Abstract
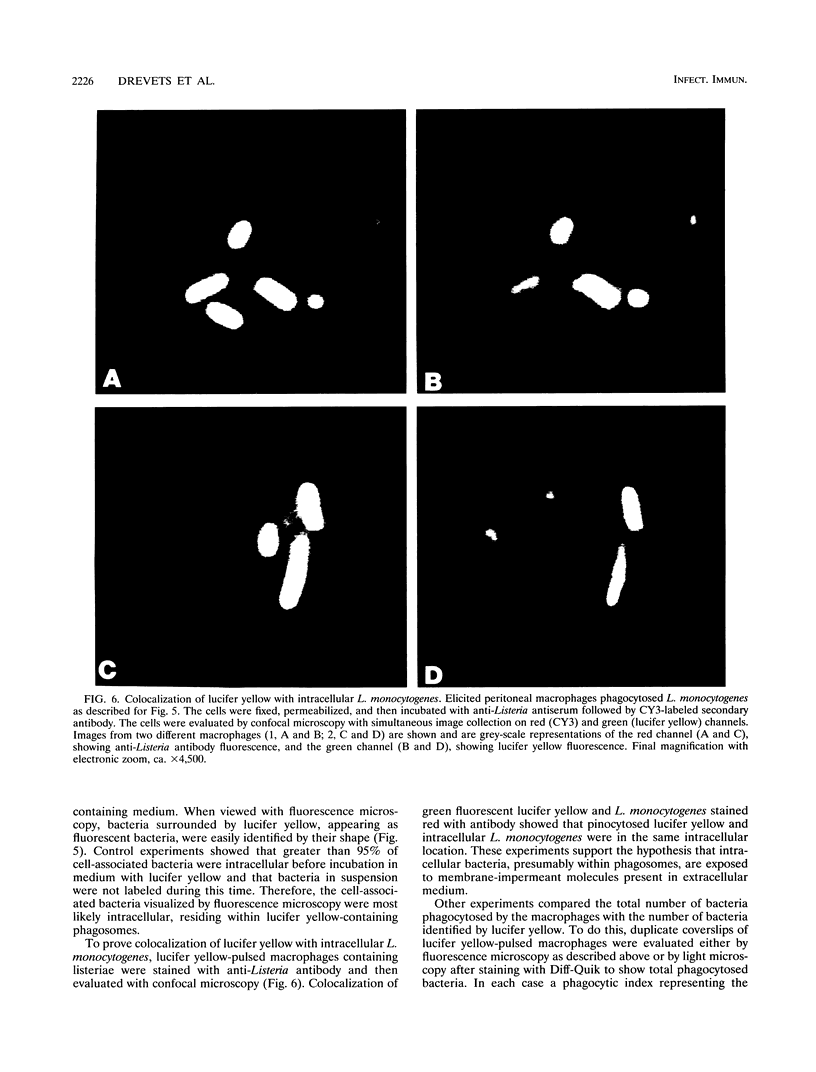
The purpose of the experiments described here was to test whether membrane-impermeant antibiotics present in the extracellular milieu could kill bacteria within macrophages. For this, mouse macrophage hybrids and elicited mouse peritoneal macrophages first were allowed to phagocytose the facultative intracellular bacterium Listeria monocytogenes. The cells were incubated with or without gentamicin, and their bactericidal activity was measured. The results show that gentamicin caused normally nonbactericidal macrophages to kill L. monocytogenes. In addition, gentamicin caused listericidal cells to kill significantly more bacteria. To determine whether gentamicin accumulated within macrophages during culture, we tested whether lysates of macrophage hybrids cultured for 72 h in gentamicin-containing medium and then washed could kill Listeria cells. When cultured with 50 to 100 micrograms of gentamicin per ml, but not when cultured with 0 to 5 micrograms of gentamicin per ml, cell lysates were extremely listericidal, demonstrating the presence of intracellular gentamicin. Because gentamicin does not penetrate cell membranes, we hypothesized that it can be internalized by the cell through pinocytosis and can enter the same intracellular compartment as does phagocytosed L. monocytogenes. To test this, macrophages which had phagocytosed L. monocytogenes were incubated with the fluorochrome lucifer yellow to trace pinocytosed medium. About half of the Listeria cells within the macrophages were surrounded by lucifer yellow, indicating delivery of pinocytosed fluid, which could contain antibiotics, to phagosomes containing bacteria. The experiments described here indicate that membrane-impermeant antibiotics can enter macrophages and kill intracellular bacteria. Thus, the use of gentamicin in macrophage bactericidal assays can interfere with the results and interpretation of experiments designed to study macrophage bactericidal activity.
