Abstract
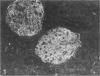
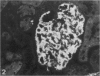
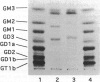
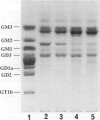
Puromycin aminonucleoside nephrosis (PAN) is a model for human minimal change nephropathy induced in rats by injection of puromycin. In PAN, defective sialylation of a major sialoprotein of podocytes, podocalyxin, has been demonstrated and the consequent decrease of anionic charge suggested as a causative factor for increased glomerular permeability and proteinuria. Whether defective sialylation is a general feature of PAN affecting also glomerular glycosphingolipids is not known. We have shown that rat glomeruli are rich in disialogangliosides GD3 and O-acetyl GD3, the functions of which are not known. Here, we made a sequential analysis of the glomerular gangliosides, especially of GD3 and its O-acetyl derivative in acute PAN using immunohistochemical and biochemical techniques and compared the results with another rat model of glomerular disease, Heymann nephritis. The prominent immunohistochemical finding was the almost total disappearance of glomerular O-acetyl GD3 and a substantial decrease of its precursor GD3 peaking at 10 days after injection of puromycin. Segmental areas lacking these gangliosides remained in glomeruli still at 30 days after injection. The response was dose dependent. Semiquantitative analysis by thin layer chromatograms showed that O-acetyl GD3 was decreased by 41% already at 3 days and by 60% at 10 days after injection of puromycin. Also GD3, the immediate precursor of O-acetyl GD3, was decreased by 20 and 19%, respectively, at 3 and 10 days after injection. At 3 days after injection, overt proteinuria had not started. At these times, no other changes were observed in the glomerular gangliosides. The decrease of glomerular GD3 and O-acetyl GD3 indicates a decrease of GD3 synthase activity and perhaps of O-acetyltransferase activity in PAN nephrosis. As these changes preceded the overt proteinuria, they may have a causal relationship to it. In the glomeruli of Heymann nephritic rats, no similar changes were seen, suggesting that the sialylation defect is not due to proteinuria but is a consequence of targeted puromycin action on cells.
Full text
PDF


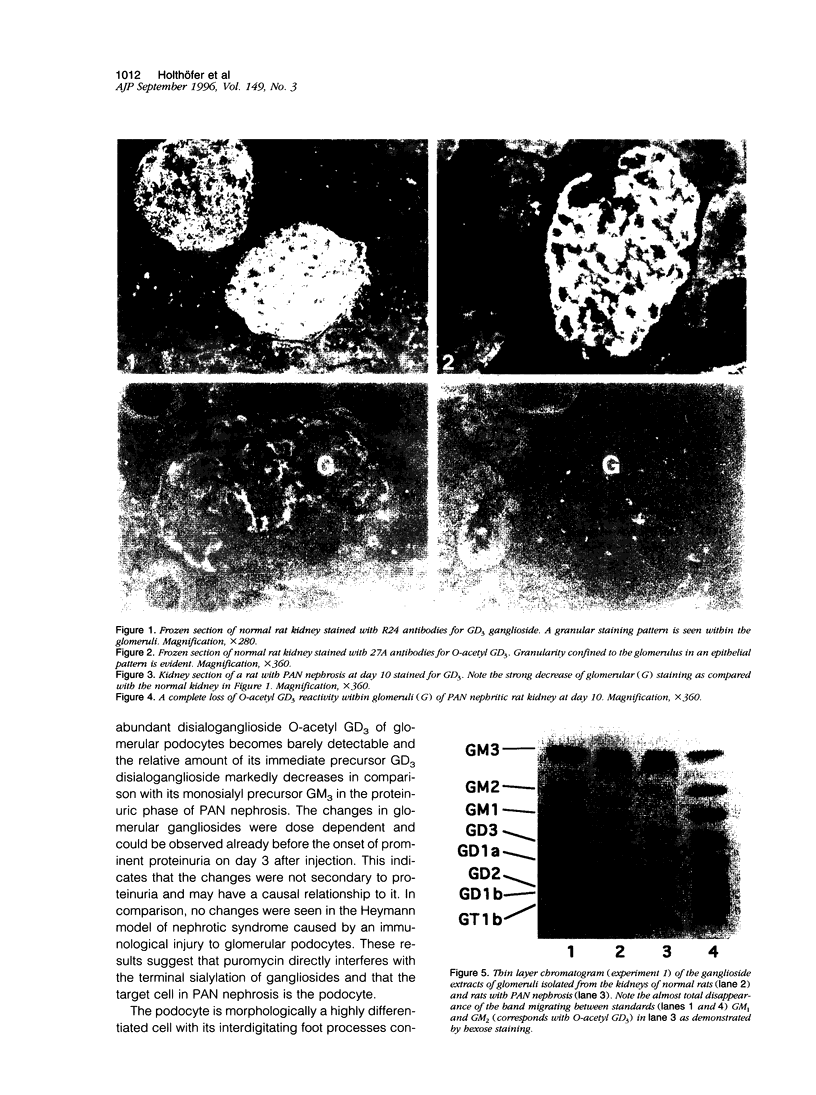
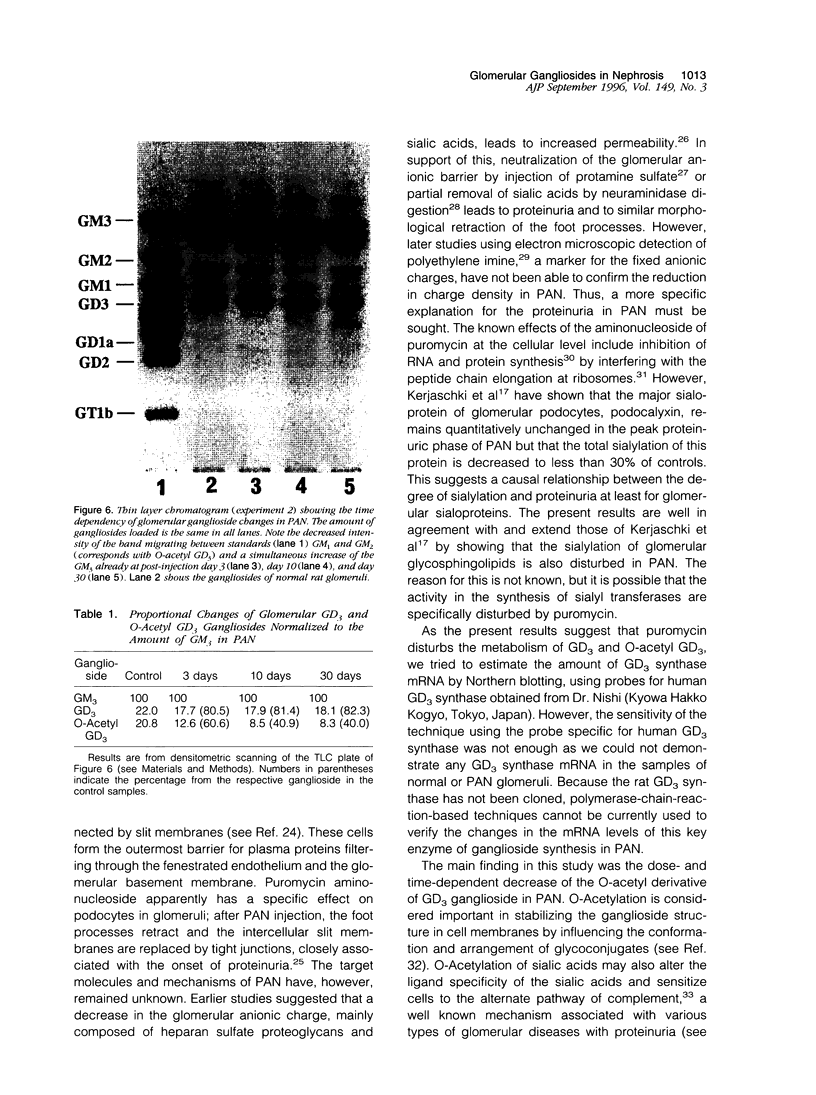


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. M. Glomerular epithelial alterations resulting from sialic acid surface coat removal. Kidney Int. 1979 Apr;15(4):376–385. doi: 10.1038/ki.1979.49. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Hakomori S., Bowen-Pope D. F., Raines E., Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984 Jun 10;259(11):6818–6825. [PubMed] [Google Scholar]
