Abstract
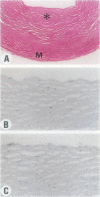
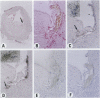
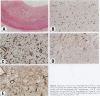
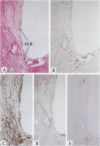
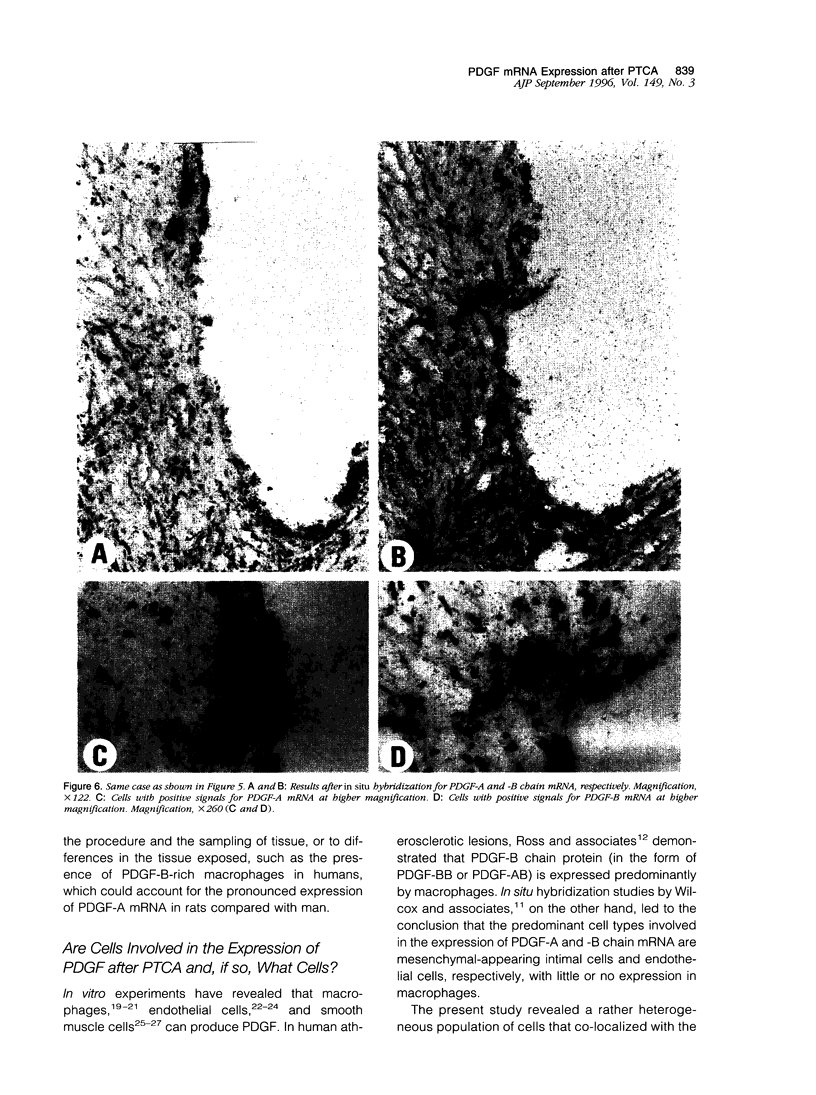
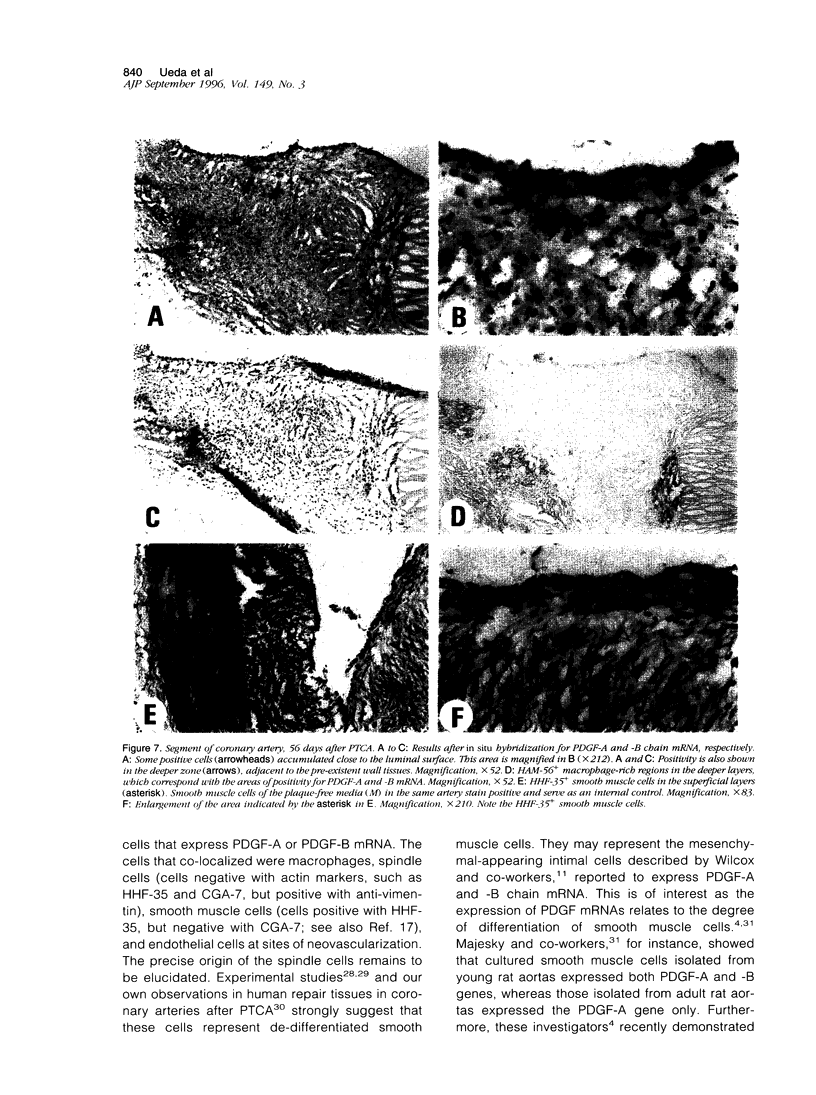
Experimental studies have shown that platelet-derived growth factor (PDGF) plays a role in wound-healing processes after angioplasty. In humans, after percutaneous transluminal coronary angioplasty (PTCA), this has not yet been documented. Six coronary arteries of five patients who died after PTCA were studied. The angioplasty sites were sliced serially, and the slices were studied using immunocytochemistry and in situ hybridization. Monoclonal antibodies were directed against muscle actin, vimentin, macrophages, and endothelium. In situ hybridization was performed using a synthetic oligonucleotide probe complementary to the PDGF-A and -B chain mRNAs. The identification of cells was based on a comparison with immune-stained sections. Positive autoradiographic signals for PDGF-A and -B chain mRNAs were found at the site of the PTCA injury and related to areas that contained macrophages, spindle cells, smooth muscle cells, and endothelial cells of neovascularization. In humans, both PDGF-A and -B chain mRNAs are expressed at sites of PTCA injury. The expression relates to the reparative response, and it appears that the cells involved are macrophages, spindle cells, smooth muscle cells, and endothelial cells of neovascularization. This is the first study to document the expression of PDGF-A and -B mRNAs at sites of repair in human coronary arteries after PTCA. It suggests strongly that PDGF is involved in the repair process after PTCA.
