performed by a one-way analysis of variance followed by post hoc Tukey’s test. Between-group comparisons of the means were performed by one-way analysis of variance followed by post hoc t-test. A difference of P < 0.05 was considered statistically significant. All significant differences indicated by asterisks are compared with controls. Other significant differences are specifically indicated in the graphs by asterisks and brackets.
Results
Survival
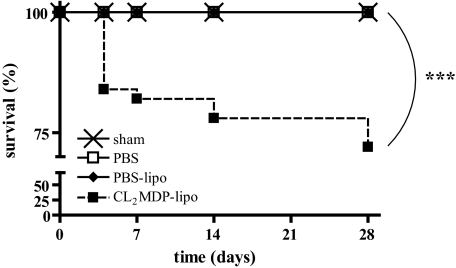
All CL2MDP liposome-injected and noninjected sham-operated mice (n = 20) and mice subjected to cryoinjury and intravenous PBS liposome (n = 12) or PBS or no injections (n = 40) survived. Survival of the cryoinjured mice that were intravenously injected with CL2MDP liposomes (n = 47) was significantly lower (P < 0.001) (Figure 1). Twenty-eight percent of the CL2MDP liposome-injected mice died. Eighteen percent died in the 1st week during the period of CL2MDP liposome injection and 10% after this period. The typical finding on autopsy of the animals was a hemothorax, indicating possible myocardial rupture.
Figure 1.
Kaplan-Meier analysis of survival of sham-operated mice (n = 20) and of cryoinjured mice intravenously injected with PBS (n = 40), PBS liposomes (n = 12), and CL2MDP liposomes (n = 47). Significant differences are indicated as ***P < 0.001.
White Blood Cell Counts
All differential white blood cell counts obtained by the automated hematology analyzer were confirmed by the manual differential counts. The total peripheral white blood cell count remained unchanged in all sham-operated mice and mice injected with PBS, PBS liposomes, or Cl2MDP liposomes and were, respectively, 4.78 × 109/L, 4.28 × 109/L, 4.60 × 109/L, and 4.74 × 109/L on average within groups.
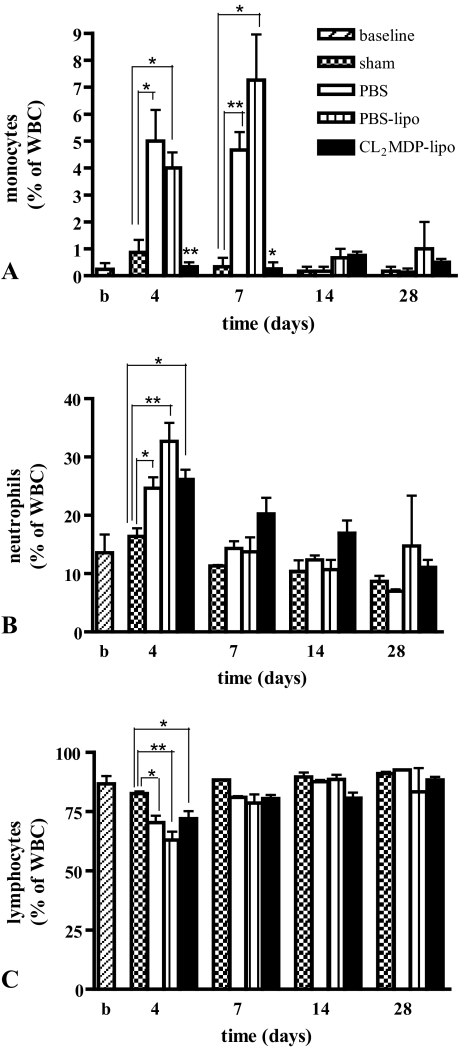
After cryoinjury, levels of circulating monocytes were significantly increased in PBS and PBS liposome-injected mice on days 4 and 7 compared with sham-operated mice (Figure 2A). In the Cl2MDP liposome-treated mice, monocyte levels were significantly reduced on days 4 and 7 after cryoinjury compared with PBS and PBS liposome-injected mice (Figure 2A). After the 1st week, monocyte levels were similar in all groups.
Figure 2.
