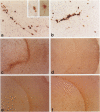
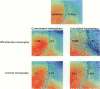
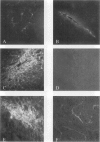
Abstract
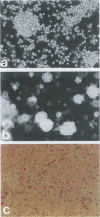
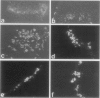
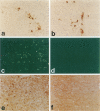
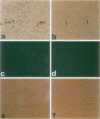
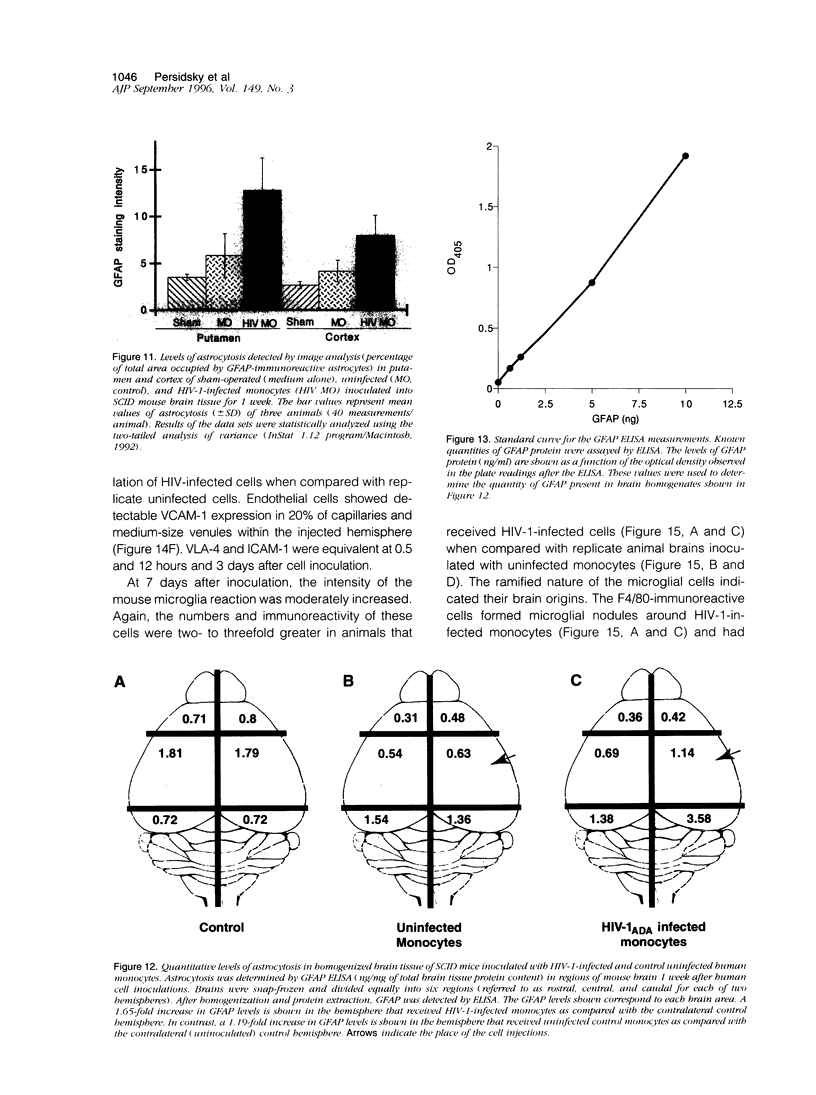
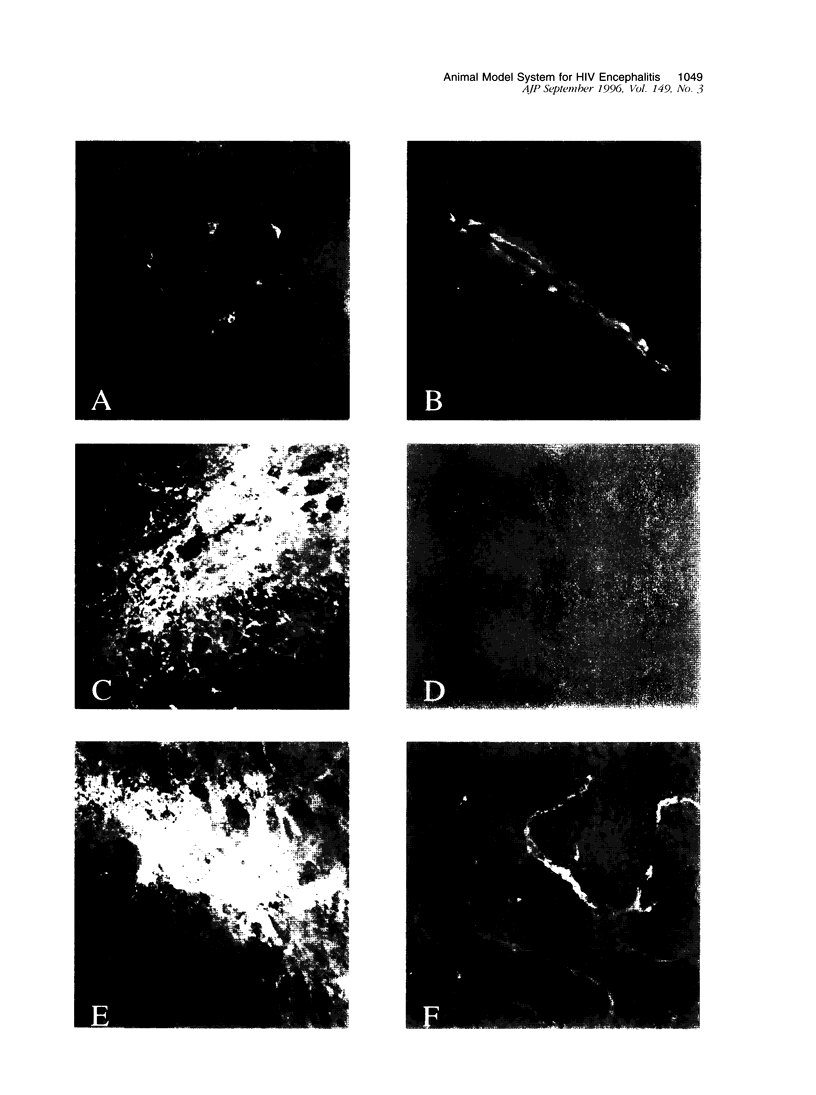
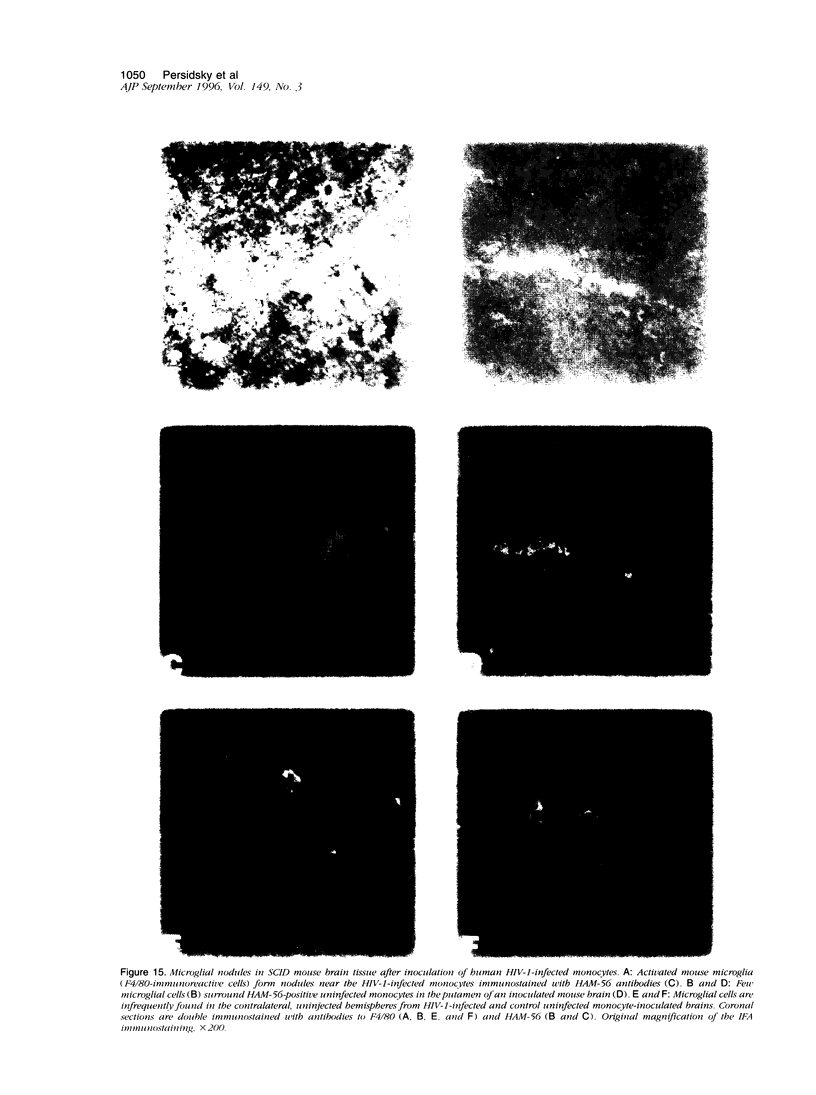
The human immunodeficiency virus (HIV) is neuroinvasive and commonly causes cognitive and motor deficits during the later stages of viral infection. (referred to as HIV dementia). The mechanism(s) for disease revolves around secretory products produced from immune-activated brain macrophages/microglia. Recently, we developed an animal model system for HIV dementia that contains xenografts of HIV-1-infected