Full text
PDF

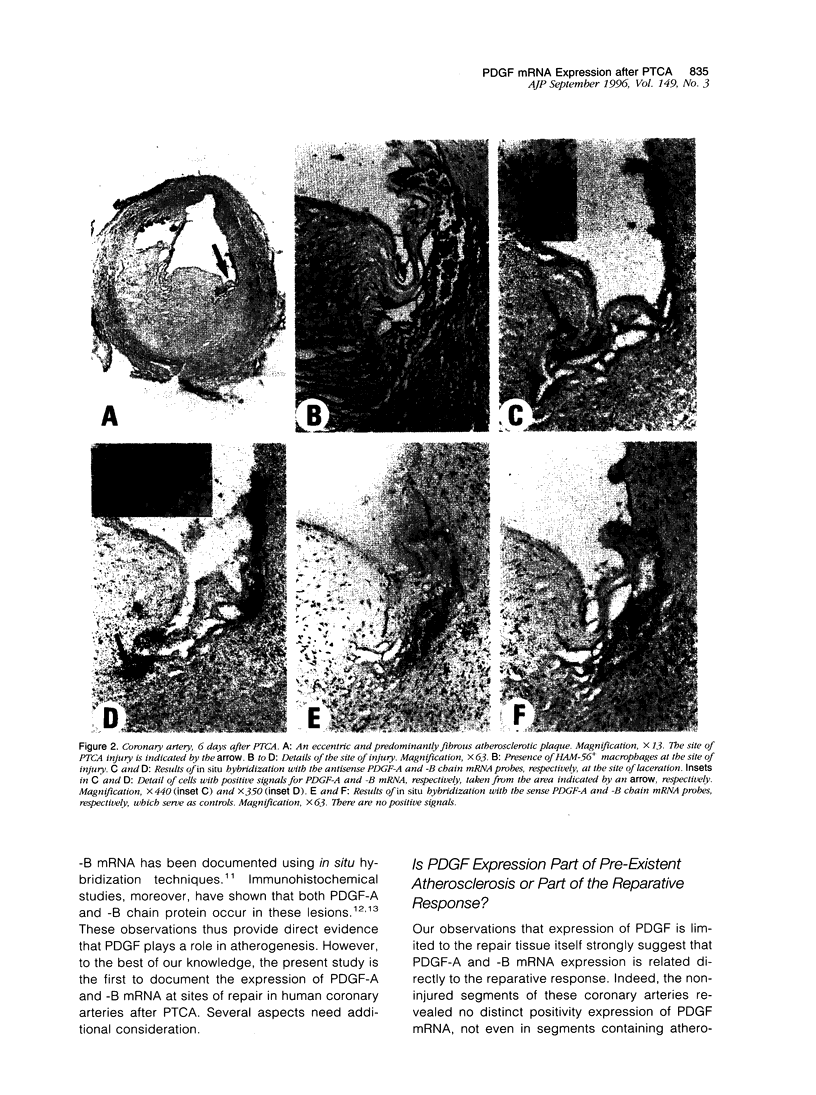
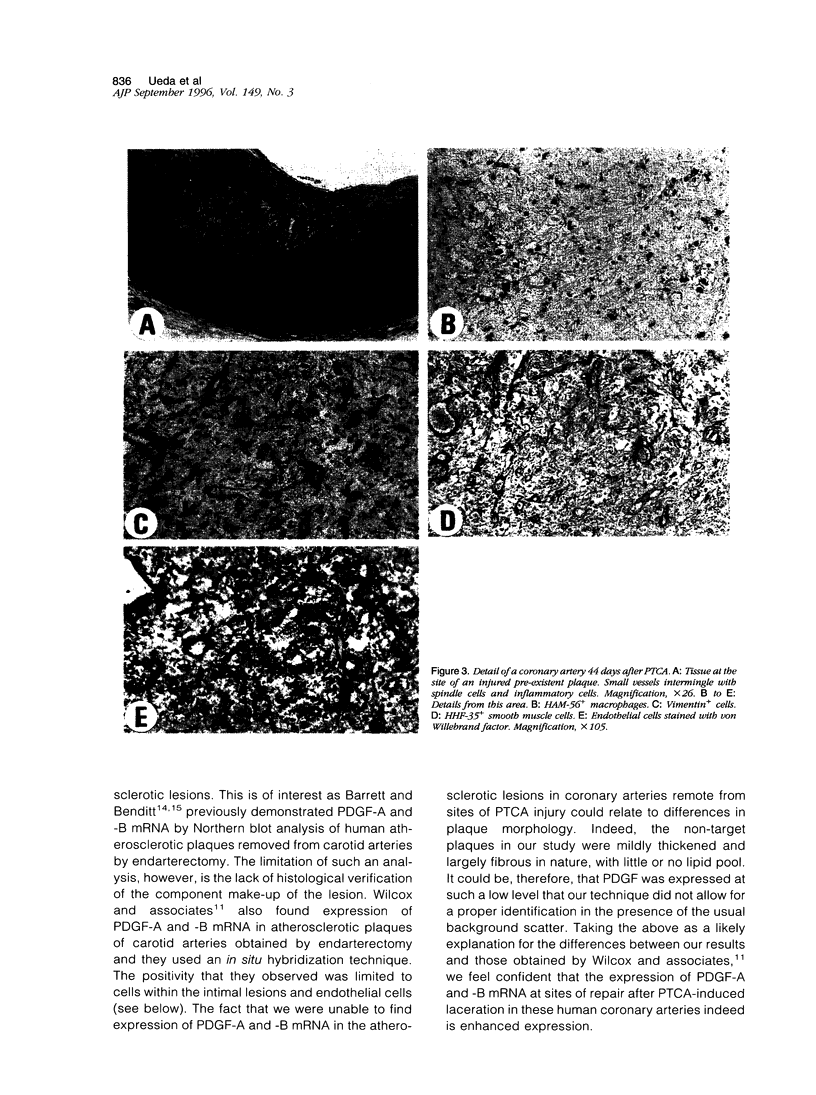
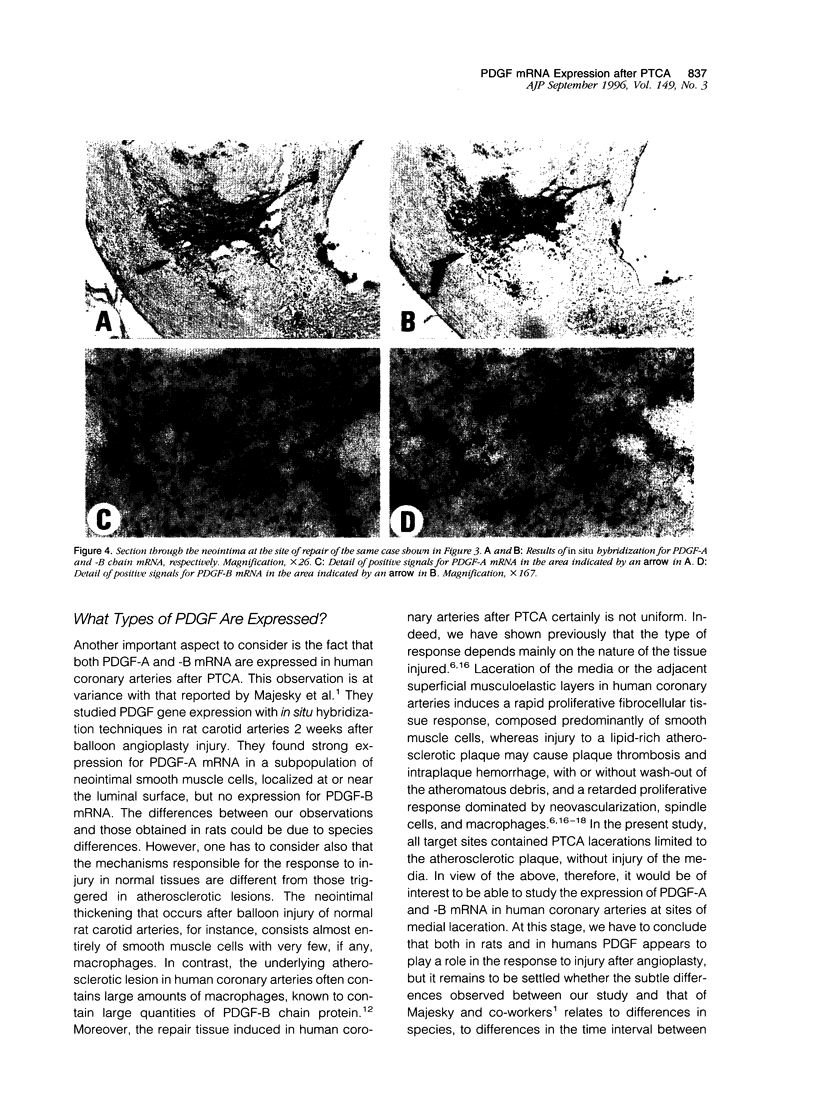
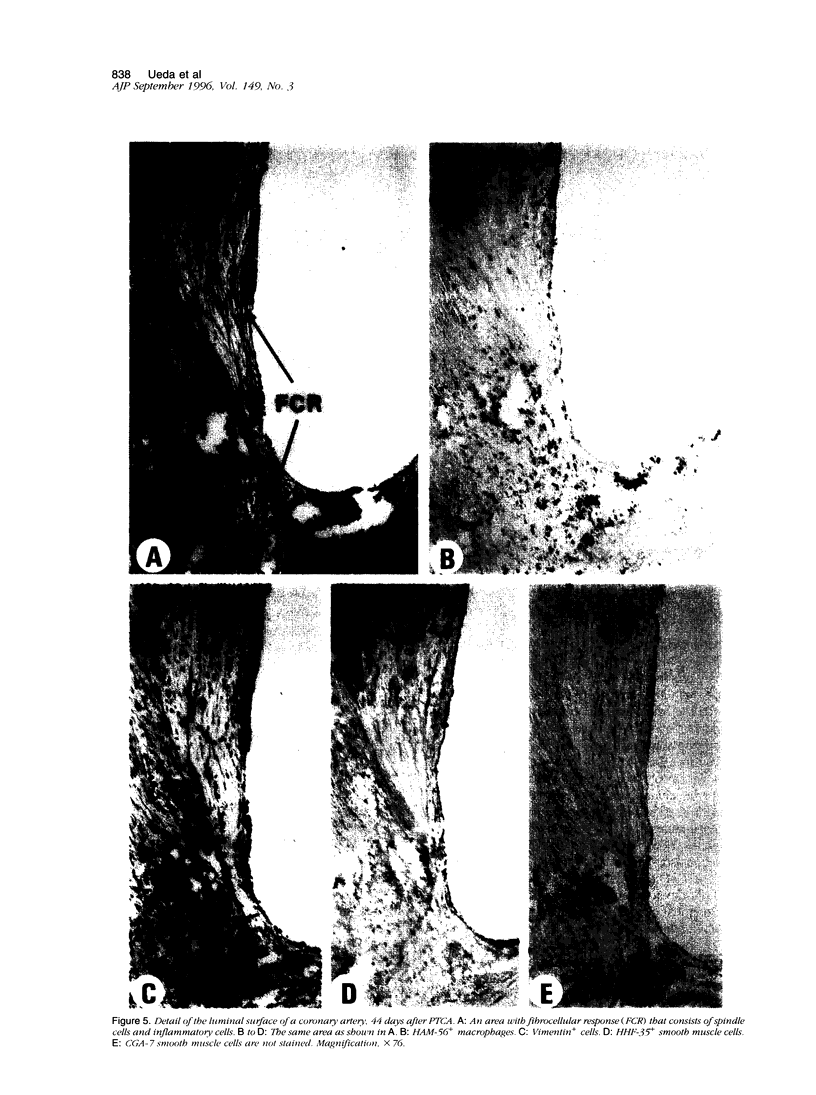



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. B., Benditt E. P. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. B., Benditt E. P. sis (platelet-derived growth factor B chain) gene transcript levels are elevated in human atherosclerotic lesions compared to normal artery. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1099–1103. doi: 10.1073/pnas.84.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank R. S., Owens G. K. Platelet-derived growth factor regulates actin isoform expression and growth state in cultured rat aortic smooth muscle cells. J Cell Physiol. 1990 Mar;142(3):635–642. doi: 10.1002/jcp.1041420325. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Corjay M. H., Blank R. S., Owens G. K. Platelet-derived growth factor-induced destabilization of smooth muscle alpha-actin mRNA. J Cell Physiol. 1990 Dec;145(3):391–397. doi: 10.1002/jcp.1041450302. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E. Cultured endothelial cells produce multiple growth factors for connective tissue cells. Exp Cell Res. 1984 Jul;153(1):167–172. doi: 10.1016/0014-4827(84)90458-0. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Gajdusek C. M. Release of endothelial cell-derived growth factor (ECDGF) by heparin. J Cell Physiol. 1984 Oct;121(1):13–21. doi: 10.1002/jcp.1041210104. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holycross B. J., Blank R. S., Thompson M. M., Peach M. J., Owens G. K. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res. 1992 Dec;71(6):1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Schumacher L., Eichner W., Weich H. A. The long 3'-untranslated regions of the PDGF-A and -B mRNAs are only distantly related. FEBS Lett. 1987 Nov 2;223(2):243–246. doi: 10.1016/0014-5793(87)80297-1. [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Raines E. W., Ross R., Reidy M. A. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993 Aug;13(8):1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye M., McConathy E., Drohan W., Tong B., Deuel T., Maciag T. Modulation of the sis gene transcript during endothelial cell differentiation in vitro. Science. 1985 May 17;228(4701):882–885. doi: 10.1126/science.3890179. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani F., Gabbiani G., Reidy M. A., Cokay M. S., Peters H., Hüttner I. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest. 1991 Oct;65(4):459–470. [PubMed] [Google Scholar]
- Koyama N., Morisaki N., Saito Y., Yoshida S. Regulatory effects of platelet-derived growth factor-AA homodimer on migration of vascular smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22806–22812. [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresta A., Balducelli M., Cantini L., Casari A., Chioin R., Fabbri M., Fontanelli A., Monici Preti P. A., Repetto S., De Servi S. Trapidil (triazolopyrimidine), a platelet-derived growth factor antagonist, reduces restenosis after percutaneous transluminal coronary angioplasty. Results of the randomized, double-blind STARC study. Studio Trapidil versus Aspirin nella Restenosi Coronarica. Circulation. 1994 Dec;90(6):2710–2715. doi: 10.1161/01.cir.90.6.2710. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekhter M. D., Gordon D. Does platelet-derived growth factor-A chain stimulate proliferation of arterial mesenchymal cells in human atherosclerotic plaques? Circ Res. 1994 Sep;75(3):410–417. doi: 10.1161/01.res.75.3.410. [DOI] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Siegbahn A., Hammacher A., Westermark B., Heldin C. H. Differential effects of the various isoforms of platelet-derived growth factor on chemotaxis of fibroblasts, monocytes, and granulocytes. J Clin Invest. 1990 Mar;85(3):916–920. doi: 10.1172/JCI114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Fujimoto T. Pathological changes induced by repeated percutaneous transluminal coronary angioplasty. Br Heart J. 1987 Dec;58(6):635–643. doi: 10.1136/hrt.58.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Fujimoto T., Tsukada T. The early phenomena of restenosis following percutaneous transluminal coronary angioplasty. Eur Heart J. 1991 Aug;12(8):937–945. [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Naruko T., Kojima A. Smooth muscle cell de-differentiation is a fundamental change preceding wound healing after percutaneous transluminal coronary angioplasty in humans. Coron Artery Dis. 1995 Jan;6(1):71–81. doi: 10.1097/00019501-199501000-00011. [DOI] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Tsukada T., Numano F., Fujimoto T. Fibrocellular tissue response after percutaneous transluminal coronary angioplasty. An immunocytochemical analysis of the cellular composition. Circulation. 1991 Apr;83(4):1327–1332. doi: 10.1161/01.cir.83.4.1327. [DOI] [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanibuchi H., Dingemans K. P., Becker A. E., Ueda M., Naruko T., Tanizawa S., Nakamura K. Is the Watanabe heritable hyperlipidemic rabbit a suitable experimental model for percutaneous transluminal coronary angioplasty in humans? A light microscopic, immunohistochemical and ultrastructural study. J Am Coll Cardiol. 1993 May;21(6):1490–1496. doi: 10.1016/0735-1097(93)90329-y. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]