Differential white blood cell counts in the peripheral blood of untreated, unoperated mice (baseline), sham-operated mice and cryoinjured mice intravenously injected with PBS, PBS liposomes, and CL2MDP liposomes. A: In the Cl2MDP liposome-treated mice, monocyte levels were significantly reduced on days 4 and 7 after cryoinjury compared with PBS and PBS liposome-injected mice. Levels of neutrophils (B) and lymphocytes (C) were similar in mice intravenously injected with PBS, PBS liposomes, and CL2MDP liposomes at all time points. Significant differences are indicated as *P < 0.05, **P < 0.01. Bars represent mean ± SEM.
On day 4 after cryoinjury, an increase in level of circulating neutrophils (Figure 2B) together with a decrease in lymphocyte levels (Figure 2C) was observed in all groups compared with sham-operated mice. Thereafter, levels returned to similar levels as in sham-operated mice. These findings demonstrate that, besides monocytes, the Cl2MDP liposomes did not affect other blood cells. White blood cell levels of sham-operated mice were similar to the baseline data in untreated, unoperated mice at all time points.
Histology
No differences were found in the evaluations of the different investigators. Furthermore, no differences were observed in the morphology of the myocardium of PBS liposome-injected, PBS-injected, or noninjected mice; therefore, they were analyzed as one control group.
LV Lumen Area and Wall Thickness
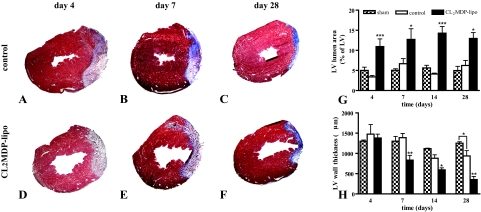
Representative cross sections of the left ventricles of cryoinjured control mice or mice injected with CL2MDP liposomes 4, 7, and 28 days after injury are shown in Figure 3. In controls, cryoinjury did not result in lumen dilatation within the time period investigated, and wall thinning was only apparent on day 28 after injury compared with sham-operated mice (Figure 3, G and H). CL2MDP liposome-mediated macrophage depletion resulted in a significant increase in area of LV lumen as of day 4 (Figure 3G) and wall thinning as of day 7 (Figure 3H) compared with controls and sham-operated mice.
Figure 3.
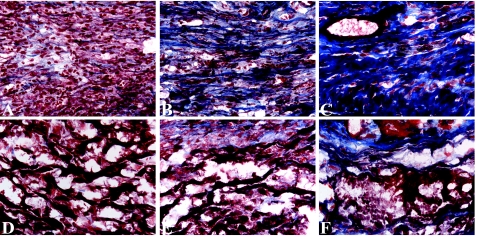
Left ventricular lumen area and wall thickness in the hearts of sham-operated mice, control mice, and mice intravenously injected with CL2MDP liposomes. A–F: Representative Masson’s trichrome stained cross sections of the left ventricles of control (A–C) and CL2MDP liposome-treated (D–F) mice on days 4 (A and D), 7 (B and E), and 28 (C and F) after cryoinjury. Original magnification, ×15. G: LV lumen area. H: LV wall thickness. CL2MDP liposome-mediated macrophage depletion results in a significant wall thinning and increase in LV lumen area compared with controls and sham-operated mice. Significant differences compared with controls are indicated as *P < 0.05, **P < 0.01, ***P < 0.001. Bars represent mean ± SEM.
Macrophage Infiltration
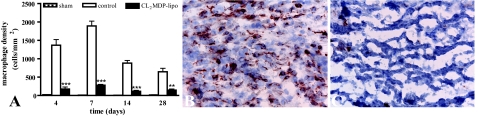
Numbers of macrophages were low in the myocardium of sham-operated mice (Figure 4A). In controls, high numbers of macrophages were present in the cryolesion during the 1st week after injury (Figure 4, A and B). After the 1st week, numbers of macrophages decreased, but they remained present in the cryolesions during the entire study period. Compared with these controls, the number of macrophages in the cryolesions of mice injected with CL2MDP liposomes was significantly lower on all time points investigated (Figure 4), indicating a high efficacy of CL2MDP liposome-mediated macrophage depletion.
Figure 4.