cells inoculated into brains of mice with severe combined immunodeficiency (SCID). This animal system was used to quantitatively evaluate HIV-induced neuropathology. Xenografts of HIV-1-infected human monocytes (placed into the putamen and cortex of SCID mice) remained viable for 5 weeks. HIV-1 p24 antigen expression in mouse brain was persistent. Progressive inflammatory responses (including astrogliosis and cytokine production), which began at 3 days, peaked at day 12. The range of astrocyte proliferative reactions exceeded the inoculation site by > 1000 microns. Brains with virus-infected monocytes showed a > or = 1.6-fold increase in glial fibrillary acidic protein (staining distribution and intensity) as compared with similarly inoculated brains with uninfected control monocytes. These findings paralleled the accumulation and activation of murine microglia (increased branching of cell processes, formation of microglial nodules, interleukin (IL)-1 beta and IL-6 expression). An inflammatory reaction of human monocytes (as defined by HLA-DR, IL-1 beta, IL-6, and tumor necrosis factor-alpha expression) and neuronal injury (apoptosis) also developed after virus-infected monocyte xenograft placement into mouse brain tissue. These data, taken together, demonstrate that this SCID mouse model of HIV-1 neuropathogenesis can reproduce key aspects of disease (virus-infected macrophages, astrocytosis, microglial activation, and neuronal damage). This model may serve as an important means for therapeutic development directed toward improving mental function in HIV-infected subjects with cognitive and motor dysfunction.
