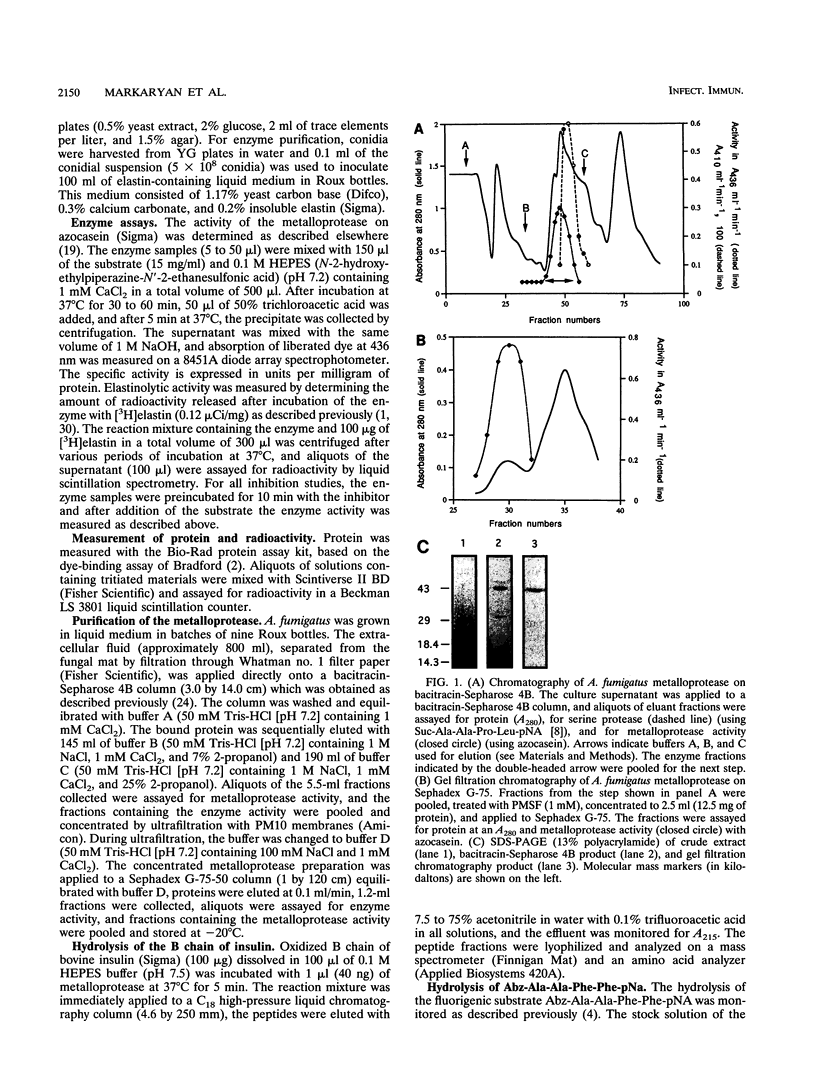
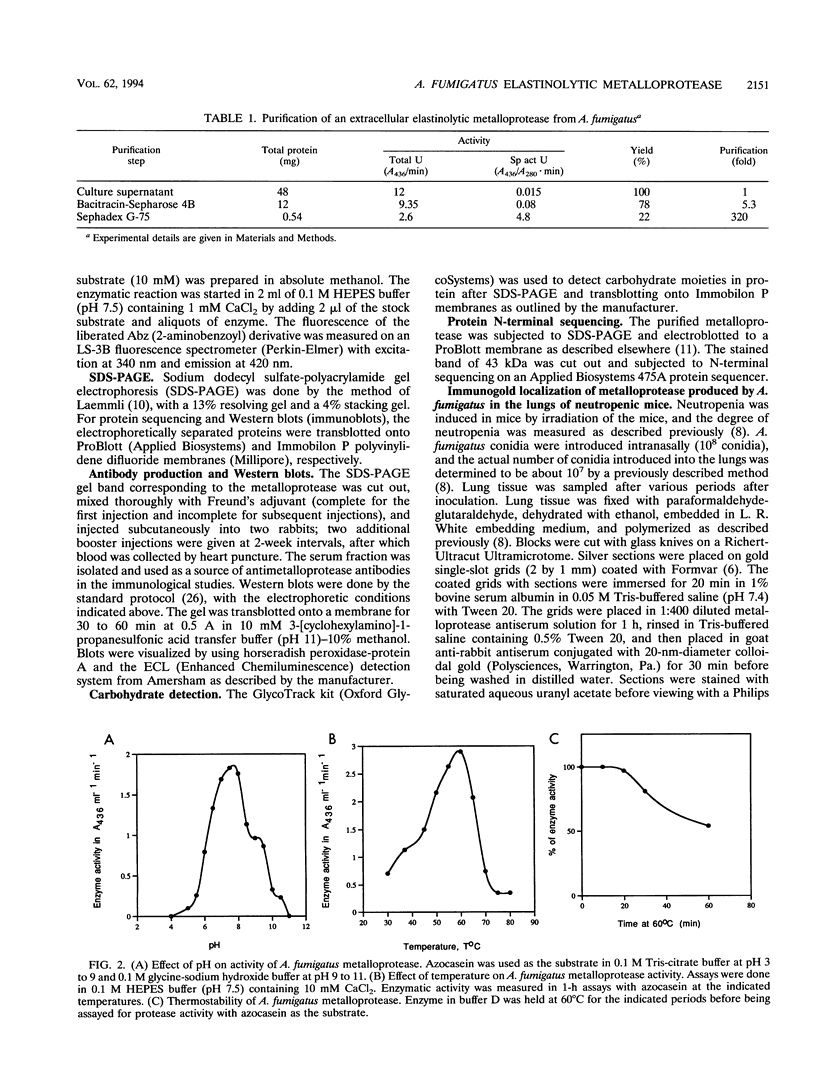
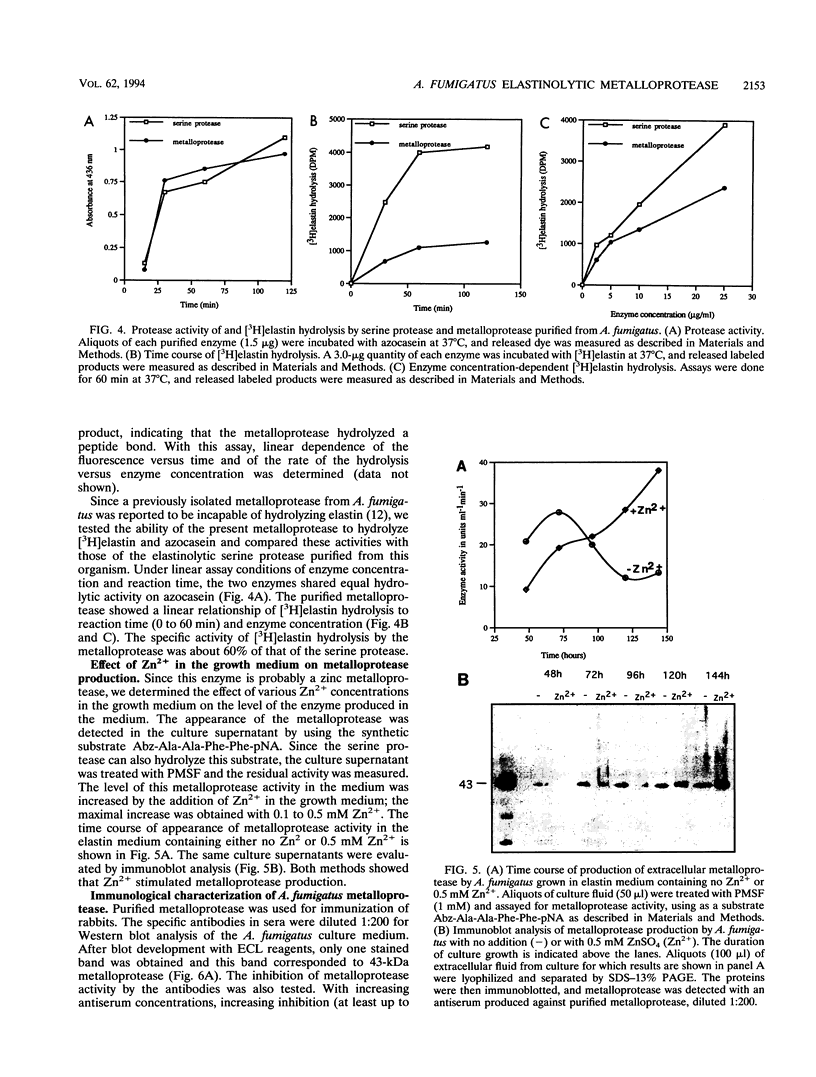
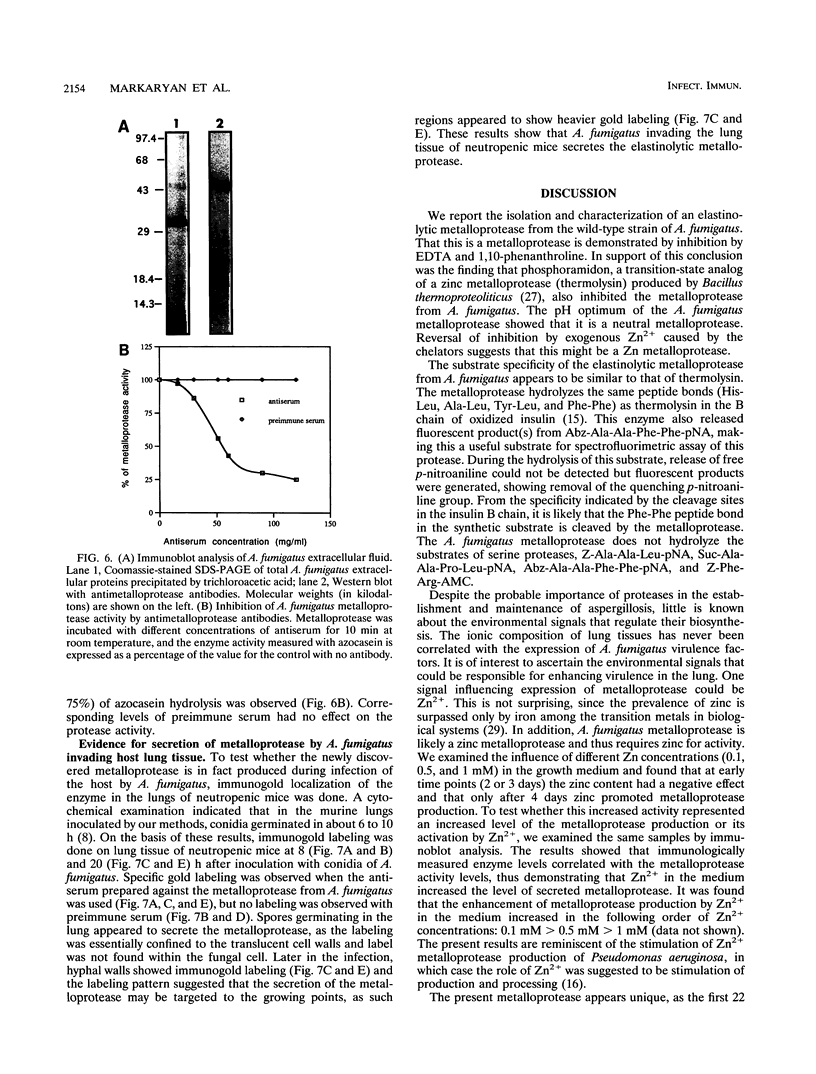
Abstract
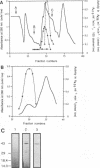
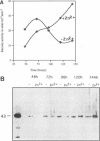
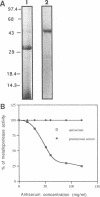
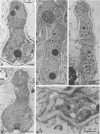
Extracellular proteases have been suggested to be virulence factors in invasive aspergillosis. Since serine protease gene-disrupted mutants retain virulence, other proteases are suspected to be also involved in the degradation of lung structural material. An elastinolytic neutral metalloprotease was purified 320-fold from the extracellular fluid of Aspergillus fumigatus grown on elastin by affinity chromatography on bacitracin-Sepharose 4B and gel filtration on Sephadex G-75. The molecular mass was determined to be 43 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. No carbohydrate was attached to this metalloprotease, and its first 22 N-terminal amino acids did not show any homology with the known metalloproteases. The enzyme was completely inhibited by EDTA, 1,10-phenanthroline, and phosphoramidon but not by inhibitors specific for serine, aspartate, and cysteine proteases. Zn2+ and, to a lesser extent, Co2+ reversed the inhibition caused by 1,10-phenanthroline. The protease hydrolyzed the peptide bonds His-Leu, Ala-Leu, Tyr-Leu, Gly-Phe, and Phe-Phe in the B chain of insulin. Synthetic substrate Abz-Ala-Ala-Phe-Phe-pNA could be used for the fluorimetric assay of the A. fumigatus metalloprotease. This enzyme had maximum activity in the pH range 7.5 to 8.0 and at 60 degrees C. It retained 50% of the protease activity when held at 60 degrees C for 