Macrophage density in the myocardium of sham-operated mice and in the myocardial cryolesions of control mice and mice intravenously injected with CL2MDP liposomes. A: Quantitative analysis of the macrophage density in the cryolesions. Significant differences compared with controls are indicated as **P < 0.01, ***P < 0.001. Bars represent mean ± SEM. B and C: F4/80 staining in the cryolesion of a control (B) and a CL2MDP liposome-injected (C) mouse 7 days after injury. High numbers of macrophages are present in the cryolesion during the 1st week after injury, whereas they are significantly reduced after CL2MDP liposome injection. Original magnification, ×400.
Neutrophil Infiltration
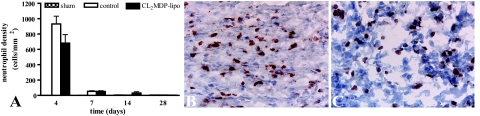
Administration of CL2MDP liposomes did not change the numbers of infiltrating neutrophils after cryoinjury (Figure 5). Neutrophils were present in cryolesions of both CL2MDP liposome-injected and control mice in equally high numbers on day 4 (compare Figure 5, B and C) and decreased rapidly thereafter in both groups. Numbers of neutrophils were low in the myocardium of sham-operated mice (Figure 5A).
Figure 5.
Neutrophil density in the myocardium of sham-operated mice and in the myocardial cryolesions of control mice and mice intravenously injected with CL2MDP liposomes. A: Quantitative analysis of the neutrophil density in the cryolesions. Bars represent mean ± SEM. B and C: Anti-neutrophil staining in the cryolesion of a control (B) and a CL2MDP liposome-injected (C) mouse 4 days after injury. Neutrophil density is similar in both groups. Original magnification, ×400.
Cardiomyocyte Replacement
Four days after cryoinjury, an extensive inflammatory cell infiltration and a complete absence of necrotic cardiomyocytes were observed in the cryolesions of controls (Figure 6A). In contrast, on day 4, the cryolesions of macrophage-depleted mice showed only low numbers of inflammatory cells and contained numerous remnants of necrotic cardiomyocytes (Figure 6D). These remnants could still be observed on days 14 and 28 after cryoinjury (Figure 6, E and F), demonstrating a considerably slower progression of cardiomyocyte replacement by granulation tissue.
Figure 6.
Cardiomyocyte clearance and collagen deposition in the myocardial cryolesions of control mice (A–C) and mice intravenously injected with CL2MDP liposomes (D–F) stained by Masson’s trichrome (blue, collagen; red, myocardial cells) on days 4 (A and D), 7 (B and E), and 28 (C and F) after cryoinjury. Four days after cryoinjury extensive inflammatory cell infiltration and a complete clearance of necrotic cardiomyocytes can be observed in the cryolesions of control mice (A). Furthermore, small collagen fibrils are present throughout the cryolesion. After day 4, the amount of collagen progressively increases (B and C), resulting in a highly collagenous scar on day 28. In contrast, on day 4, the cryolesion of macrophage-depleted mice shows only low numbers of inflammatory cells and numerous remnants of necrotic cardiomyocytes (D). These remnants can still be observed on days 14 (E) and 28 (F) after cryoinjury. Moreover, the amount of deposited collagen is low compared with controls at all time points. Magnification, ×400.
Collagen Deposition
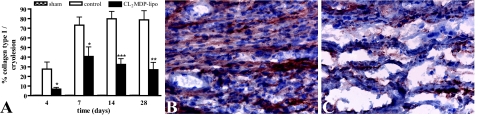
Only very low amounts of collagen could be detected in the myocardium of sham-operated mice (Figure 7A). On day 4, deposited collagen fibrils were present throughout the cryolesion (Figure 6A) but were hardly observed after macrophage depletion (Figure 6D). This observation was confirmed by morphometric quantification of deposited collagen type I, a major component of myocardial scar. In 4-day-old cryolesions, 27 ± 7% surface area collagen type I was present, but after macrophage depletion, the amount of collagen type I was significantly lower, with only 7 ± 2% surface area present (P < 0.05; Figure 7A). After day 4, in controls, the total amount of collagen, as observed by Masson’s trichrome staining (Figure 6), and the amount of collagen type I (P < 0.001; Figure 7A) further increased, which resulted in a highly collagenous scar on day 28 (Figure 6C). Although an increase in collagen deposition was observed in the cryolesions of macrophage-depleted mice over time, the amount of deposited collagen was significantly lower compared with controls at all time points investigated, as observed by Masson’s trichrome staining (Figure 6) and by the amount of collagen type I (Figure 7A). Thus, macrophage depletion resulted in a scar with low collagen content.
