Abstract
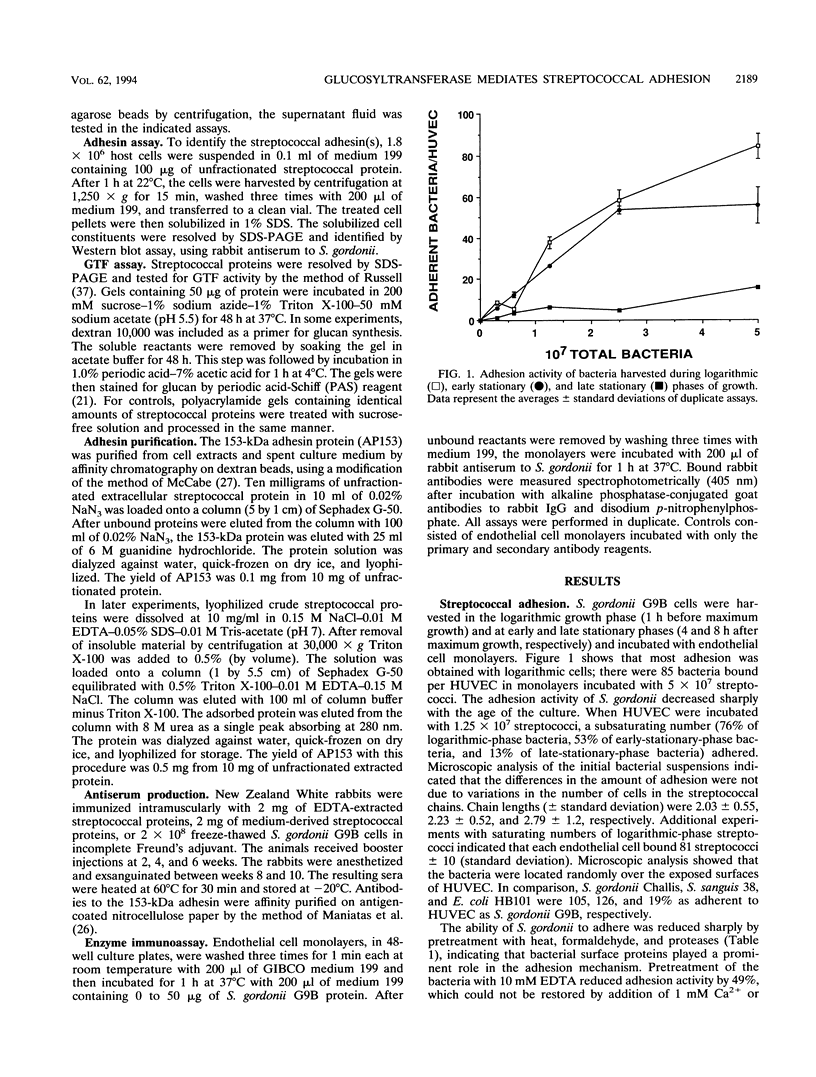
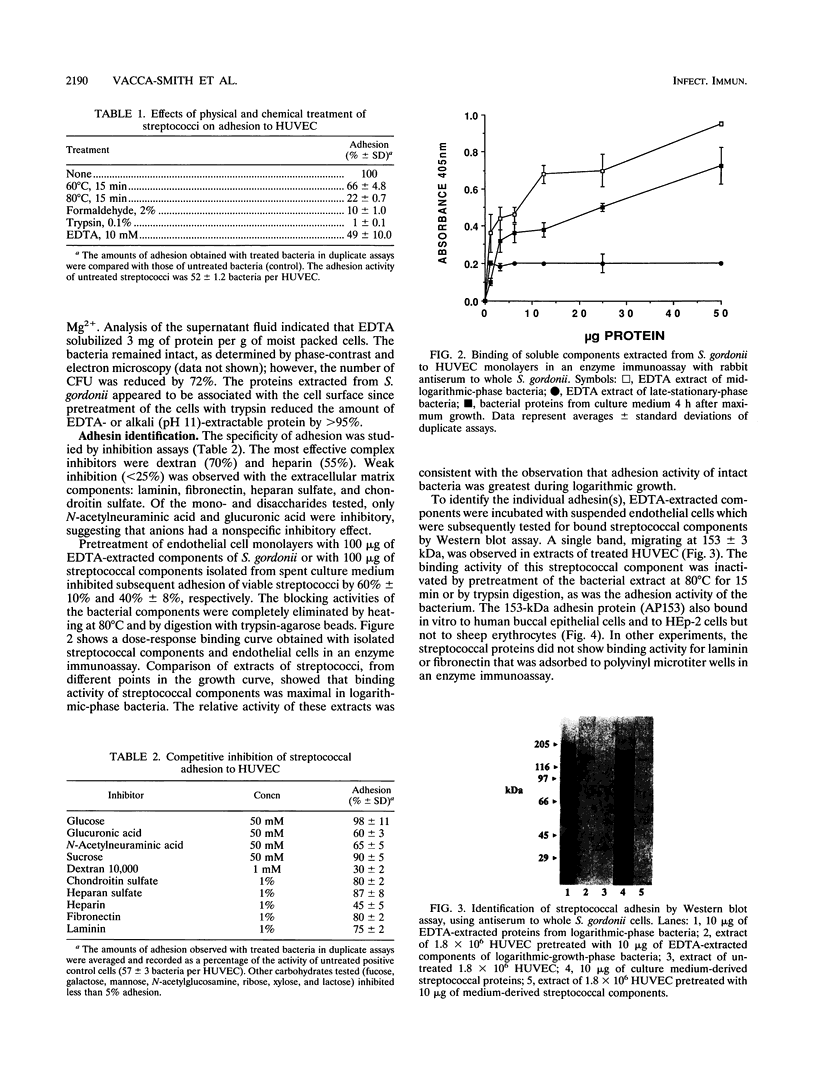
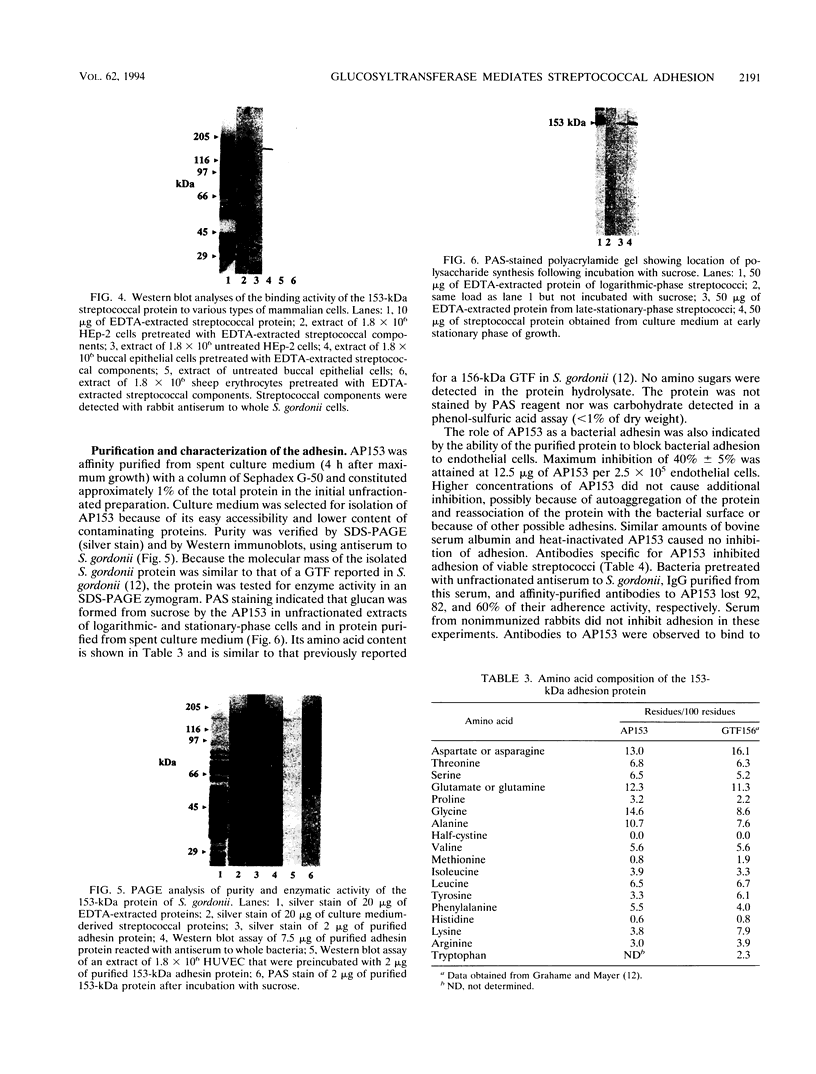
Human umbilical vein endothelial cells (HUVEC) were used as an experimental host model to investigate the mechanism(s) of streptococcal adhesion in infective endocarditis. Adhesion activity of Streptococcus gordonii was maximal during the logarithmic phase of growth and was greatly reduced or eliminated by pretreatment of bacteria with heat, formaldehyde, or trypsin. At saturating numbers of streptococci, an average of 81 bacteria were bound per HUVEC. Streptococcal adhesion was inhibited by low-molecular-weight dextran and heparin but not by sucrose, fibronectin, or laminin. Adhesion was also prevented by pretreatment of HUVEC with proteins dissociated from the surface of S. gordonii with 10 mM EDTA or isolated from spent culture medium. Western blot (immunoblot) assays detected a single adhesion protein of 153 kDa (AP153) on HUVEC after incubation with unfractionated extracts of streptococci. The adhesin exhibited glucosyltransferase (GTF) activity when incubated with sucrose and Triton X-100 after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The AP153 was purified by affinity chromatography on dextran beads and show to have binding activity for HUVEC, GTF activity, an amino acid composition similar to that reported for GTF of S. gordonii, and the ability to inhibit S. gordonii adhesion. Incubation of the streptococci with antibodies to the adhesin inhibited bacterial attachment to HUVEC monolayers. These results indicate that surface-localized GTF mediates adhesion of S. gordonii to HUVEC in vitro and may serve as a mechanism for colonization of the endocardium in infective endocarditis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddour L. M., Christensen G. D., Lowrance J. H., Simpson W. A. Pathogenesis of experimental endocarditis. Rev Infect Dis. 1989 May-Jun;11(3):452–463. doi: 10.1093/clinids/11.3.452. [DOI] [PubMed] [Google Scholar]
- Baddour L. M. Twelve-year review of recurrent native-valve infective endocarditis: a disease of the modern antibiotic era. Rev Infect Dis. 1988 Nov-Dec;10(6):1163–1170. doi: 10.1093/clinids/10.6.1163. [DOI] [PubMed] [Google Scholar]
