Abstract
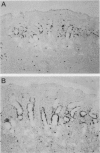
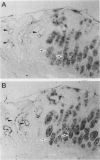
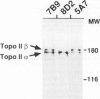
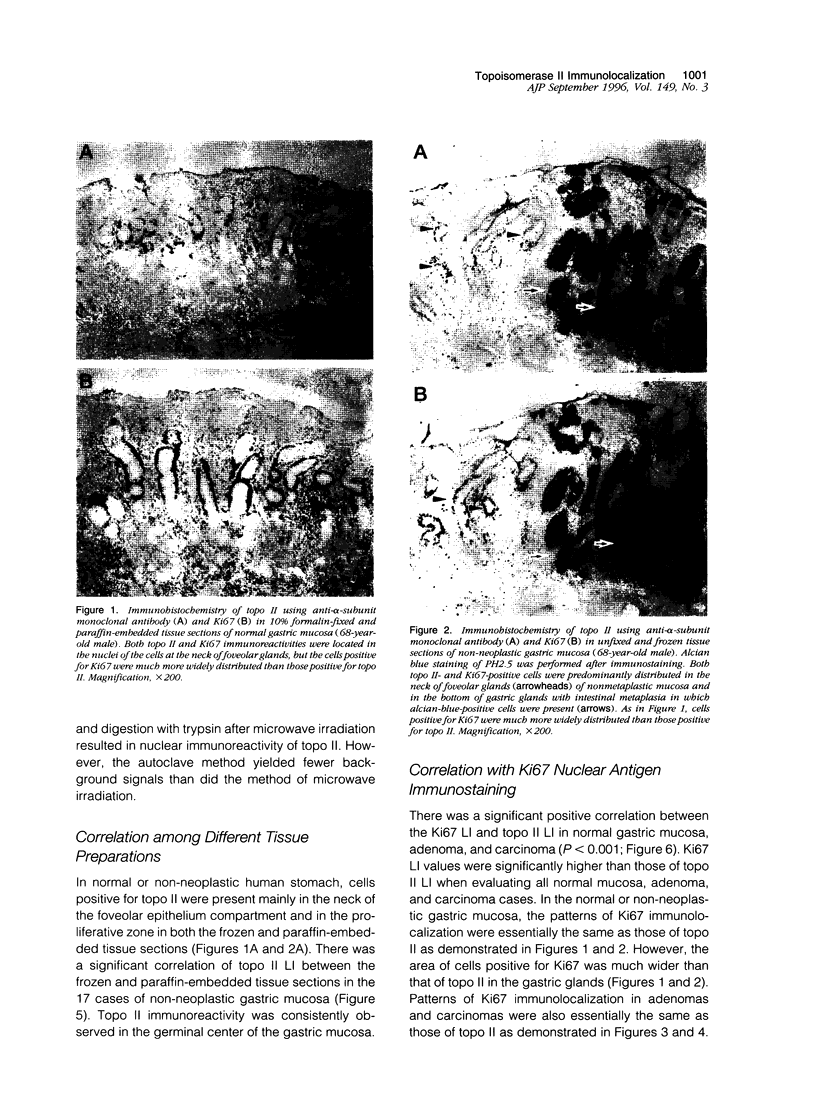
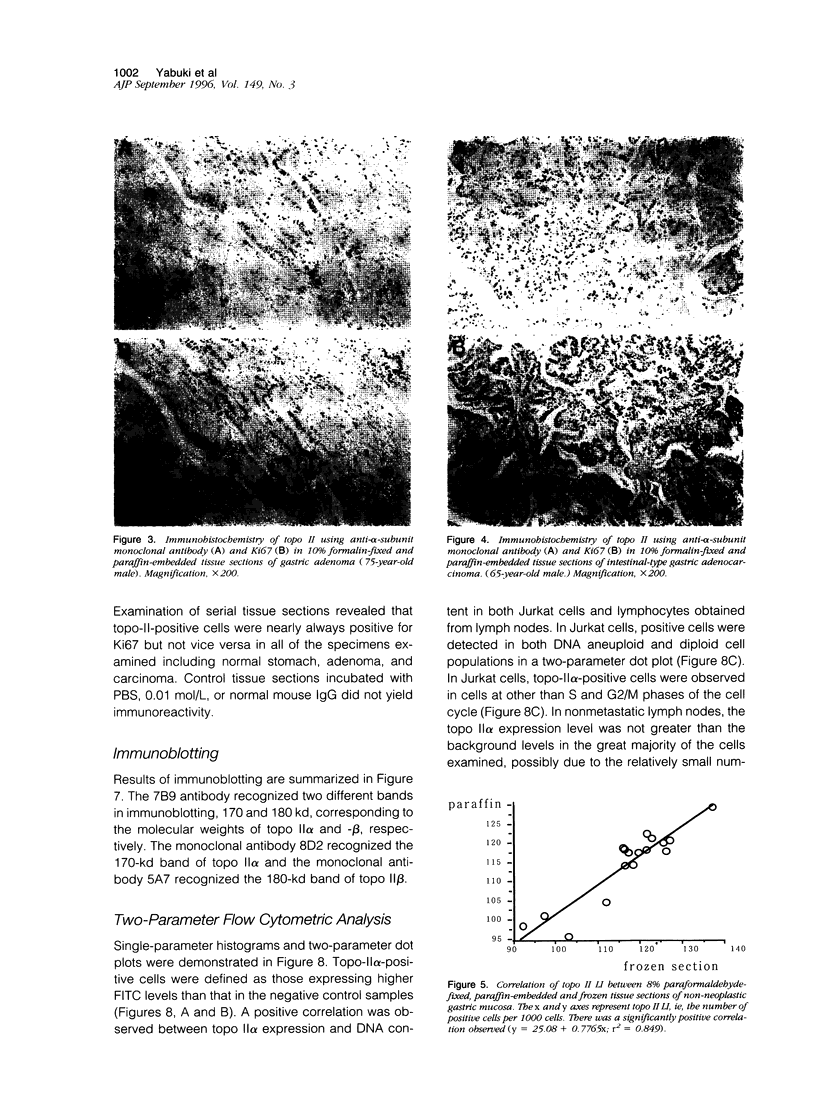
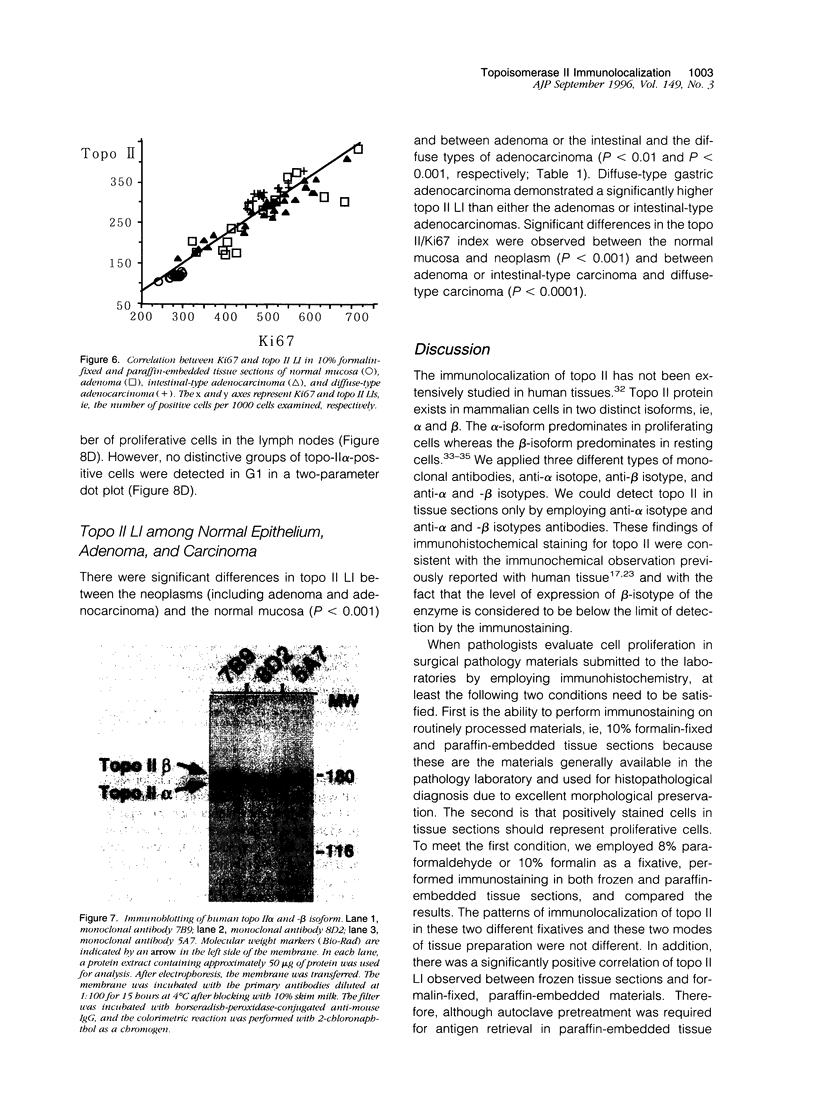
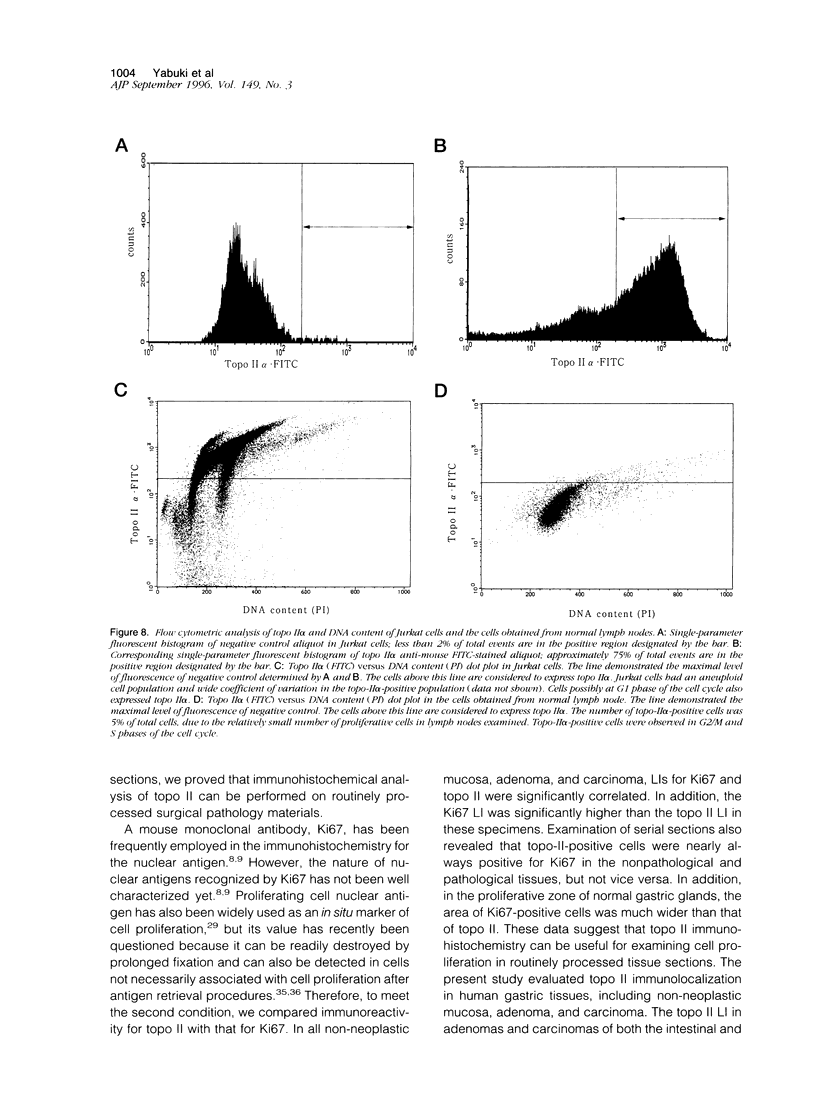
Topoisomerase II (topo II) separates chromosomes at the end of mitosis and is also the target for various chemotherapeutic agents. Expression of this enzyme has been demonstrated to increase rapidly at the end of the S to G2/M phase and decrease after the completion of mitosis. We immunolocalized topo II in specimens of both normal and neoplastic human gastric mucosas to evaluate expression of this enzyme. Three different antibodies were used for the immunostaining of topo II (anti-topo II alpha isoform, anti-topo II beta isoform and anti-topo II alpha and -beta isoforms). There were no significant differences in topo II labeling index (LI) between frozen and paraffin-embedded tissue sections obtained from the same cases. Topo II LI was significantly correlated with Ki67 LI in all of the specimens examined. The area of cells positive for Topo II was much narrower than that of Ki67 in the normal gastric glands, and the pattern of Topo II immunolocalization in both adenomas and adenocarcinomas was also essentially the same as that of Ki67. The topo II LI values (positive cells/1000 cells) for normal gastric gland, adenoma, intestinal-type adenocarcinoma, and diffuse-type adenocarcinoma were 114.7 +/- 2.2, 266.7 +/- 18.8, 277.6 +/- 19.2, and 324.5 +/- 5.3, respectively. Significant differences in topo II LI and topo II/Ki67 index were observed between normal and neoplastic mucosas (P < 0.0001) and between adenomas or intestinal-type adenocarcinoma and diffuse-type adenocarcinoma (P < 0.001 and P < 