Figure 7.
Collagen type I expression in the myocardium of sham-operated mice and in the myocardial cryolesions of control mice and mice intravenously injected with CL2MDP liposomes. A: Quantitative analysis of collagen type I deposition in the cryolesions. Significant differences compared with controls are indicated as *P < 0.05, **P < 0.01, ***P < 0.001. Bars represent mean ± SEM. B and C: Collagen type I staining in the cryolesion of a control mouse (B) and a CL2MDP liposome-injected mouse (C) 7 days after injury. Collagen type I deposition is low in liposome-mediated macrophage-depleted mice compared with controls. Original magnification, ×400.
Myofibroblast Infiltration
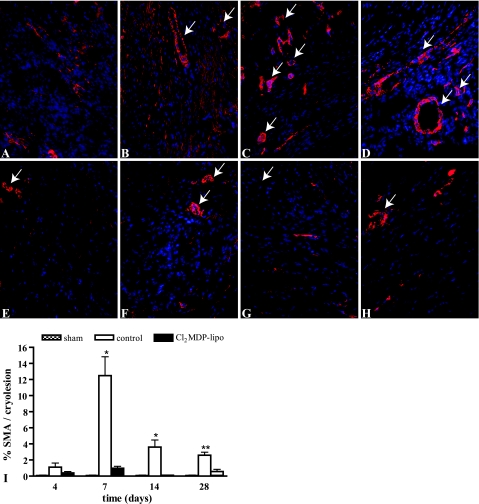
Because myofibroblasts are known to be important for collagen deposition during scar formation after myocardial injury, we determined the numbers of myofibroblasts in the cryolesions. Myofibroblasts were not observed in the myocardium of sham-operated mice (Figure 8I). Myofibroblasts, identified as spindle-shaped α-SMA positive cells, accumulated in controls in the border zone of the cryolesion 4 days after cryoinjury (Figure 8A). Myofibroblast infiltration in the cryolesions was a transient response (Figure 8I), with high numbers of accumulating myofibroblasts on day 7 (P < 0.01 compared with days 4 and 14; Figure 8, B and I) that markedly decreased thereafter. On days 14 and 28, α-SMA staining was predominantly localized in smooth muscle cells of blood vessels, indicating maturation of the neovasculature in the cryolesion (Figure 8, C and D).
Figure 8.
α-SMA staining in the myocardial cryolesions. A–H: α-SMA staining (red) in the myocardium of sham-operated mice and in the cryolesion of control mice (A–D) and mice intravenously injected with CL2MDP liposomes (E–H) 4 (A and E), 7 (B and F), 14 (C and G), and 28 (D and H) days after injury. Original magnification, ×200. Spindle-shaped α-SMA-positive myofibroblasts accumulated in the border zone of the cryolesion 4 days after cryoinjury (A) and were present throughout the cryolesion on day 7 (B). On days 14 (C) and 28 (D), α-SMA staining was predominantly localized in smooth muscle cells of blood vessels. Only low numbers of myofibroblasts infiltrated into the cryolesion of macrophage-depleted mice (E–H). Furthermore, fewer α-SMA-positive vascular structures were observed in the cryolesions of macrophage-depleted mice compared with controls on days 14 and 28 (G and H). Arrows indicate examples of α-SMA-positive blood vessels, which were excluded from semiquantitative analysis. Nuclei are stained by DAPI (blue). I: Quantitative analysis of the α-SMA staining in the cryolesions. Significant differences compared with controls are indicated as *P < 0.01, **P < 0.001. Bars represent mean ± SEM.
Only low numbers of myofibroblasts were present in the cryolesion of macrophage-depleted mice, which were significantly lower compared with controls on days 7, 14, and 28 (Figure 8I). Furthermore, in the macrophage-depleted mice, less α-SMA-positive vascular structures were observed in the cryolesions compared with controls on days 14 and 28 (Figure 8, G and H).