- Buchan R. A., Jenkinson H. F. Glucosyltransferase production by Streptococcus sanguis Challis and comparison with other oral streptococci. Oral Microbiol Immunol. 1990 Apr;5(2):63–71. doi: 10.1111/j.1399-302x.1990.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Crawford I., Russell C. Comparative adhesion of seven species of streptococci isolated from the blood of patients with sub-acute bacterial endocarditis to fibrin-platelet clots in vitro. J Appl Bacteriol. 1986 Feb;60(2):127–133. doi: 10.1111/j.1365-2672.1986.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Dall L. H., Herndon B. L. Association of cell-adherent glycocalyx and endocarditis production by viridans group streptococci. J Clin Microbiol. 1990 Aug;28(8):1698–1700. doi: 10.1128/jcm.28.8.1698-1700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall L., Barnes W. G., Lane J. W., Mills J. Enzymatic modification of glycocalyx in the treatment of experimental endocarditis due to viridans streptococci. J Infect Dis. 1987 Nov;156(5):736–740. doi: 10.1093/infdis/156.5.736. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Gilliland B. C., Petersdorf R. G. Effect of immunization on susceptibility to experimental Streptococcus mutans and Streptococcus sanguis endocarditis. Infect Immun. 1978 Oct;22(1):52–56. doi: 10.1128/iai.22.1.52-56.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. V., Pedrazzoli V., Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991 Jun;6(3):129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Freedman L. R. The pathogenesis of infective endocarditis. J Antimicrob Chemother. 1987 Sep;20 (Suppl A):1–6. doi: 10.1093/jac/20.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Grahame D. A., Mayer R. M. Purification, and comparison, of two forms of dextransucrase from Streptococcus sanguis. Carbohydr Res. 1985 Oct 15;142(2):285–298. doi: 10.1016/0008-6215(85)85030-8. [DOI] [PubMed] [Google Scholar]
- Grahame D. A., Mayer R. M. The origin and composition of multiple forms of dextransucrase from Streptococcus sanguis. Biochim Biophys Acta. 1984 Apr 27;786(1-2):42–48. doi: 10.1016/0167-4838(84)90151-1. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Cell-free released components of Streptococcus sanguis inhibit human platelet aggregation. Infect Immun. 1983 Oct;42(1):394–401. doi: 10.1128/iai.42.1.394-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Gong K., MacFarlane G. D., Erickson P. R., Soberay A. H., Krebsbach P. H., Manjula G., Schilling K., Bowen W. H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990 Feb;58(2):515–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Sande M. A. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect Immun. 