0.01, respectively). Simultaneous measurement of topo II alpha and nuclear DNA content by two-parameter flow cytometry revealed that the Jurkat cell line established from acute lymphocytic leukemia cells expressed the enzyme in cells at other than S and G2/M phases of the cell cycle whereas topo-II alpha-positive cells were predominantly observed in S and G2/M phases of the cell cycle in the cells from normal lymph nodes. These findings suggest that dys-regulation or qualitative changes of topo II alpha expression are associated with malignancy. Topo II immunostaining can thus detect proliferating cells in routinely processed tissue sections and can indicate the altered topo II alpha expression in human cancers, which may be related to the sensitivity to topo-II-targeted chemotherapeutic agents.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binaschi M., Capranico G., De Isabella P., Mariani M., Supino R., Tinelli S., Zunino F. Comparison of DNA cleavage induced by etoposide and doxorubicin in two human small-cell lung cancer lines with different sensitivities to topoisomerase II inhibitors. Int J Cancer. 1990 Feb 15;45(2):347–352. doi: 10.1002/ijc.2910450223. [DOI] [PubMed] [Google Scholar]
- Coon J. S., Landay A. L., Weinstein R. S. Advances in flow cytometry for diagnostic pathology. Lab Invest. 1987 Nov;57(5):453–479. [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Bartus H. F., Mattern M. R., Crooke S. T., Mirabelli C. K. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989 Oct 3;28(20):8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- Drake F. H., Zimmerman J. P., McCabe F. L., Bartus H. F., Per S. R., Sullivan D. M., Ross W. E., Mattern M. R., Johnson R. K., Crooke S. T. Purification of topoisomerase II from amsacrine-resistant P388 leukemia cells. Evidence for two forms of the enzyme. J Biol Chem. 1987 Dec 5;262(34):16739–16747. [PubMed] [Google Scholar]