Neovascularization
Vascularization of the cryolesion was determined by counting the small, medium-sized, and large blood vessels, defined, respectively, as vessels comprising one, two to six, or more than six endothelial cell nuclei. The cryolesion of controls as well as that of CL2MDP liposome-injected mice exhibited a low capillary density 4 days after injury, with 311 ± 22 and 254 ± 38 small vessels/mm2, respectively (Figure 9A). In contrast, healthy myocardium contains 2650 ± 172 small vessels/mm2, as we have demonstrated previously.7 In the cryolesion of control mice, the number of capillaries remained constant until day 7. After day 7, the number of small vessels decreased. In the cryolesions of macrophage-depleted mice, the number of small capillary vessels had already decreased on day 7 and was significantly lower compared with controls (compare Figure 9, B and C; P < 0.05). Therefore, macrophage depletion led to a faster regression of small vessels compared with controls. Furthermore, most small vessels of controls contained erythrocytes, indicating perfusion, whereas the small vessels of macrophage-depleted mice only sporadically contained erythrocytes, suggesting a lack of perfusion.
Figure 9.
Vascularization in myocardial cryolesions of control mice and mice intravenously injected with CL2MDP liposomes. A, D, and G: Number of small (A), medium (D), and large (G) vessels present in the cryolesion. Significant differences compared with controls are indicated as *P < 0.05, **P < 0.01. Bars represent mean ± SEM. B and C: Micrographs of small vessels (selection indicated by arrows) in the cryolesion of a toluidine blue-stained, 2-μm-thick T-7100 section of a control mouse (B) and a mouse intravenously injected with CL2MDP liposomes (C) 7 days after injury. In some small vessels, erythrocytes can be observed (light blue). The number of small vessels is lower in liposome-mediated macrophage-depleted mice compared with controls. Magnification, ×1000. E and F: Medium-sized vessels (selection indicated by arrowheads) in the cryolesion of a control mouse (E) and a mouse intravenously injected with CL2MDP liposomes (F) 14 days after injury. The number of medium-sized vessels is lower in liposome-mediated macrophage-depleted mice compared with controls. Magnification, ×400. H and I: Large vessels (asterisks) in the cryolesion of a control mouse (H) and a mouse intravenously injected with CL2MDP liposomes (I) 28 days after injury. Magnification, ×400.
Whereas small vessels were only sporadically observed in the cryolesion after the 1st week, the number of medium-sized vessels was high in the cryolesion during all time points investigated, with 50 ± 4 vessels/mm2 on day 4, 120 ± 3 vessels/mm2 on day 7, 136 ± 6 vessels/mm2 on day 14, and 86 ± 11 vessels/mm2 on day 28 (Figure 9D). To compare, healthy myocardium contains 18 ± 1 medium-sized vessels/mm2.7 In contrast to controls, no increase in medium-sized vessels was observed after macrophage depletion (Figure 9D).
Although the number of large vessels tended to be higher in controls, no significant differences were found between controls and CL2MDP liposome-injected mice (Figure 9, G–I). Both medium-sized and large vessels were functional because they contained erythrocytes.
TGF-β1 and VEGF-A Expression
The observation that both scar formation and neovascularization were markedly impaired after macrophage depletion demonstrates that macrophages are key regulators of these two important aspects of myocardial wound healing. To further substantiate this result, we investigated the expression patterns in the cryolesions of two important factors in neovascularization and scar formation, ie, TGF-β1, a key player in both scar formation and neovascularization, and VEGF-A, an endothelial cell-specific mitogen that is highly angiogenic.