1 h. Zn2+ and Co2+ at 1 mM did not inhibit the protease activity. The metalloprotease was able to hydrolyze elastin, and its elastinolytic activity was comparable to that of the serine protease from this organism. The presence of Zn2+ in the culture medium stimulated the metalloprotease production. Rabbit antibodies prepared against the enzyme severely inhibited the enzyme activity. Immunogold electron microscopy revealed that A. fumigatus invading neutropenic mouse lungs secretes this metalloprotease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Filippova I. Iu, Lysogorskaia E. N., Oksenoit E. S., Troshchenkova E. P., Stepanov V. M. Novye fluorestsentnye substraty metalloéndopeptidaz s vnutrennim tusheniem fluorestsentsii. Bioorg Khim. 1988 Apr;14(4):467–471. [PubMed] [Google Scholar]
- Frosco M., Chase T., Jr, Macmillan J. D. Purification and properties of the elastase from Aspergillus fumigatus. Infect Immun. 1992 Mar;60(3):728–734. doi: 10.1128/iai.60.3.728-734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaton-Ogay K., Suter M., Crameri R., Falchetto R., Fatih A., Monod M. Nucleotide sequence of a genomic and a cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiol Lett. 1992 Apr 15;71(2):163–168. doi: 10.1016/0378-1097(92)90506-j. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Lee J. D., Rogers L. M., Zimmerman P., Ceselski S., Fox B., Stein B., Copelan E. A. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect Immun. 1993 Jun;61(6):2357–2368. doi: 10.1128/iai.61.6.2357-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M. H., Chase T., Jr, Macmillan J. D. Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect Immun. 