Abstract
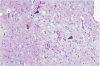
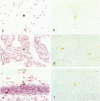
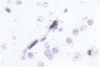
Neurological dysfunction has been shown to be associated with human immunodeficiency virus (HIV) infection. The incidence of these abnormalities is greater in HIV-infected children when compared with adults, and the patterns of neurological disease are also known to differ from those observed in the adult population. The reasons for these differences are unclear but are most likely related to the immaturity of the host's immune and central nervous systems at the time of infection. This is thought to be particularly true for infants infected with HIV prenatally. To examine these questions, the brains of fetal rhesus macaques that were infected with SIVmac251 at various time points in utero were examined. Direct fetal inoculations were performed on gestational day (GD) 65 (n = 8; early second trimester), GD 110 (n = 4; early third trimester) and GD 130 (n = 2; mid third trimester), with harvest of fetal tissues on GD 80, 100, 130, or 145. Eleven sham controls were included with harvest at correlative time points. Specimens were examined by routine histology, immunohistochemistry, and in situ hybridization to localize viral antigens and SIV nucleic acid. Histologically, scattered glial nodules, spongiosis, and mineralization were found in the basal ganglia and deep white matter in 4 of the 14 fetuses (3 inoculated on GD 65 and one on GD 110). These fetuses and those without histological lesions had viral nucleic acid and SIV antigen in the stroma of the choroid plexus, meninges, and external granular layer of the cerebellum and in columns of cells in the cortical plate. In contrast to juvenile and adult macaques, very few SIV-positive perivascular mononuclear cells were present. These findings suggest that SIV has a different distribution in the brain of fetal macaques after direct infection when compared with adult or juvenile animals. Furthermore, the results of these studies suggest that differences in neurological disease between pediatric and adult patients with acquired immune deficiency syndrome are most likely related to the time of infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belman A. L., Diamond G., Dickson D., Horoupian D., Llena J., Lantos G., Rubinstein A. Pediatric acquired immunodeficiency syndrome. Neurologic syndromes. Am J Dis Child. 1988 Jan;142(1):29–35. doi: 10.1001/archpedi.1988.02150010039017. [DOI] [PubMed] [Google Scholar]
- Belman A. L. HIV-1-associated CNS disease in infants and children. Res Publ Assoc Res Nerv Ment Dis. 1994;72:289–310. [PubMed] [Google Scholar]
- Boche D., Gray F., Chakrabarti L., Hurtrel M., Montagnier L., Hurtrel B. Low susceptibility of resident microglia to simian immunodeficiency virus replication during the early stages of infection. Neuropathol Appl Neurobiol. 1995 Dec;21(6):535–539. doi: 10.1111/j.1365-2990.1995.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Cameron R. S., Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4(2):124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Hurtrel M., Maire M. A., Vazeux R., Dormont D., Montagnier L., Hurtrel B. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991 Dec;139(6):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Luciw P. A. Simian immunodeficiency viruses and their relationship to the human immunodeficiency viruses. AIDS. 1988;2 (Suppl 1):S3–10. doi: 10.1097/00002030-198800001-00002. [DOI] [PubMed] [Google Scholar]
- Gibbs J. S., Lackner A. A., Lang S. M., Simon M. A., Sehgal P. K., Daniel M. D., Desrosiers R. C. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995 Apr;69(4):2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschkowitz N. Brain development in the fetus, neonate and infant. Biol Neonate. 1988;54(1):1–19. doi: 10.1159/000242818. [DOI] [PubMed] [Google Scholar]
- Ilyinskii P. O., Daniel M. D., Simon M. A., Lackner A. A., Desrosiers R. C. The role of upstream U3 sequences in the pathogenesis of simian immunodeficiency virus-induced AIDS in rhesus monkeys. J Virol. 1994 Sep;68(9):5933–5944. doi: 10.1128/jvi.68.9.5933-5944.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Lackner A. A., Vogel P., Ramos R. A., Kluge J. D., Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994 Aug;145(2):428–439. [PMC free article] [PubMed] [Google Scholar]