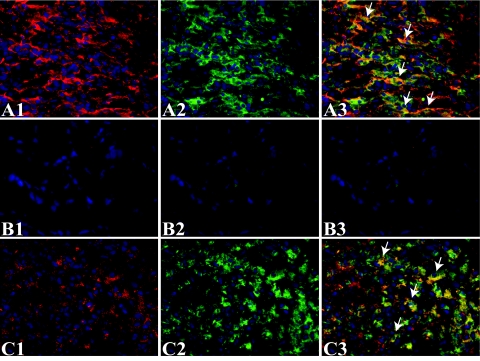
On days 4 and 7, high levels of TGF-β and VEGF-A were present in the control cryolesions (Figure 10, A1 and C1). Immunofluorescent double staining for TGF-β and VEGF with F4/80 showed that both factors co-localized with macrophages in the control cryolesion (Figure 10, A and C). Although myofibroblasts are also known to be able to produce TGF-β, co-localization of TGF-β and myofibroblasts, as detected by α-SMA, was only sporadically observed (data not shown). Co-localization of TGF-β and α-SMA was, however, frequently observed in smooth muscle cells of blood vessels (data not shown). As expected due to the observation that macrophages produce high amounts of TGF-β and VEGF-A, TGF-β (Figure 10B) and VEGF-A (data not shown) were markedly reduced in the cryolesions of macrophage-depleted mice.
Figure 10.
Macrophage TGF-β and VEGF-A expression in myocardial cryolesions of control mice and mice intravenously injected with CL2MDP liposomes 7 days after injury. A and B: Immunofluorescent double staining using an anti-TGF-β antibody (A1 and B1, red) and an anti-F4/80 antibody for macrophages (A2 and B2, green) after PBS (A) or CL2MDP liposome (B) injection. Arrows in the merged micrographs (A3 and B3) indicate a selection of co-localization of TGF-β and macrophages. C: Immunofluorescent double staining using an anti-VEGF-A antibody (C1, red) and an anti-F4/80 antibody (C2, green) for macrophages after PBS (A) injection. Arrows in the merged micrograph (C3) indicate a selection of co-localization of VEGF-A and macrophages. A high level of TGF-β and VEGF-A is present in the cryolesion (A1 and C1). The merged micrographs show co-localization of TGF-β (A3) and VEGF-A (C3) with macrophages in the cryolesion. TGF-β (B) and VEGF-A (data not shown) expression was markedly reduced in the cryolesions of macrophage-depleted mice. Magnification, ×400.
Discussion
Effective myocardial wound healing is characterized by rapid removal of cell debris, a strong but transient inflammatory response, neovascularization, myofibroblast infiltration, and, ultimately, effective scar formation. Here, we show that liposome-mediated macrophage depletion during the 1st week after myocardial injury markedly impairs all of these processes, which demonstrates that macrophages are pivotal for myocardial wound healing.
Clodronate-containing liposomes are commonly used to study the role of macrophages in various organs. Because vascular endothelium of capillaries cannot be crossed by liposomes, the intravenously injected clodronate liposomes have no access to resident macrophages in the heart itself. However, monocytes, the precursors of macrophages, can be depleted by intravenous injection of clodronate liposomes in the circulation,18 and subsequently, macrophage infiltration into organs during an inflammatory reaction can be prevented. Galeazzi et al17 described an effective depletion of macrophages in the inflamed jejunum of mice after serial intravenous injections of clodronate liposomes. Using the same approach, we reached substantial and sufficient macrophage depletion in the injured myocardium, as shown by immunohistochemistry.
In controls, presence of high numbers of macrophages led to a rapid removal of necrotic cardiomyocytes and replacement by granulation tissue, containing inflammatory cells and blood vessels, followed by deposition of collagen. This process was considerably slower when macrophage infiltration was prevented by administration of clodronate liposomes during the 1st week after injury. In the macrophage-depleted hearts, nonresorbed cell debris was still observed 4 weeks after cryoinjury, and markedly less granulation tissue, including blood vessels and scar, were formed. Therefore, macrophages provide the main phagocytotic capacity for the removal of cellular debris in the injured myocardium, and absence of macrophages cannot be compensated by the presence of other cells like neutrophils, which are also known to have phagocytotic capacity.