1974 Dec;10(6):1433–1438. doi: 10.1128/iai.10.6.1433-1438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Synthesis of factor VIII by endothelial cells. Ann N Y Acad Sci. 1982;401:163–170. doi: 10.1111/j.1749-6632.1982.tb25715.x. [DOI] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamont R. J., Rosan B., Murphy G. M., Baker C. T. Streptococcus sanguis surface antigens and their interactions with saliva. Infect Immun. 1988 Jan;56(1):64–70. doi: 10.1128/iai.56.1.64-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance J. H., Baddour L. M., Simpson W. A. The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J Clin Invest. 1990 Jul;86(1):7–13. doi: 10.1172/JCI114717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance J. H., Hasty D. L., Simpson W. A. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect Immun. 1988 Sep;56(9):2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M. Purification and characterization of a primer-independent glucosyltransferase from Streptococcus mutans 6715-13 mutant 27. Infect Immun. 1985 Dec;50(3):771–777. doi: 10.1128/iai.50.3.771-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J., Pulliam L., Dall L., Marzouk J., Wilson W., Costerton J. W. Exopolysaccharide production by viridans streptococci in experimental endocarditis. Infect Immun. 1984 Jan;43(1):359–367. doi: 10.1128/iai.43.1.359-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Munro C. L., Macrina F. L. Sucrose-derived exopolysaccharides of Streptococcus mutans V403 contribute to infectivity in endocarditis. Mol Microbiol. 1993 Apr;8(1):133–142. doi: 10.1111/j.1365-2958.1993.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Ness P. M., Perkins H. A. Transient bacteremia after dental procedures and other minor manipulations. Transfusion. 1980 Jan-Feb;20(1):82–85. doi: 10.1046/j.1537-2995.1980.20180125046.x. [DOI] [PubMed] [Google Scholar]
- Ozols J. Amino acid analysis. Methods Enzymol. 1990;182:587–601. doi: 10.1016/0076-6879(90)82046-5. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J Clin Invest. 1978 Oct;62(4):805–814. doi: 10.1172/JCI109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Effects of molecular weight of dextran on the adherence of Streptococcus sanguis to damaged heart valves. Infect Immun. 1980 Jul;29(1):1–7. doi: 10.1128/iai.29.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Use of triton X-100 to overcome the inhibition of fructosyltransferase by SDS. Anal Biochem. 1979 Aug;97(1):173–175. doi: 10.1016/0003-2697(79)90342-7. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schollin J. Adherence of alpha-hemolytic streptococci to human endocardial, endothelial and buccal cells. Acta Paediatr Scand. 1988 Sep;77(5):705–710. doi: 10.1111/j.1651-2227.1988.tb10734.x. [DOI] [PubMed] [Google Scholar]
