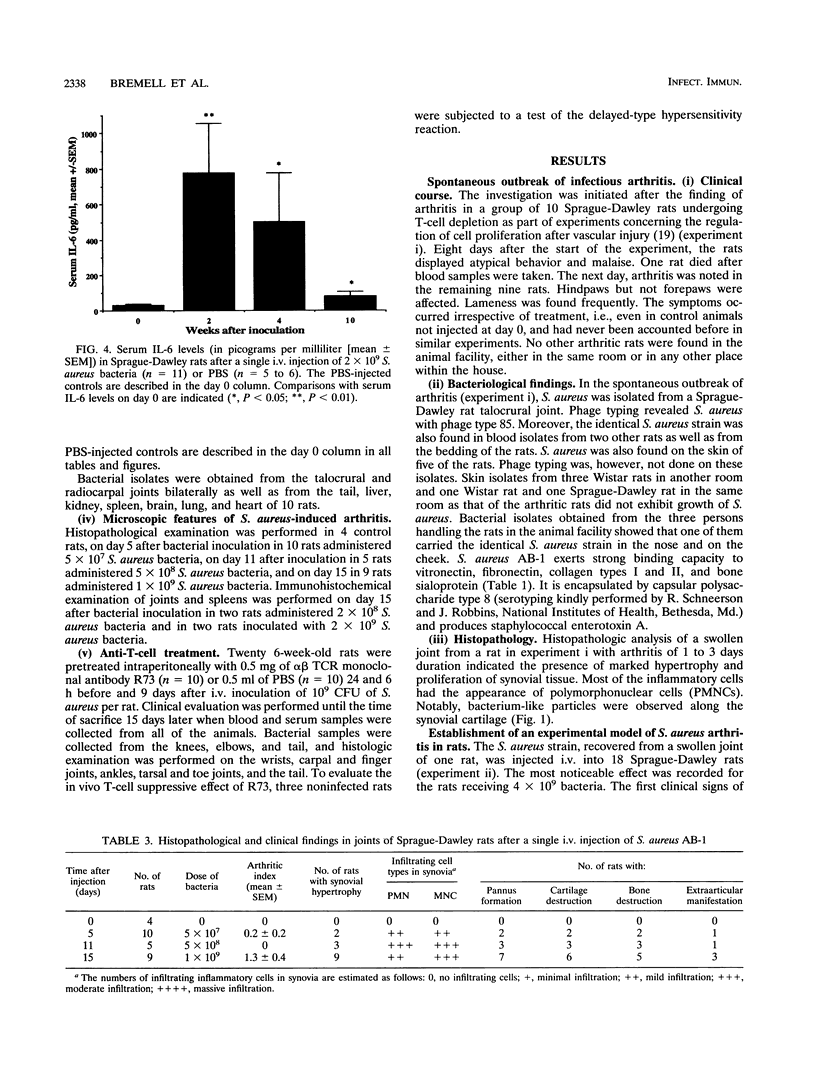
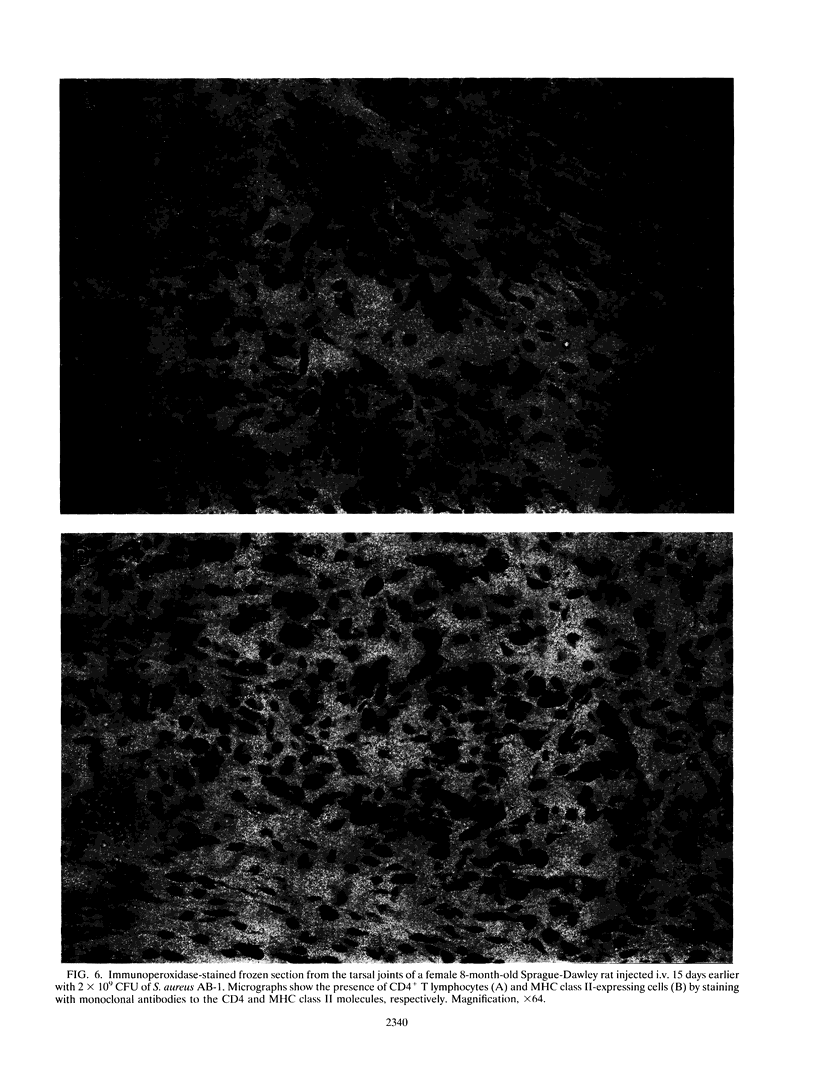
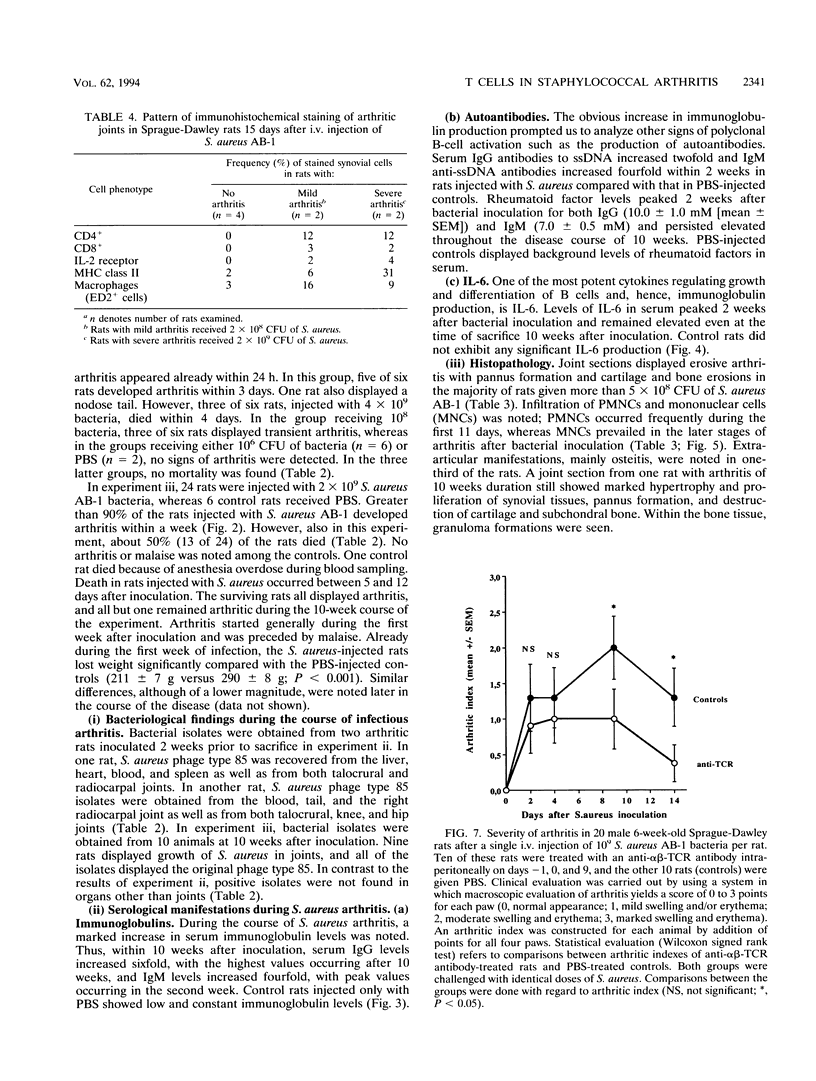
Abstract
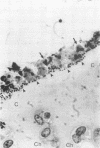
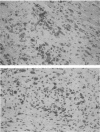
Staphylococcus aureus is the most common bacterial species found in nongonococcal bacterial arthritis in humans. We present the first description, to our knowledge, of an outbreak of spontaneous staphylococcal arthritis in a rat colony. In a group of 10 rats, 9 displayed arthritis. Clinically, the most obvious findings were arthritis of one or both hindpaws and malaise. Bacteriophage typing showed the common phage type 85 in isolates recovered from the joints, blood, and bedding of rats and from the nose and cheeks of one person from the staff of the animal facility. The S. aureus strain proved to produce staphylococcal enterotoxin A and exhibited strong binding to collagen types I and II and bone sialoprotein, which are potentially important virulence factors. When the recovered S. aureus strain was injected intravenously into healthy rats, severe septic arthritis was induced in almost all of the animals. The arthritic lesions were characterized by infiltration of phagocytic cells and T lymphocytes into the synovium. Many of the synovial cells strongly expressed major histocompatibility complex class II molecules. Increased levels of interleukin 6 in serum as well as a prominent polyclonal B-cell activation were noted throughout the disease course. Pretreatment of S. aureus-injected rats in vivo with an antibody to the alpha beta T-cell receptor significantly decreased the severity of the arthritis. Our results indicate that alpha beta + T lymphocytes contribute to an erosive and persistent course of S. aureus arthritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Abdelnour A., Arvidson S., Bremell T., Rydén C., Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993 Sep;61(9):3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Boetner A. G., Binder M., Bille-Hansen V. Streptococcus suis infections in Danish pigs and experimental infection with Streptococcus suis serotype 7. Acta Pathol Microbiol Immunol Scand B. 1987 Aug;95(4):233–239. doi: 10.1111/j.1699-0463.1987.tb03118.x. [DOI] [PubMed] [Google Scholar]