- Fry A. M., Chresta C. M., Davies S. M., Walker M. C., Harris A. L., Hartley J. A., Masters J. R., Hickson I. D. Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res. 1991 Dec 15;51(24):6592–6595. [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Gazdar A. F., Beck H., Zunino F., Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992 Apr 1;52(7):1666–1674. [PubMed] [Google Scholar]
- Goukon Y., Sasano H., Nishihira T., Nagura H., Mori S. p53 overexpression in human esophageal carcinoma: a correlation with tumor DNA ploidy and two parameter flow cytometric study. Anticancer Res. 1994 May-Jun;14(3B):1305–1312. [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Heck M. M., Hittelman W. N., Earnshaw W. C. Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1086–1090. doi: 10.1073/pnas.85.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Wu H. Y., Liu L. F. Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res. 1988 Jun 1;48(11):3230–3235. [PubMed] [Google Scholar]
- Hwang J. L., Shyy S. H., Chen A. Y., Juan C. C., Whang-Peng J. Studies of topoisomerase-specific antitumor drugs in human lymphocytes using rabbit antisera against recombinant human topoisomerase II polypeptide. Cancer Res. 1989 Feb 15;49(4):958–962. [PubMed] [Google Scholar]
- Itakura Y., Sasano H., Mori S., Nagura H. DNA ploidy in human esophageal squamous dysplasias and squamous cell carcinomas as determined by image analysis. Mod Pathol. 1994 Oct;7(8):867–873. [PubMed] [Google Scholar]
- Kasahara K., Fujiwara Y., Sugimoto Y., Nishio K., Tamura T., Matsuda T., Saijo N. Determinants of response to the DNA topoisomerase II inhibitors doxorubicin and etoposide in human lung cancer cell lines. J Natl Cancer Inst. 1992 Jan 15;84(2):113–118. doi: 10.1093/jnci/84.2.113. [DOI] [PubMed] [Google Scholar]