1984 Jan;43(1):320–325. doi: 10.1128/iai.43.1.320-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Monod M., Paris S., Sanglard D., Jaton-Ogay K., Bille J., Latgé J. P. Isolation and characterization of a secreted metalloprotease of Aspergillus fumigatus. Infect Immun. 1993 Oct;61(10):4099–4104. doi: 10.1128/iai.61.10.4099-4104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod M., Paris S., Sarfati J., Jaton-Ogay K., Ave P., Latgé J. P. Virulence of alkaline protease-deficient mutants of Aspergillus fumigatus. FEMS Microbiol Lett. 1993 Jan 1;106(1):39–46. doi: 10.1111/j.1574-6968.1993.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Monod M., Togni G., Rahalison L., Frenk E. Isolation and characterisation of an extracellular alkaline protease of Aspergillus fumigatus. J Med Microbiol. 1991 Jul;35(1):23–28. doi: 10.1099/00222615-35-1-23. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Comparative study of various neutral proteinases from microorganisms: specificity with oligopeptides. Arch Biochem Biophys. 1971 Sep;146(1):291–296. doi: 10.1016/s0003-9861(71)80066-8. [DOI] [PubMed] [Google Scholar]
- Olson J. C., Ohman D. E. Efficient production and processing of elastase and LasA by Pseudomonas aeruginosa require zinc and calcium ions. J Bacteriol. 1992 Jun;174(12):4140–4147. doi: 10.1128/jb.174.12.4140-4147.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam M., Dhar S. C. Physico-chemical properties of the acid proteinase from A. fumigatus. Ital J Biochem. 1981 Jan-Feb;30(1):63–74. [PubMed] [Google Scholar]