- Lyman W. D., Kress Y., Kure K., Rashbaum W. K., Rubinstein A., Soeiro R. Detection of HIV in fetal central nervous system tissue. AIDS. 1990 Sep;4(9):917–920. doi: 10.1097/00002030-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Mankowski J. L., Spelman J. P., Ressetar H. G., Strandberg J. D., Laterra J., Carter D. L., Clements J. E., Zink M. C. Neurovirulent simian immunodeficiency virus replicates productively in endothelial cells of the central nervous system in vivo and in vitro. J Virol. 1994 Dec;68(12):8202–8208. doi: 10.1128/jvi.68.12.8202-8208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. J., Alexander N. J., Sutjipto S., Lackner A. A., Gettie A., Hendrickx A. G., Lowenstine L. J., Jennings M., Marx P. A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989 Oct;63(10):4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. J., Marthas M., Torten J., Alexander N. J., Moore J. P., Doncel G. F., Hendrickx A. G. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994 Oct;68(10):6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottet H. S., Persidsky Y., Sasseville V. G., Nukuna A. N., Bock P., Zhai Q. H., Sharer L. R., McComb R. D., Swindells S., Soderland C. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996 Feb 1;156(3):1284–1295. [PubMed] [Google Scholar]
- Nuovo G. J., Gallery F., MacConnell P., Braun A. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am J Pathol. 1994 Apr;144(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972 May;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Saito Y., Sharer L. R., Epstein L. G., Michaels J., Mintz M., Louder M., Golding K., Cvetkovich T. A., Blumberg B. M. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994 Mar;44(3 Pt 1):474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sasseville V. G., Lane J. H., Walsh D., Ringler D. J., Lackner A. A. VCAM-1 expression and leukocyte trafficking to the CNS occur early in infection with pathogenic isolates of SIV. J Med Primatol. 1995 May;24(3):123–131. doi: 10.1111/j.1600-0684.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W. A., Lackner A. A., Smith M. O., Lausen N. C., Beall D., Ringler D. J. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am J Pathol. 1992 Nov;141(5):1021–1030. [PMC free article] [PubMed] [Google Scholar]
- Sharer L. R., Dowling P. C., Michaels J., Cook S. D., Menonna J., Blumberg B. M., Epstein L. G. Spinal cord disease in children with HIV-1 infection: a combined molecular biological and neuropathological study. Neuropathol Appl Neurobiol. 1990 Aug;16(4):317–331. doi: 10.1111/j.1365-2990.1990.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Sharer L. R., Epstein L. G., Cho E. S., Joshi V. V., Meyenhofer M. F., Rankin L. F., Petito C. K. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986 Mar;17(3):271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Smith M. O., Heyes M. P., Lackner A. A. Early intrathecal events in rhesus macaques (Macaca mulatta) infected with pathogenic or nonpathogenic molecular clones of simian immunodeficiency virus. Lab Invest. 1995 May;72(5):547–558. [PubMed] [Google Scholar]
- Tarantal A. F., Marthas M. L., Gargosky S. E., Otysula M., McChesney M. B., Miller C. J., Hendrickx A. G. Effects of viral virulence on intrauterine growth in SIV-infected fetal rhesus macaques (Macaca mulatta). J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Oct 1;10(2):129–138. doi: 10.1097/00042560-199510020-00004. [DOI] [PubMed] [Google Scholar]
- Tornatore C., Chandra R., Berger J. R., Major E. O. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994 Mar;44(3 Pt 1):481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Tornatore C., Meyers K., Atwood W., Conant K., Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994 Jan;68(1):93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeux R., Lacroix-Ciaudo C., Blanche S., Cumont M. C., Henin D., Gray F., Boccon-Gibod L., Tardieu M. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol. 1992 Jan;140(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Achim C. L. Human immunodeficiency virus encephalitis and dementia. Ann Neurol. 1995 Oct;38(4):559–560. doi: 10.1002/ana.410380402. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Belman A. L., Dickson D. W., Rubinstein A., Nelson J. A. Human immunodeficiency virus within the brains of children with AIDS. Clin Neuropathol. 1990 Jan-Feb;9(1):1–6. [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]