- Katoh R., Bray C. E., Suzuki K., Komiyama A., Hemmi A., Kawaoi A., Oyama T., Sugai T., Sasou S. Growth activity in hyperplastic and neoplastic human thyroid determined by an immunohistochemical staining procedure using monoclonal antibody MIB-1. Hum Pathol. 1995 Feb;26(2):139–146. doi: 10.1016/0046-8177(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., McLaughlin S. J., Kastan M. B., Liu L. F., Karp J. E., Burke P. J. Topoisomerase II levels during granulocytic maturation in vitro and in vivo. Cancer Res. 1991 Jul 1;51(13):3534–3543. [PubMed] [Google Scholar]
- Kim R., Hirabayashi N., Nishiyama M., Saeki S., Toge T., Okada K. Expression of MDR1, GST-pi and topoisomerase II as an indicator of clinical response to adriamycin. Anticancer Res. 1991 Jan-Feb;11(1):429–431. [PubMed] [Google Scholar]
- Kimura K., Nozaki N., Saijo M., Kikuchi A., Ui M., Enomoto T. Identification of the nature of modification that causes the shift of DNA topoisomerase II beta to apparent higher molecular weight forms in the M phase. J Biol Chem. 1994 Oct 7;269(40):24523–24526. [PubMed] [Google Scholar]
- Kobayashi S., Nishimura J., Kanaide H. Cytosolic Ca2+ transients are not required for platelet-derived growth factor to induce cell cycle progression of vascular smooth muscle cells in primary culture. Actions of tyrosine kinase. J Biol Chem. 1994 Mar 25;269(12):9011–9018. [PubMed] [Google Scholar]
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Nitiss J. L., Liu Y. X., Harbury P., Jannatipour M., Wasserman R., Wang J. C. Amsacrine and etoposide hypersensitivity of yeast cells overexpressing DNA topoisomerase II. Cancer Res. 1992 Aug 15;52(16):4467–4472. [PubMed] [Google Scholar]