- Brakenhoff J. P., de Groot E. R., Evers R. F., Pannekoek H., Aarden L. A. Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J Immunol. 1987 Dec 15;139(12):4116–4121. [PubMed] [Google Scholar]
- Bremell T., Abdelnour A., Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992 Jul;60(7):2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremell T., Lange S., Svensson L., Jennische E., Gröndahl K., Carlsten H., Tarkowski A. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990 Nov;33(11):1739–1744. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- Bremell T., Lange S., Yacoub A., Rydén C., Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991 Aug;59(8):2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Carlsten H., Nilsson L. A., Tarkowski A. Impaired cutaneous delayed-type hypersensitivity in autoimmune MRL lpr/lpr mice. Int Arch Allergy Appl Immunol. 1986;81(4):322–325. doi: 10.1159/000234156. [DOI] [PubMed] [Google Scholar]
- Carlsten H., Tarkowski A., Jonsson R., Nilsson L. A. Expression of heterozygous lpr gene in MRL mice. II. Acceleration of glomerulonephritis, sialadenitis, and autoantibody production. Scand J Immunol. 1990 Jul;32(1):21–28. doi: 10.1111/j.1365-3083.1990.tb02887.x. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Elwing H., Nygren H. Diffusion in gel-enzyme linked immunosorbent assay (DIG-ELISA): a simple method for quantitation of class-specific antibodies. J Immunol Methods. 1979;31(1-2):101–107. doi: 10.1016/0022-1759(79)90290-4. [DOI] [PubMed] [Google Scholar]
- Feinstein R. E., Eld K. Naturally occurring erysipelas in rats. Lab Anim. 1989 Jul;23(3):256–260. doi: 10.1258/002367789780810473. [DOI] [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D. L., Chisholm P. L., Rice P. A. Experimental models of bacterial arthritis: a microbiologic and histopathologic characterization of the arthritis after the intraarticular injections of Neisseria gonorrhoeae, Staphylococcus aureus, group A streptococci, and Escherichia coli. J Rheumatol. 1983 Feb;10(1):5–11. [PubMed] [Google Scholar]