Besides their role in removal of cellular debris, macrophages were shown to secrete high levels of VEGF-A and TGF-β in the cryolesions. These growth factors are both known to be important for neovascularization.19,20 Subsequently, depletion of macrophages led to a marked reduction of these growth factors together with a marked reduction in neovascularization, which demonstrates that macrophages are key regulators of vessel formation during myocardial wound healing via secretion of angiogenic growth factors. This finding is in line with the study of Pipp et al,21 showing that monocyte depletion totally abolishes collateral growth in both rabbit and mouse. In addition to the secretion of growth factors, the ability of macrophage progenitors, ie, monocytes, to differentiate into endothelium-like cells may also contribute to their angiogenic effects.22,23,24 Moreover, Moldovan et al25 showed that macrophages drill tunnels in ischemic myocardium using macrophage metalloelastase and suggested that these tunnels might evolve to become capillaries, which may therefore represent another mechanism by which macrophages participate in neovascularization in the injured myocardium.
Furthermore, macrophages may not only be regulators of formation of new blood vessels in injured myocardium but also of subsequent maturation of these newly formed vessels. We can substantiate this with our observation of a reduction in the number of vessels containing smooth muscle cells, which are usually present around larger vessels, and the fact that the total number of large vessels was not reduced after macrophage depletion. Danenberg et al26 showed that macrophage depletion during neointima formation significantly reduced proliferation of smooth muscle cells, which suggests that although we did not study smooth muscle cell proliferation, macrophage depletion may have reduced smooth muscle cell proliferation and thereby vessel maturation.
Macrophage depletion also led to a markedly low presence of myofibroblasts in the cryolesions, indicating that macrophages are essential for myofibroblast formation and recruitment after injury. Macrophages probably regulate myofibroblast formation via TGF-β secretion, because our results demonstrated that macrophages express high levels of TGF-β in the injured area and TGF-β is known to induce fibroblast to myofibroblast differentiation after injury.27 Furthermore, macrophages have been shown to express tenascin-C,28 which regulates recruitment of myofibroblasts during wound healing after myocardial injury.29
Myofibroblasts are regarded as major contributors to scar formation both indirectly by regulating scar formation via secretion of fibrogenic growth factors30,31 and directly by collagen secretion.32 Therefore, the reduced presence of myofibroblasts because of the absence of macrophages impaired collagen deposition in the cryolesion.
Fast and adequate replacement of necrotic tissue by granulation tissue, and ultimately scar, is suggested to be beneficial because it could shorten the period of vulnerability to most common complications that occur during the healing of myocardial injury, such as lumen dilatation, wall thinning, and aneurisms, as well as cardiac rupture.10 Our results confirm this hypothesis, because the slower healing and scar formation after injury resulting from the depletion of macrophage lead to increased lumen dilatation and wall thinning and a significantly decreased survival of the macrophage-depleted mice. This major influence of wound healing on outcome after myocardial injury further substantiates the importance of macrophages.
The cryoinjury model was selected in this study over the coronary artery ligation model. In contrast to the latter, cryoinjury produces a homogenous lesion in a defined location and of a reproducible size, which facilitates the comparison of infiltrating cells, scar formation, and remodeling due to the small variability between animals. Nevertheless, the nature of the injury is different from that produced by ischemic myocardial infarction, and therefore the resultant scar after cryoinjury can differ from that found clinically.
Based on our findings, we suggest that early administration of additional macrophages after myocardial infarction could be a relevant option for improving myocardial wound healing and subsequent functional outcome after myocardial infarction. Recently, this suggestion has been substantiated by the demonstration that intracardiac injection of human macrophages into the infarcted myocardium of rats immediately after permanent coronary ligation improves vascularization, myofibroblast accumulation, scar thickening, and accumulation of resident macrophages and, moreover, reduces remodeling and ventricular dysfunction.33 Several studies have focused on injection of macrophage-related growth factors and cytokines improve healing after myocardial infarction.8,34,35,36 However, treatment with macrophages has advantages over treatment with growth factors and cytokines. Besides their phagocytotic capacity, macrophages release most, if not all, of the required growth factors and cytokines for adequate myocardial wound healing.
In conclusion, we acquired a better understanding of the multifunctional role of macrophages in myocardial wound healing. Based on these findings, we propose that increasing macrophage numbers early after myocardial infarction could be a clinically relevant option to promote myocardial wound healing and subsequently to reduce remodeling and heart failure.
Footnotes
Address reprint requests to Machteld J. van Amerongen, M.Sc., University Medical Center Groningen, Faculty of Medical Sciences, Department of Pathology and Laboratory Medicine, Medical Biology Section, Hanzeplein 1, 9713 GZ Groningen, The Netherlands. E-mail: m.van.amerongen@med.umcg.nl.