- Ramesh M. V., Sirakova T., Kolattukudy P. E. Isolation, characterization, and cloning of cDNA and the gene for an elastinolytic serine proteinase from Aspergillus flavus. Infect Immun. 1994 Jan;62(1):79–85. doi: 10.1128/iai.62.1.79-85.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Amlung T. W., Miller M. S. Isolation and characterization of an elastinolytic proteinase from Aspergillus flavus. Infect Immun. 1990 Aug;58(8):2529–2534. doi: 10.1128/iai.58.8.2529-2534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Bode R. B., McCuan-Kirsch C. M. Elastase production in clinical isolates of Aspergillus. Diagn Microbiol Infect Dis. 1988 Jul;10(3):165–170. doi: 10.1016/0732-8893(88)90036-3. [DOI] [PubMed] [Google Scholar]
- Rinaldi M. G. Invasive aspergillosis. Rev Infect Dis. 1983 Nov-Dec;5(6):1061–1077. doi: 10.1093/clinids/5.6.1061. [DOI] [PubMed] [Google Scholar]
- Starcher B. C. Elastin and the lung. Thorax. 1986 Aug;41(8):577–585. doi: 10.1136/thx.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Cohen J., Krausz T., Van Noorden S., Holden D. W. The alkaline protease of Aspergillus fumigatus is not a virulence determinant in two murine models of invasive pulmonary aspergillosis. Infect Immun. 1993 May;61(5):1650–1656. doi: 10.1128/iai.61.5.1650-1656.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronrud D. E., Roderick S. L., Matthews B. W. Structural basis for the action of thermolysin. Matrix Suppl. 1992;1:107–111. [PubMed] [Google Scholar]
- Vallee B. L. Zinc: biochemistry, physiology, toxicology and clinical pathology. Biofactors. 1988 Jan;1(1):31–36. [PubMed] [Google Scholar]
- Wewers M. D., Herzyk D. J., Gadek J. E. Alveolar fluid neutrophil elastase activity in the adult respiratory distress syndrome is complexed to alpha-2-macroglobulin. J Clin Invest. 1988 Oct;82(4):1260–1267. doi: 10.1172/JCI113724. [DOI] [PMC free article] [PubMed] [Google Scholar]