- Schollin J., Danielsson D. Bacterial adherence to endothelial cells from rat heart, with special regard to alpha-hemolytic streptococci. APMIS. 1988 May;96(5):428–432. doi: 10.1111/j.1699-0463.1988.tb05326.x. [DOI] [PubMed] [Google Scholar]
- Sommer P., Gleyzal C., Guerret S., Etienne J., Grimaud J. A. Induction of a putative laminin-binding protein of Streptococcus gordonii in human infective endocarditis. Infect Immun. 1992 Feb;60(2):360–365. doi: 10.1128/iai.60.2.360-365.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. C. Protease production by Streptococcus sanguis associated with subacute bacterial endocarditis. Infect Immun. 1982 Dec;38(3):1037–1045. doi: 10.1128/iai.38.3.1037-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. I., Baron E. J., Tenenbaum M. J., Kaplan M. H., Greenspan J., Facklam R. R., Tyburski M. B., Goldman M. A., Kanzer B. F., Pizzarello R. A. Viridans streptococcal endocarditis: clinical, microbiological, and echocardiographic correlations. J Infect Dis. 1986 Oct;154(4):597–603. doi: 10.1093/infdis/154.4.597. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992 Sep;56(3):395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J. B., Lindholm P., Peterson P. K., Jacob H. S., Furcht L. T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985 Jul;120(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Vickerman M. M., Clewell D. B., Jones G. W. Sucrose-promoted accumulation of growing glucosyltransferase variants of Streptococcus gordonii on hydroxyapatite surfaces. Infect Immun. 1991 Oct;59(10):3523–3530. doi: 10.1128/iai.59.10.3523-3530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells V. D., Munro C. L., Sulavik M. C., Clewell D. B., Macrina F. L. Infectivity of a glucan synthesis-defective mutant of Streptococcus gordonii (Challis) in a rat endocarditis model. FEMS Microbiol Lett. 1993 Sep 15;112(3):301–305. doi: 10.1111/j.1574-6968.1993.tb06466.x. [DOI] [PubMed] [Google Scholar]
- Yersin B. R., Glauser M. P., Freedman L. R. Effect of nitrogen mustard on natural history of right-sided streptococcal endocarditis in rabbits: role for cellular host defenses. Infect Immun. 1982 Jan;35(1):320–325. doi: 10.1128/iai.35.1.320-325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Davee T., Fierer J., Morey M. K. Streptococcus sanguis II (viridans) prosthetic valve endocarditis with myocardial, splenic and cerebral abscesses. West J Med. 1987 Apr;146(4):479–481. [PMC free article] [PubMed] [Google Scholar]