Full text
PDF
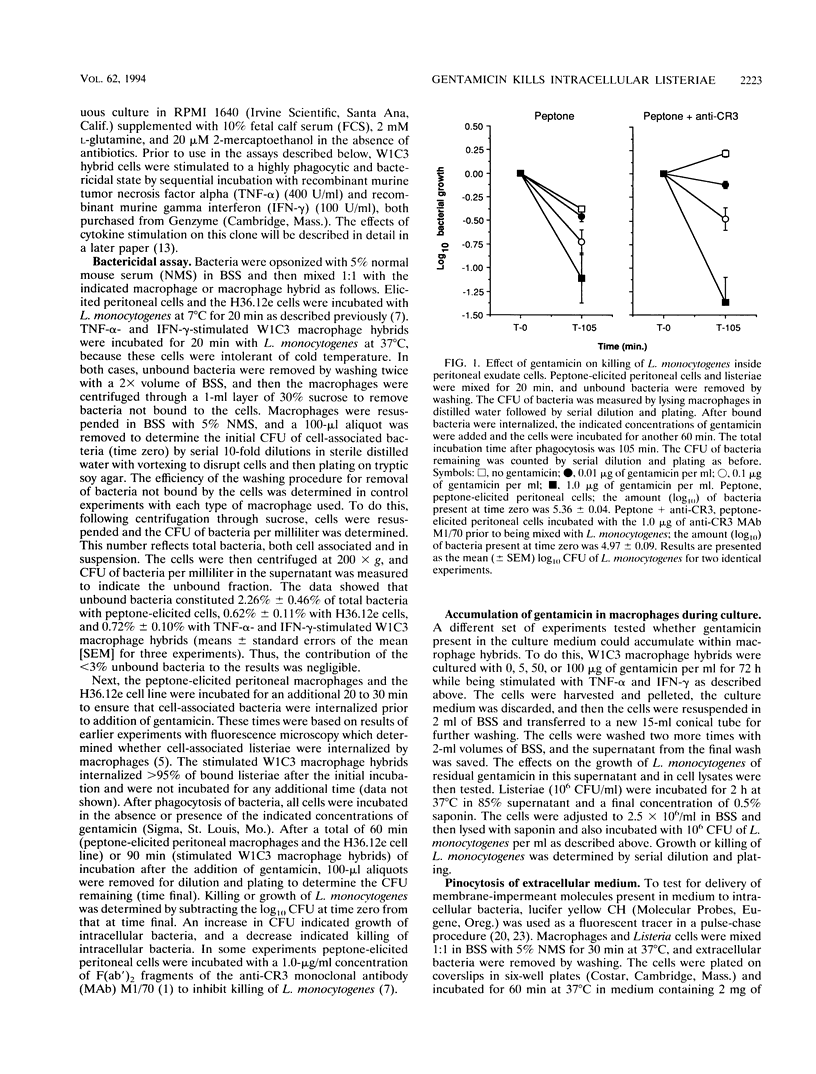
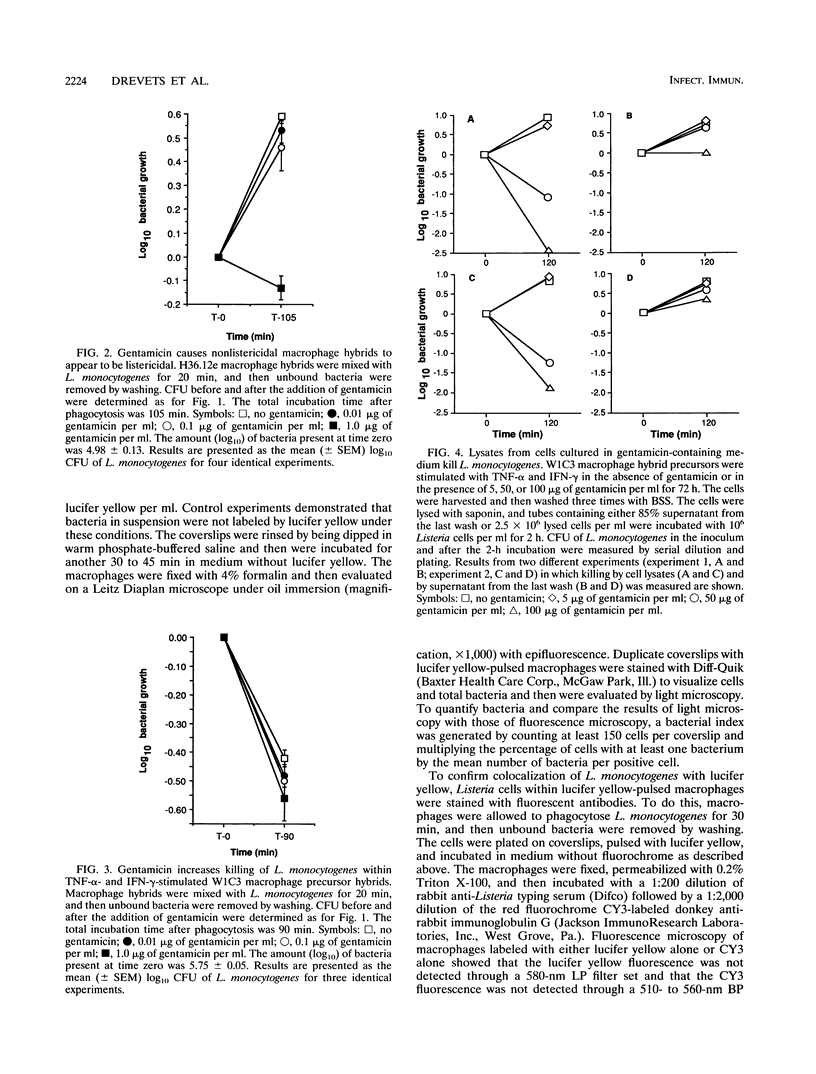



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P., Brostoff J. Intracellular killing of Listeria monocytogenes by activated macrophages (Mackaness system) is due to antibiotic. Nature. 1975 Aug 7;256(5517):515–517. doi: 10.1038/256515a0. [DOI] [PubMed] [Google Scholar]
- Drevets D. A., Campbell P. A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J Immunol Methods. 1991 Aug 28;142(1):31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- Drevets D. A., Canono B. P., Campbell P. A. Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L. monocytogenes and in preventing escape of the bacteria into the cytoplasm. J Leukoc Biol. 1992 Jul;52(1):70–79. doi: 10.1002/jlb.52.1.70. [DOI] [PubMed] [Google Scholar]
- Drevets D. A., Leenen P. J., Campbell P. A. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J Immunol. 1993 Nov 15;151(10):5431–5439. [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., Corwin R. W., Steinberg T. H., Grossman G. D. Uptake of antibiotics by human alveolar macrophages. Am Rev Respir Dis. 1984 Jun;129(6):933–937. doi: 10.1164/arrd.1984.129.6.933. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic mycobacterium and a nonpathogenic yeast. J Exp Med. 1991 Oct 1;174(4):881–889. doi: 10.1084/jem.174.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenen P. J., Slieker W. A., Melis M., Van Ewijk W. Murine macrophage precursor characterization. I. Production, phenotype and differentiation of macrophage precursor hybrids. Eur J Immunol. 