- Cheresh D. A., Pytela R., Pierschbacher M. D., Klier F. G., Ruoslahti E., Reisfeld R. A. An Arg-Gly-Asp-directed receptor on the surface of human melanoma cells exists in an divalent cation-dependent functional complex with the disialoganglioside GD2. J Cell Biol. 1987 Sep;105(3):1163–1173. doi: 10.1083/jcb.105.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couser W. G., Baker P. J., Adler S. Complement and the direct mediation of immune glomerular injury: a new perspective. Kidney Int. 1985 Dec;28(6):879–890. doi: 10.1038/ki.1985.214. [DOI] [PubMed] [Google Scholar]
- GORSKI J., AIZAWA Y., MUELLER G. C. Effect of puromycin in vivo on the synthesis of protein, RNA and phospholipids in rat tissues. Arch Biochem Biophys. 1961 Dec;95:508–511. doi: 10.1016/0003-9861(61)90183-7. [DOI] [PubMed] [Google Scholar]
- Groggel G. C., Hovingh P., Border W. A., Linker A. Changes in glomerular heparan sulfate in puromycin aminonucleoside nephrosis. Am J Pathol. 1987 Sep;128(3):521–527. [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hanai N., Nores G. A., MacLeod C., Torres-Mendez C. R., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 and lyso-GM3 in tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1988 Aug 5;263(22):10915–10921. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Rothman-Denes L. B. Protein synthesis. Annu Rev Biochem. 1973;42:397–438. doi: 10.1146/annurev.bi.42.070173.002145. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Reivinen J., Miettinen A. Nephron segment and cell-type specific expression of gangliosides in the developing and adult kidney. Kidney Int. 1994 Jan;45(1):123–130. doi: 10.1038/ki.1994.14. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Detachment of endothelium and epithelium from the glomerular basement membrane produced by kidney perfusion with neuraminidase. Lab Invest. 1980 Mar;42(3):375–384. [PubMed] [Google Scholar]
- Kerjaschki D., Sharkey D. J., Farquhar M. G. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984 Apr;98(4):1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Vernillo A. T., Farquhar M. G. Reduced sialylation of podocalyxin--the major sialoprotein of the rat kidney glomerulus--in aminonucleoside nephrosis. Am J Pathol. 1985 Mar;118(3):343–349. [PMC free article] [PubMed] [Google Scholar]
- Kreutter D., Kim J. Y., Goldenring J. R., Rasmussen H., Ukomadu C., DeLorenzo R. J., Yu R. K. Regulation of protein kinase C activity by gangliosides. J Biol Chem. 1987 Feb 5;262(4):1633–1637. [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Miettinen A., Dekan G., Farquhar M. G. Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol. 1990 Oct;137(4):929–944. [PMC free article] [PubMed] [Google Scholar]
- Miettinen A., Törnroth T., Tikkanen I., Virtanen I., Linder E. Heymann nephritis induced by kidney brush border glycoproteins. Lab Invest. 1980 Dec;43(6):547–555. [PubMed] [Google Scholar]