Full text
PDF




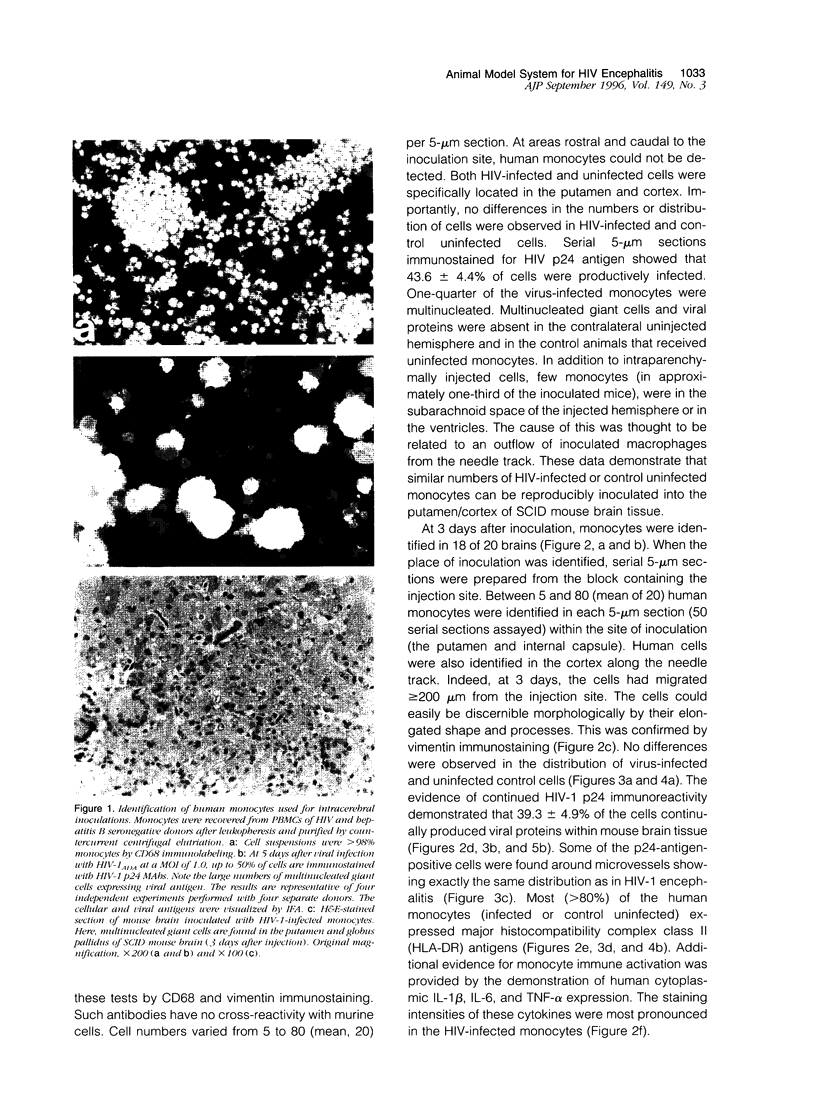
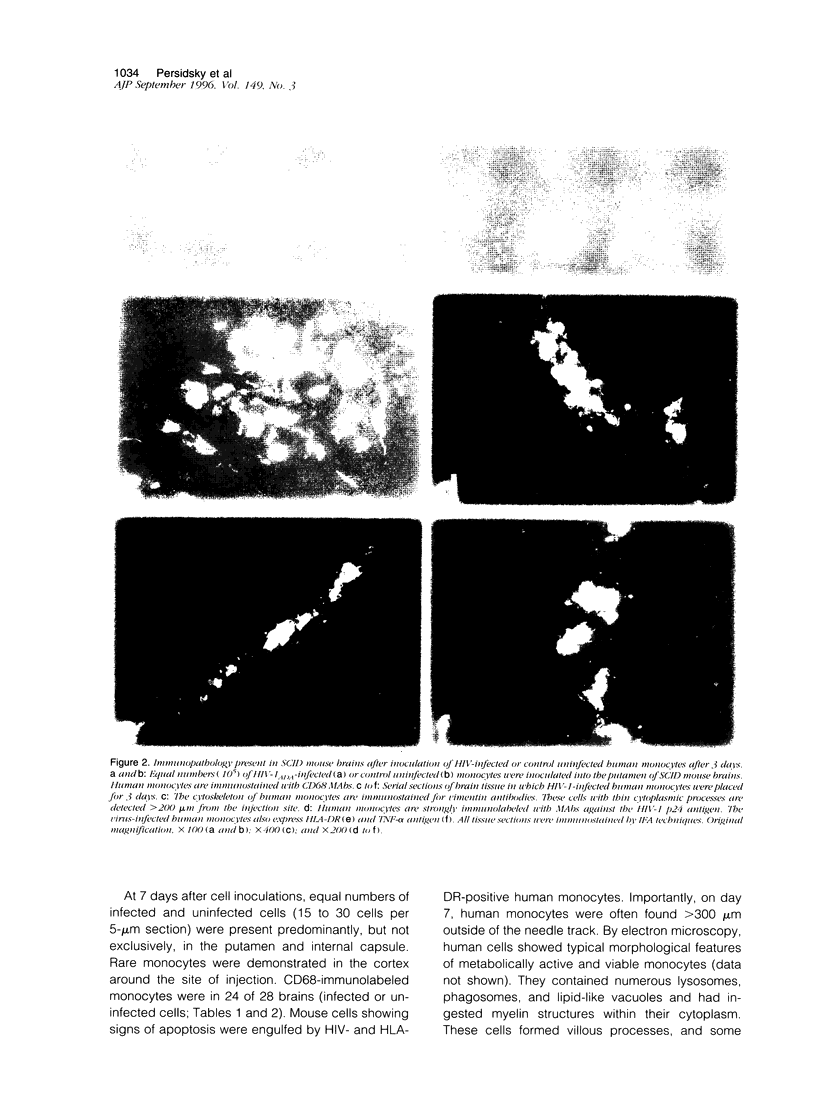
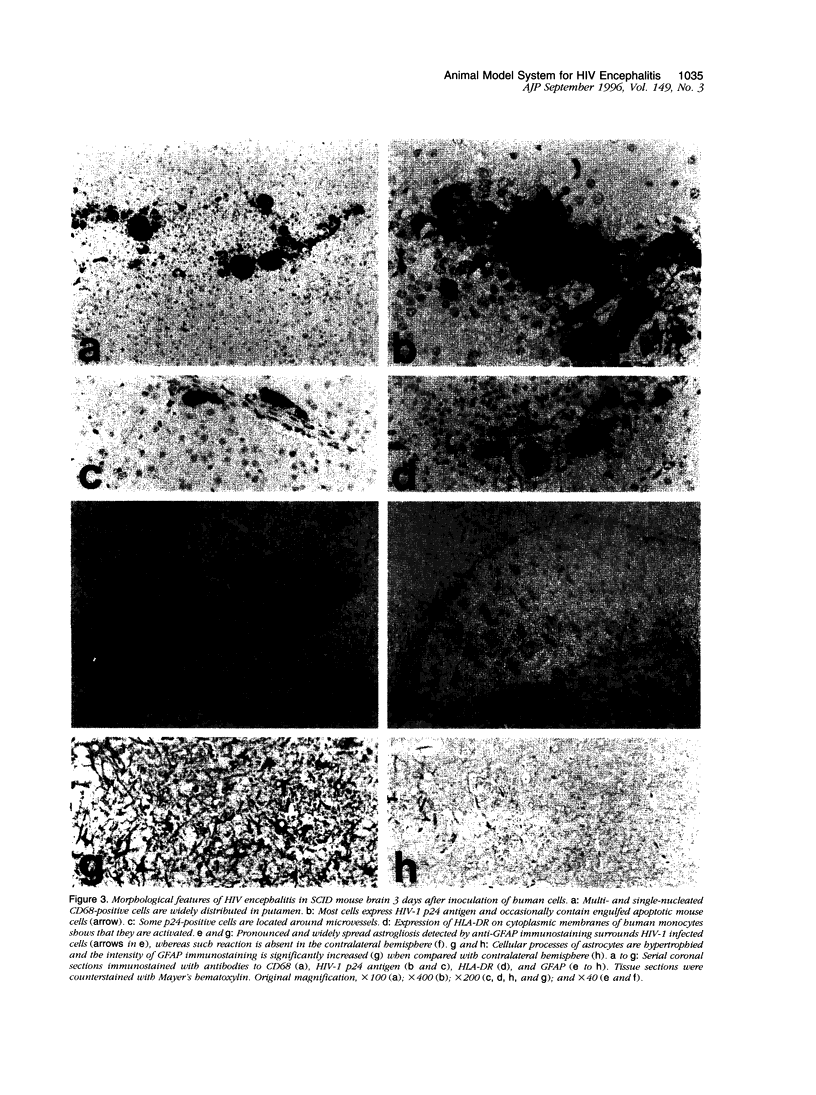

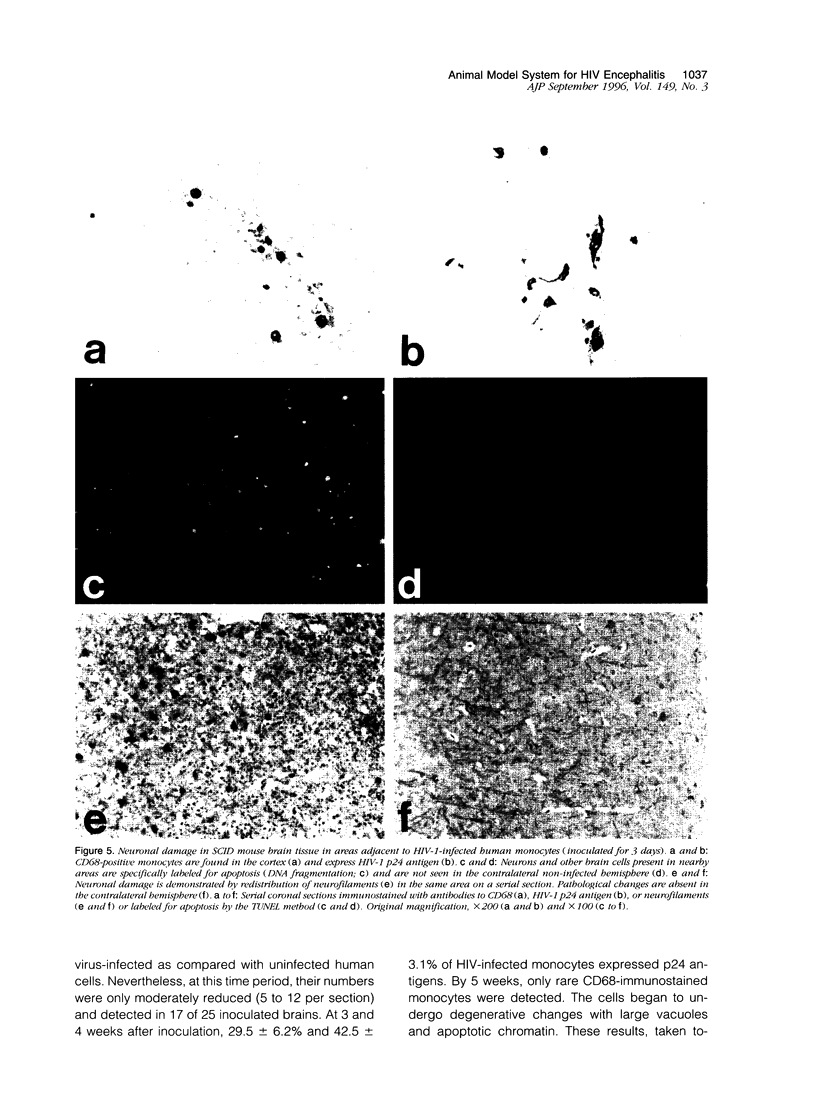









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskin G. B., Murphey-Corb M., Roberts E. D., Didier P. J., Martin L. N. Correlates of SIV encephalitis in rhesus monkeys. J Med Primatol. 1992 Feb-May;21(2-3):59–63. [PubMed] [Google Scholar]
- Bukrinsky M. I., Nottet H. S., Schmidtmayerova H., Dubrovsky L., Flanagan C. R., Mullins M. E., Lipton S. A., Gendelman H. E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995 Feb 1;181(2):735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. G., McKay R. D. A hypothermic miniaturized stereotaxic instrument for surgery in newborn rats. J Neurosci Methods. 1993 Apr;47(1-2):105–114. doi: 10.1016/0165-0270(93)90026-n. [DOI] [PubMed] [Google Scholar]
- Dickson D. W. Apoptosis in the brain. Physiology and pathology. Am J Pathol. 1995 May;146(5):1040–1044. [PMC free article] [PubMed] [Google Scholar]
- Dickson D. W., Mattiace L. A., Kure K., Hutchins K., Lyman W. D., Brosnan C. F. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991 Feb;64(2):135–156. [PubMed] [Google Scholar]
- Epstein L. G., Cvetkovich T. A., Lazar E., Dehlinger K., Dzenko K., del Cerro C., del Cerro M. Successful xenografts of second trimester human fetal brain and retinal tissue in the anterior chamber of the eye of adult immunosuppressed rats. J Neural Transplant Plast. 1992 Apr-Sep;3(2-3):151–158. doi: 10.1155/NP.1992.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I. P., Luthert P. J., Lantos P. L. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991 May 11;337(8750):1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Gelbard H. A., Nottet H. S., Swindells S., Jett M., Dzenko K. A., Genis P., White R., Wang L., Choi Y. B., Zhang D. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994 Jul;68(7):4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Lipton S. A., Tardieu M., Bukrinsky M. I., Nottet H. S. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994 Sep;56(3):389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Glass J. D., Fedor H., Wesselingh S. L., McArthur J. C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995 Nov;38(5):755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Wesselingh S. L., McArthur J. C. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994 May;35(5):592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Brew B. J., Martin A., Price R. W., Salazar A. M., Sidtis J. J., Yergey J. A., Mouradian M. M., Sadler A. E., Keilp J. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991 Feb;29(2):202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Hurtrel M., Ganière J. P., Guelfi J. F., Chakrabarti L., Maire M. A., Gray F., Montagnier L., Hurtrel B. Comparison of early and late feline immunodeficiency virus encephalopathies. AIDS. 1992 Apr;6(4):399–406. doi: 10.1097/00002030-199204000-00007. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. Neurovirology: evolution of a new discipline. J Neurovirol. 1995 Mar;1(1):2–4. doi: 10.3109/13550289509111005. [DOI] [PubMed] [Google Scholar]
- Kalter D. C., Nakamura M., Turpin J. A., Baca L. M., Hoover D. L., Dieffenbach C., Ralph P., Gendelman H. E., Meltzer M. S. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J Immunol. 1991 Jan 1;146(1):298–306. [PubMed] [Google Scholar]
- Kennedy P. G., Narayan O., Ghotbi Z., Hopkins J., Gendelman H. E., Clements J. E. Persistent expression of Ia antigen and viral genome in visna-maedi virus-induced inflammatory cells. Possible role of lentivirus-induced interferon. J Exp Med. 1985 Dec 1;162(6):1970–1982. doi: 