References
- Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–3638. doi: 10.1242/dev.124.18.3633. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Wiseman DM. Macrophages, wound repair and angiogenesis. Prog Clin Biol Res. 1988;266:131–145. [PubMed] [Google Scholar]
- Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc. 1980;27:1–11. [PubMed] [Google Scholar]
- Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci USA. 1989;86:2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. Increased inflammatory response and neovascularization in reperfused vs. nonreperfused murine myocardial infarction. Cardiovasc Pathol. 2006;15:83–90. doi: 10.1016/j.carpath.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Minatoguchi S, Takemura G, Chen XH, Wang N, Uno Y, Koda M, Arai M, Misao Y, Lu C, Suzuki K, Goto K, Komada A, Takahashi T, Kosai K, Fujiwara T, Fujiwara H. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. 2004;109:2572–2580. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- Morita M, Kawashima S, Ueno M, Kubota A, Iwasaki T. Effects of late reperfusion on infarct expansion and infarct healing in conscious rats. Am J Pathol. 1993;143:419–430. [PMC free article] [PubMed] [Google Scholar]
- Richard V, Murry CE, Reimer KA. Healing of myocardial infarcts in dogs. Effects of late reperfusion. Circulation. 1995;92:1891–1901. doi: 10.1161/01.cir.92.7.1891. [DOI] [PubMed] [Google Scholar]
- Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, Ichikawa Y, Ikeda Y, Kobayashi H, Ohori K, Shimamoto K. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. J Am Coll Cardiol. 2006;47:626–634. doi: 10.1016/j.jacc.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Jensen JA, Kosek JC, Hunt TK, Goodson WH, 3rd, Miller DC. Cardiac cryolesions as an experimental model of myocardial wound healing. Ann Surg. 1987;206:798–803. doi: 10.1097/00000658-198712000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen MJ, Harmsen MC, Petersen AH, Kors G, van Luyn MJ. The enzymatic degradation of scaffolds and their replacement by vascularized extracellular matrix in the murine myocardium. Biomaterials. 2006;27:2247–2257. doi: 10.1016/j.biomaterials.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Naito M, Nagai H, Kawano S, Umezu H, Zhu H, Moriyama H, Yamamoto T, Takatsuka H, Takei Y. Liposome-encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J Leukoc Biol. 1996;60:337–344. doi: 10.1002/jlb.60.3.337. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Galeazzi F, Haapala EM, Van Rooijen N, Vallance BA, Collins SM. Inflammation-induced impairment of enteric nerve function in nematode-infected mice is macrophage dependent. Am J Physiol. 2000;278:G259–G265. doi: 10.1152/ajpgi.2000.278.2.G259. [DOI] [PubMed] [Google Scholar]
- Sunderkötter C, Nikolic T, Dillon MJ, van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996;271:603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, Adamkiewicz J, Elsasser HP, Muller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87:378–384. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R, Gati I, Moerman E, Golomb G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S, Sharifi BG. Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation. 1999;99:1284–1289. doi: 10.1161/01.cir.99.10.1284. [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- Katwa LC. Cardiac myofibroblasts isolated from the site of myocardial infarction express endothelin de novo. Am J Physiol. 2003;285:H1132–H1139. doi: 10.1152/ajpheart.01141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, Kachel E, Holbova R, Mardor Y, Daniels D, Ocherashvilli A, Orenstein A, Danon D. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114:94–100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2003;108:2134–2140. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, Ichikawa Y, Ikeda Y, Kobayashi H, Ohori K, Shimamoto K. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. J Am Coll Cardiol. 2006;47:626–634. doi: 10.1016/j.jacc.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Hughes GC, Biswas SS, Yin B, Coleman RE, DeGrado TR, Landolfo CK, Lowe JE, Annex BH, Landolfo KP. Therapeutic angiogenesis in chronically ischemic porcine myocardium: comparative effects of bFGF and VEGF. Ann Thorac Surg. 2004;77:812–818. doi: 10.1016/j.athoracsur.2003.09.060. [DOI] [PubMed] [Google Scholar]