- Miettinen A., Westerlund B., Tarkkanen A. M., Törnroth T., Ljungberg P., Renkonen O. V., Korhonen T. K. Binding of bacterial adhesins to rat glomerular mesangium in vivo. Kidney Int. 1993 Mar;43(3):592–600. doi: 10.1038/ki.1993.87. [DOI] [PubMed] [Google Scholar]
- Mohos S. C., Skoza L. Glomerular sialoprotein. Science. 1969 Jun 27;164(3887):1519–1521. doi: 10.1126/science.164.3887.1519. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Reivinen J., Holthöfer H., Miettinen A. A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int. 1992 Sep;42(3):624–631. doi: 10.1038/ki.1992.327. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Rodewald R., Karnovsky M. J. An ultrastructural study of the glomerular slit diaphragm in aminonucleoside nephrosis. Lab Invest. 1975 Nov;33(5):461–468. [PubMed] [Google Scholar]
- Sariola H., Aufderheide E., Bernhard H., Henke-Fahle S., Dippold W., Ekblom P. Antibodies to cell surface ganglioside GD3 perturb inductive epithelial-mesenchymal interactions. Cell. 1988 Jul 15;54(2):235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Schauer R. Sialic acids: metabolism of O-acetyl groups. Methods Enzymol. 1987;138:611–626. doi: 10.1016/0076-6879(87)38055-3. [DOI] [PubMed] [Google Scholar]
- Schwarzmann G., Sandhoff K. Metabolism and intracellular transport of glycosphingolipids. Biochemistry. 1990 Dec 11;29(49):10865–10871. doi: 10.1021/bi00501a001. [DOI] [PubMed] [Google Scholar]
- Seiler M. W., Rennke H. G., Venkatachalam M. A., Cotran R. S. Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest. 1977 Jan;36(1):48–61. [PubMed] [Google Scholar]
- Shayman J. A., Radin N. S. Structure and function of renal glycosphingolipids. Am J Physiol. 1991 Mar;260(3 Pt 2):F291–F302. doi: 10.1152/ajprenal.1991.260.3.F291. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Boström K., Fredman P., Månsson J. E., Rosengren B., Rynmark B. M. Human brain gangliosides: developmental changes from early fetal stage to advanced age. Biochim Biophys Acta. 1989 Sep 25;1005(2):109–117. doi: 10.1016/0005-2760(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Varki A., Kornfeld S. An autosomal dominant gene regulates the extent of 9-O-acetylation of murine erythrocyte sialic acids. A probable explanation for the variation in capacity to activate the human alternate complement pathway. J Exp Med. 1980 Sep 1;152(3):532–544. doi: 10.1084/jem.152.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Ledeen R. W. Gangliosides as modulators of neuronal calcium. Prog Brain Res. 1994;101:101–112. doi: 10.1016/s0079-6123(08)61942-1. [DOI] [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Ganglioside metabolism. Enzymology, Topology, and regulation. J Biol Chem. 1993 Mar 15;268(8):5341–5344. [PubMed] [Google Scholar]