10.1084/jem.162.6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieburtz K., Schiffer R. B. Neurologic manifestations of human immunodeficiency virus infections. Neurol Clin. 1989 Aug;7(3):447–468. [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kure K., Weidenheim K. M., Lyman W. D., Dickson D. W. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80(4):393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- Lackner A. A., Smith M. O., Munn R. J., Martfeld D. J., Gardner M. B., Marx P. A., Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991 Sep;139(3):609–621. [PMC free article] [PubMed] [Google Scholar]
- Lackner A. A., Vogel P., Ramos R. A., Kluge J. D., Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994 Aug;145(2):428–439. [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Gendelman H. E. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995 Apr 6;332(14):934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Murray E. A., Rausch D. M., Lendvay J., Sharer L. R., Eiden L. E. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science. 1992 Mar 6;255(5049):1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Zink M. C. Lentivirus-host interactions: lessons from visna and caprine arthritis-encephalitis viruses. Ann Neurol. 1988;23 (Suppl):S95–100. doi: 10.1002/ana.410230725. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Jordan B. D., Price R. W. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986 Jun;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991 Jun;41(6):778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- Nottet H. S., Gendelman H. E. Unraveling the neuroimmune mechanisms for the HIV-1-associated cognitive/motor complex. Immunol Today. 