- Oya M., Yao T., Nagai E., Tsuneyoshi M. Metastasizing intramucosal gastric carcinomas. Well differentiated type and proliferative activity using proliferative cell nuclear antigen and Ki-67. Cancer. 1995 Feb 15;75(4):926–935. doi: 10.1002/1097-0142(19950215)75:4<926::aid-cncr2820750406>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Prosperi E., Sala E., Negri C., Oliani C., Supino R., Astraldi Ricotti G. B., Bottiroli G. Topoisomerase II alpha and beta in human tumor cells grown in vitro and in vivo. Anticancer Res. 1992 Nov-Dec;12(6B):2093–2099. [PubMed] [Google Scholar]
- Quinn C. M., Wright N. A. The clinical assessment of proliferation and growth in human tumours: evaluation of methods and applications as prognostic variables. J Pathol. 1990 Feb;160(2):93–102. doi: 10.1002/path.1711600202. [DOI] [PubMed] [Google Scholar]
- Sasano H., Imatani A., Shizawa S., Suzuki T., Nagura H. Cell proliferation and apoptosis in normal and pathologic human adrenal. Mod Pathol. 1995 Jan;8(1):11–17. [PubMed] [Google Scholar]
- Sasano H., Miyazaki S., Gooukon Y., Nishihira T., Sawai T., Nagura H. Expression of p53 in human esophageal carcinoma: an immunohistochemical study with correlation to proliferating cell nuclear antigen expression. Hum Pathol. 1992 Nov;23(11):1238–1243. doi: 10.1016/0046-8177(92)90291-a. [DOI] [PubMed] [Google Scholar]
- Schlüter C., Duchrow M., Wohlenberg C., Becker M. H., Key G., Flad H. D., Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993 Nov;123(3):513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Hsiang Y. H., Liu L. F. DNA topoisomerases as anticancer drug targets. Adv Pharmacol. 1990;21:149–183. doi: 10.1016/s1054-3589(08)60342-7. [DOI] [PubMed] [Google Scholar]
- Schwarting R. Little missed markers and Ki-67. Lab Invest. 1993 Jun;68(6):597–599. [PubMed] [Google Scholar]