- Goldenberg D. L., Cohen A. S. Synovial membrane histopathology in the differential diagnosis of rheumatoid arthritis, gout, pseudogout, systemic lupus erythematosus, infectious arthritis and degenerative joint disease. Medicine (Baltimore) 1978 May;57(3):239–252. doi: 10.1097/00005792-197805000-00004. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L., Reed J. I. Bacterial arthritis. N Engl J Med. 1985 Mar 21;312(12):764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Holm S., Fotev Z., Hedrich H. J., Fingerle J. T lymphocytes inhibit the vascular response to injury. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10530–10534. doi: 10.1073/pnas.88.23.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard D. J., Jacobson E. R., Clemmons R. E., Campbell G. A. Bacteremia and septic arthritis in a West African dwarf crocodile. J Am Vet Med Assoc. 1988 May 15;192(10):1453–1454. [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hünig T., Wallny H. J., Hartley J. K., Lawetzky A., Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989 Jan 1;169(1):73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson R., Tarkowski A., Klareskog L. A demineralization procedure for immunohistopathological use. EDTA treatment preserves lymphoid cell surface antigens. J Immunol Methods. 1986 Apr 3;88(1):109–114. doi: 10.1016/0022-1759(86)90058-x. [DOI] [PubMed] [Google Scholar]
- Kaplow I. S. Letter: Substitute for benzidine in myeloperoxidase stains. Am J Clin Pathol. 1975 Mar;63(3):451–451. doi: 10.1093/ajcp/63.3.451. [DOI] [PubMed] [Google Scholar]
- Kasari T. R., Marquis H., Scanlan C. M. Septic arthritis and osteomyelitis in a bovine digit: a mixed infection of Actinomyces pyogenes and Fusobacterium necrophorum. Cornell Vet. 1988 Jul;78(3):215–219. [PubMed] [Google Scholar]
- Lansdorp P. M., Aarden L. A., Calafat J., Zeiljemaker W. P. A growth-factor dependent B-cell hybridoma. Curr Top Microbiol Immunol. 1986;132:105–113. doi: 10.1007/978-3-642-71562-4_14. [DOI] [PubMed] [Google Scholar]
- Lue C., Tarkowski A., Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J Immunol. 1988 Jun 1;140(11):3793–3800. [PubMed] [Google Scholar]
- Mahowald M. L. Animal models of infectious arthritis. Clin Rheum Dis. 1986 Aug;12(2):403–421. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Monoclonal antibodies to Ia antigens from rat thymus: cross reactions with mouse and human and use in purification of rat Ia glycoproteins. Immunol Rev. 1979;47:117–137. doi: 10.1111/j.1600-065x.1979.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Morris M. P., Fletcher O. J. Diagnostic summary of 1986 turkey, broiler breeder, and layer necropsy cases at the University of Georgia. Avian Dis. 1988 Jul-Sep;32(3):391–403. [PubMed] [Google Scholar]