1990 Jan;20(1):15–25. doi: 10.1002/eji.1830200104. [DOI] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Mayorga L. S., Bertini F., Stahl P. D. Fusion of newly formed phagosomes with endosomes in intact cells and in a cell-free system. J Biol Chem. 1991 Apr 5;266(10):6511–6517. [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Schreiber R. D., Connelly P., Tilney L. G. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989 Dec 1;170(6):2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin E. L., Swanson J. A. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989 Nov 1;170(5):1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin D. E., Gao P. X., Cao C. X., Neu H. C., Silverstein S. C. Gemfibrozil enhances the listeriacidal effects of fluoroquinolone antibiotics in J774 macrophages. J Exp Med. 1992 Nov 1;176(5):1439–1447. doi: 10.1084/jem.176.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R. D., Wong J., David J. R. Stimulation of pinocytosis in the macrophage by lymphocyte mediators. I. Description of the phenomenon. Cell Immunol. 1980 Sep 15;55(1):145–154. doi: 10.1016/0008-8749(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Swanson J. A., Yirinec B. D., Silverstein S. C. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985 Mar;100(3):851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastellier C., Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994 Feb;62(2):543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek P. J. Antimicrobial drugs, microorganisms, and phagocytes. Rev Infect Dis. 1989 Mar-Apr;11(2):213–245. doi: 10.1093/clinids/11.2.213. [DOI] [PubMed] [Google Scholar]