- Smith P. J., Morgan S. A., Fox M. E., Watson J. V. Mitoxantrone-DNA binding and the induction of topoisomerase II associated DNA damage in multi-drug resistant small cell lung cancer cells. Biochem Pharmacol. 1990 Nov 1;40(9):2069–2078. doi: 10.1016/0006-2952(90)90237-f. [DOI] [PubMed] [Google Scholar]
- Sullivan D. M., Glisson B. S., Hodges P. K., Smallwood-Kentro S., Ross W. E. Proliferation dependence of topoisomerase II mediated drug action. Biochemistry. 1986 Apr 22;25(8):2248–2256. doi: 10.1021/bi00356a060. [DOI] [PubMed] [Google Scholar]
- Sullivan D. M., Latham M. D., Ross W. E. Proliferation-dependent topoisomerase II content as a determinant of antineoplastic drug action in human, mouse, and Chinese hamster ovary cells. Cancer Res. 1987 Aug 1;47(15):3973–3979. [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987 Sep 11;50(6):917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Webb C. D., Latham M. D., Lock R. B., Sullivan D. M. Attenuated topoisomerase II content directly correlates with a low level of drug resistance in a Chinese hamster ovary cell line. Cancer Res. 1991 Dec 15;51(24):6543–6549. [PubMed] [Google Scholar]
- Yonemura Y., Ooyama S., Sugiyama K., Ninomiya I., Kamata T., Yamaguchi A., Matsumoto H., Miyazaki I. Growth fractions in gastric carcinomas determined with monoclonal antibody Ki-67. Cancer. 1990 Mar 1;65(5):1130–1134. doi: 10.1002/1097-0142(19900301)65:5<1130::aid-cncr2820650516>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- van der Zee A. G., Hollema H., de Jong S., Boonstra H., Gouw A., Willemse P. H., Zijlstra J. G., de Vries E. G. P-glycoprotein expression and DNA topoisomerase I and II activity in benign tumors of the ovary and in malignant tumors of the ovary, before and after platinum/cyclophosphamide chemotherapy. Cancer Res. 1991 Nov 1;51(21):5915–5920. [PubMed] [Google Scholar]