- Nakamura Y., Masuhara T., Ito-Kuwa S., Aoki S. Induction of experimental Candida arthritis in rats. J Med Vet Mycol. 1991;29(3):179–192. [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Patti J. M., Bremell T., Krajewska-Pietrasik D., Abdelnour A., Tarkowski A., Rydén C., Hök M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994 Jan;62(1):152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Brown T. A., Radl J., Haaijman J. J., Mestecky J. Assay of human IgA subclass antibodies in serum and secretions by means of monoclonal antibodies. J Immunol Methods. 1986 Feb 27;87(1):87–93. doi: 10.1016/0022-1759(86)90347-9. [DOI] [PubMed] [Google Scholar]
- Rydén C., Yacoub A. I., Maxe I., Heinegård D., Oldberg A., Franzén A., Ljungh A., Rubin K. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur J Biochem. 1989 Sep 15;184(2):331–336. doi: 10.1111/j.1432-1033.1989.tb15023.x. [DOI] [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Patti J. M., Butcher W., Gristina A. G., Speziale P., Hök M. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol. 1993 Jan;7(1):99–107. doi: 10.1111/j.1365-2958.1993.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Czerkinsky C., Nilsson L. A. Detection of IgG rheumatoid factor secreting cells in autoimmune MRL/1 mice: a kinetic study. Clin Exp Immunol. 1984 Oct;58(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Tissi L., Marconi P., Mosci P., Merletti L., Cornacchione P., Rosati E., Recchia S., von Hunolstein C., Orefici G. Experimental model of type IV Streptococcus agalactiae (group B streptococcus) infection in mice with early development of septic arthritis. Infect Immun. 1990 Sep;58(9):3093–3100. doi: 10.1128/iai.58.9.3093-3100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. E. O., RIPPON J. E. Bacteriophage typing of Staphylococcus aureus. J Hyg (Lond) 1952 Sep;50(3):320–353. doi: 10.1017/s002217240001963x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- van Riessen D., Daha M. R., Smeets T. J., Breedveld F. C. The influence of synovial fibroblasts on the phagocytosis of Staphylococcus by polymorphonuclear cells. Rheumatol Int. 1992;12(1):1–5. doi: 10.1007/BF00246869. [DOI] [PubMed] [Google Scholar]