1995 Sep;16(9):441–448. doi: 10.1016/0167-5699(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Nottet H. S., Jett M., Flanagan C. R., Zhai Q. H., Persidsky Y., Rizzino A., Bernton E. W., Genis P., Baldwin T., Schwartz J. A regulatory role for astrocytes in HIV-1 encephalitis. An overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-alpha by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol. 1995 Apr 1;154(7):3567–3581. [PubMed] [Google Scholar]
- Nottet H. S., Persidsky Y., Sasseville V. G., Nukuna A. N., Bock P., Zhai Q. H., Sharer L. R., McComb R. D., Swindells S., Soderland C. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996 Feb 1;156(3):1284–1295. [PubMed] [Google Scholar]
- O'Callaghan J. P. Quantification of glial fibrillary acidic protein: comparison of slot-immunobinding assays with a novel sandwich ELISA. Neurotoxicol Teratol. 1991 May-Jun;13(3):275–281. doi: 10.1016/0892-0362(91)90073-6. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Lawson L. J., Reid D. M. Biology of the mononuclear phagocyte system of the central nervous system and HIV infection. J Leukoc Biol. 1994 Sep;56(3):399–406. doi: 10.1002/jlb.56.3.399. [DOI] [PubMed] [Google Scholar]
- Persidsky Y., Nottet H. S., Sasseville V. G., Epstein L. G., Gendelman H. E. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J Neurovirol. 1995 Sep;1(3-4):229–243. doi: 10.3109/13550289509114019. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995 May;146(5):1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Herndier B. G., Tang N. M., McGrath M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991 Feb;87(2):503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch D. M., Heyes M. P., Murray E. A., Lendvay J., Sharer L. R., Ward J. M., Rehm S., Nohr D., Weihe E., Eiden L. E. Cytopathologic and neurochemical correlates of progression to motor/cognitive impairment in SIV-infected rhesus monkeys. J Neuropathol Exp Neurol. 1994 Mar;53(2):165–175. doi: 10.1097/00005072-199403000-00008. [DOI] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W. A., Lackner A. A., Smith M. O., Lausen N. C., Beall D., Ringler D. J. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am J Pathol. 1992 Nov;141(5):1021–1030. [PMC free article] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W., Brodie S. J., Hesterberg P., Pauley D., Ringler D. J. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am J Pathol. 1994 Jan;144(1):27–40. [PMC free article] [PubMed] [Google Scholar]
- Schmidtmayerova H., Nottet H. S., Nuovo G., Raabe T., Flanagan C. R., Dubrovsky L., Gendelman H. E., Cerami A., Bukrinsky M., Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler T. J., DiScenna P. The role of hippocampus in memory: a hypothesis. Neurosci Biobehav Rev. 1985 Fall;9(3):377–389. doi: 10.1016/0149-7634(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Toggas S. M., Masliah E., Rockenstein E. M., Rall G. F., Abraham C. R., Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994 Jan 13;367(6459):188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tyor W. R., Glass J. D., Griffin J. W., Becker P. S., McArthur J. C., Bezman L., Griffin D. E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992 Apr;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Tyor W. R., Power C., Gendelman H. E., Markham R. B. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh S. L., Power C., Glass J. D., Tyor W. R., McArthur J. C., Farber J. M., Griffin J. W., Griffin D. E. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993 Jun;33(6):576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994 Oct;36(4):673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M. C., Yager J. A., Myers J. D. Pathogenesis of caprine arthritis encephalitis virus. Cellular localization of viral transcripts in tissues of infected goats. Am J Pathol. 1990 Apr;136(4):843–854. [PMC free article] [PubMed] [Google Scholar]