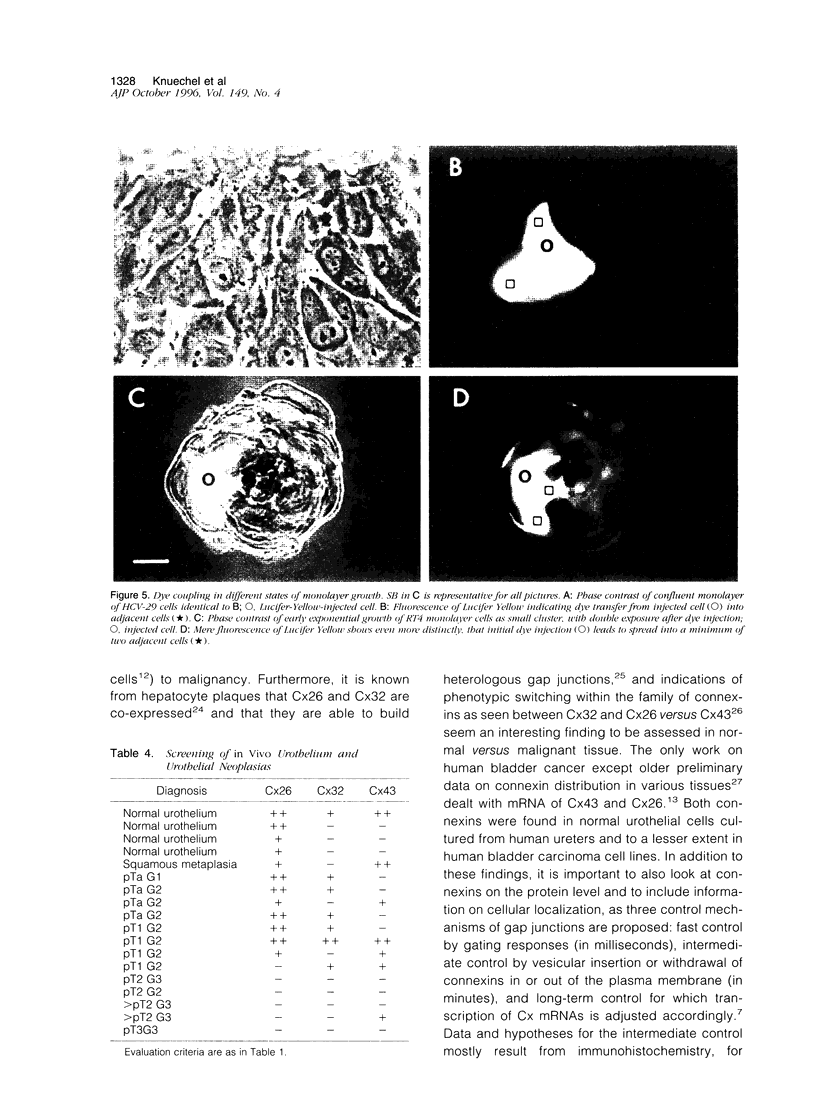
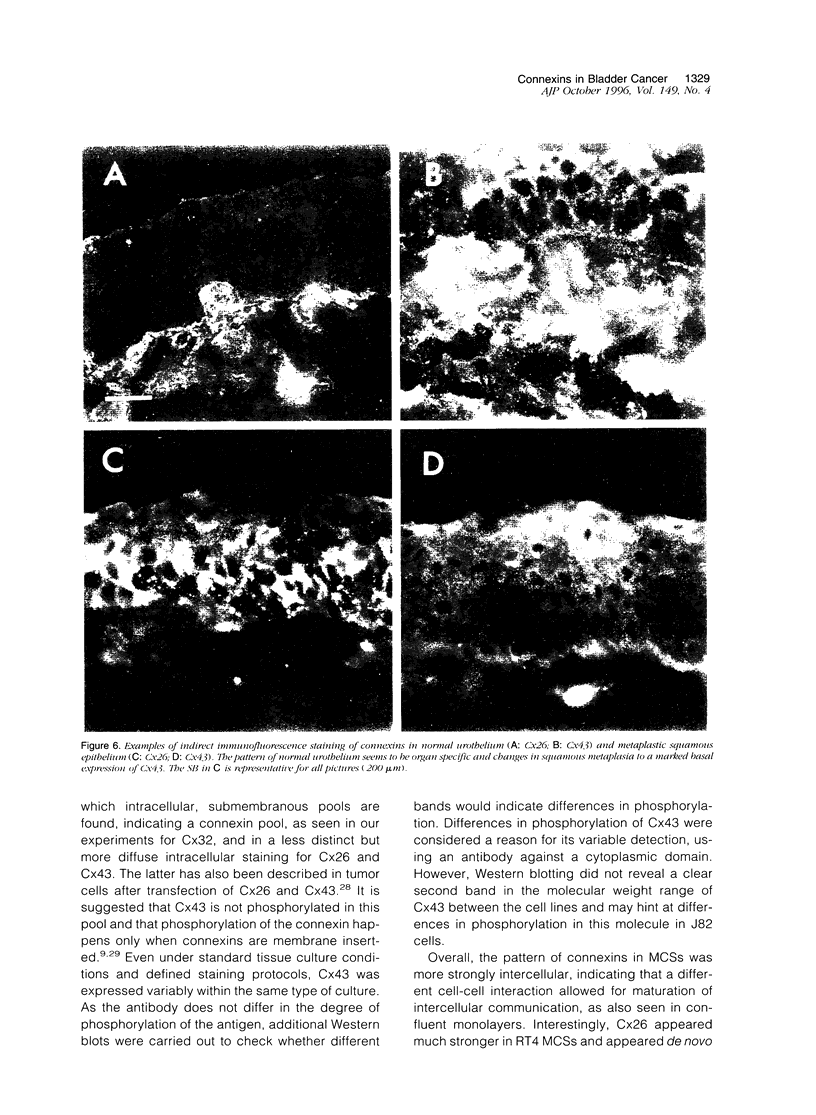
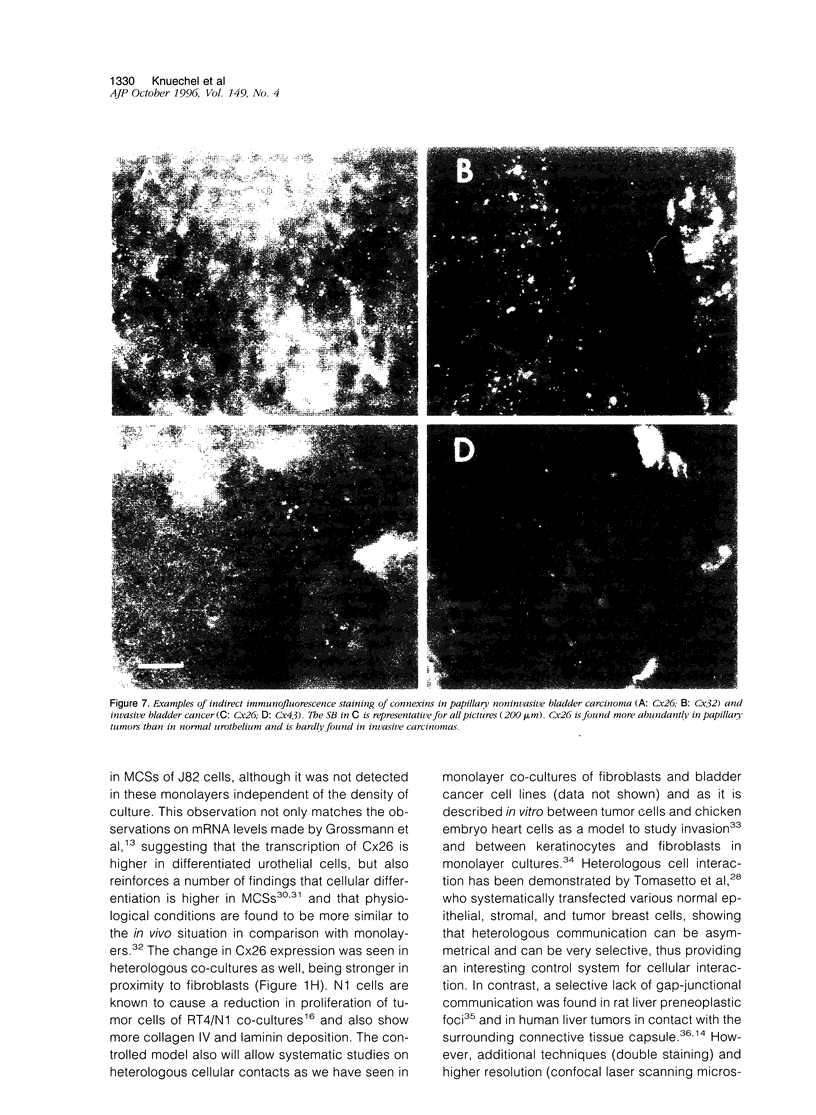
Abstract
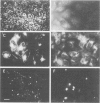
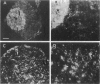
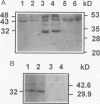
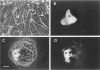
The identification of gap-junctional proteins (connexins) and the preparation of related antibodies provides new tools to study patterns of intercellular communication in tumors. Focusing on the biology of human bladder carcinoma, we compared the expression of gap-junctional proteins (connexins Cx26, Cx32, and Cx43) with a dye-coupling assay for gap-junctional intercellular communication in three cell lines with different urothelial differentiation. The cell lines HCV-29, RT4, and J82 were initially grown as monolayers of different ages. Connexin expression was found mostly positive over the time of culture and found constantly negative only in J82 cells for Cx26 and HCV-29 cells for Cx32. In HCV-29 cells, Cx26 increased in positivity over the time of culture. Western blotting with the antibodies confirmed the findings. Comparisons of dye transfer using Lucifer Yellow showed an increase of coupling in the normal urothelial cell line HCV-29 in contrast to a decrease of coupling in the tumor cell lines. Data were extended by multicellular spheroid (MCS) co-cultures with the stromal fibroblast line N1. In three-dimensional cultures as MCSs, Cx26 was increased in proximity of RT4 tumor cells to fibroblasts, and positivity was maintained in J82 cells. E-cadherin expression in cell lines showed no change in dependence of growth state. The data suggest that Cx26 plays a role in negative growth control or differentiation of urothelial cells. Preliminary comparative data on normal and neoplastic urothelium show all three connexins in normal urothelium, in contrast to varying amounts of Cx43 and low amounts of Cx32 in tumors and evident loss of Cx26 in low-grade tumors. Discrepancies between monolayer and MCS cultures are most likely due to higher differentiation in MCSs, and the continuation of systematic work with heterologous MCSs is indicated for more information on the role of gap-junctional proteins in human tumors.
Full text
PDF


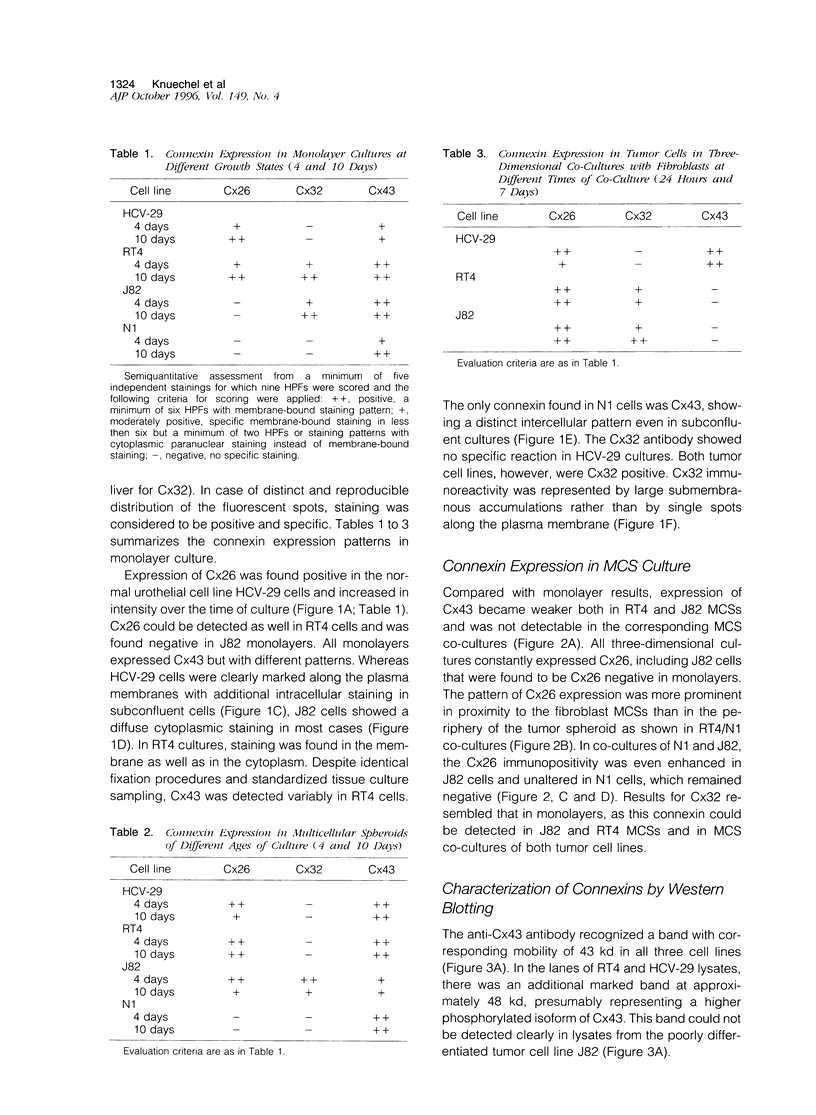

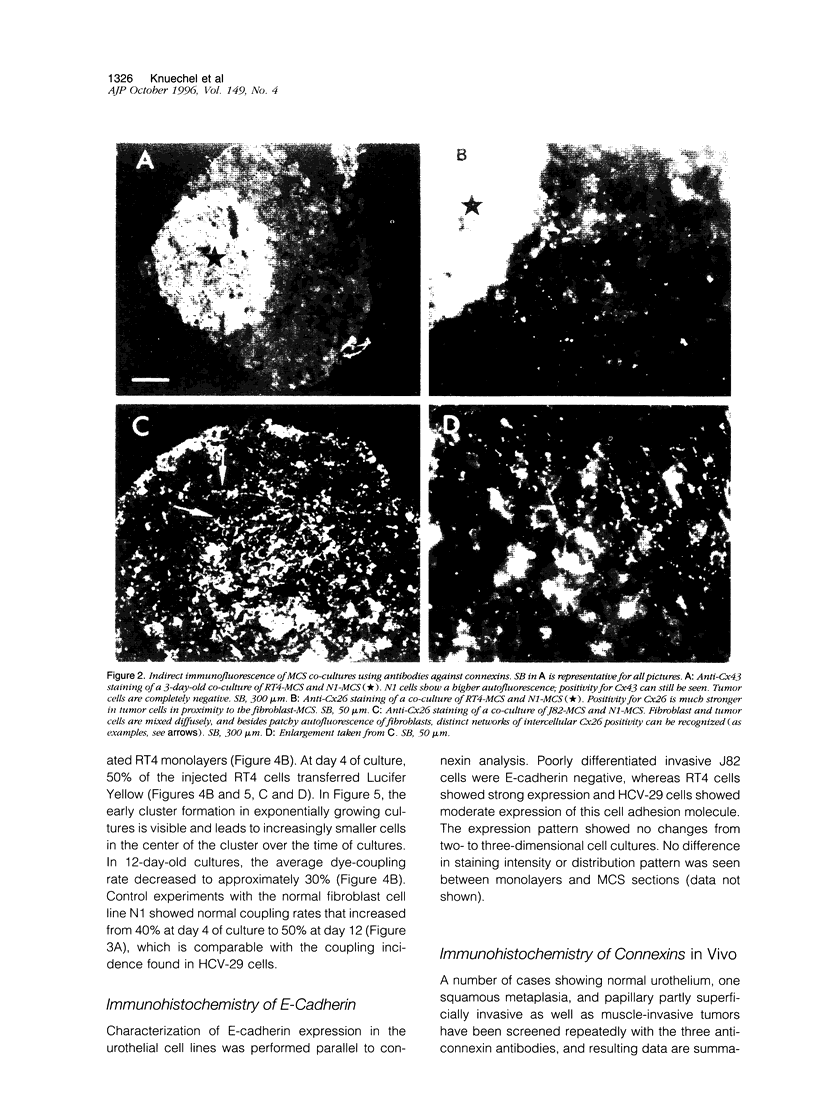
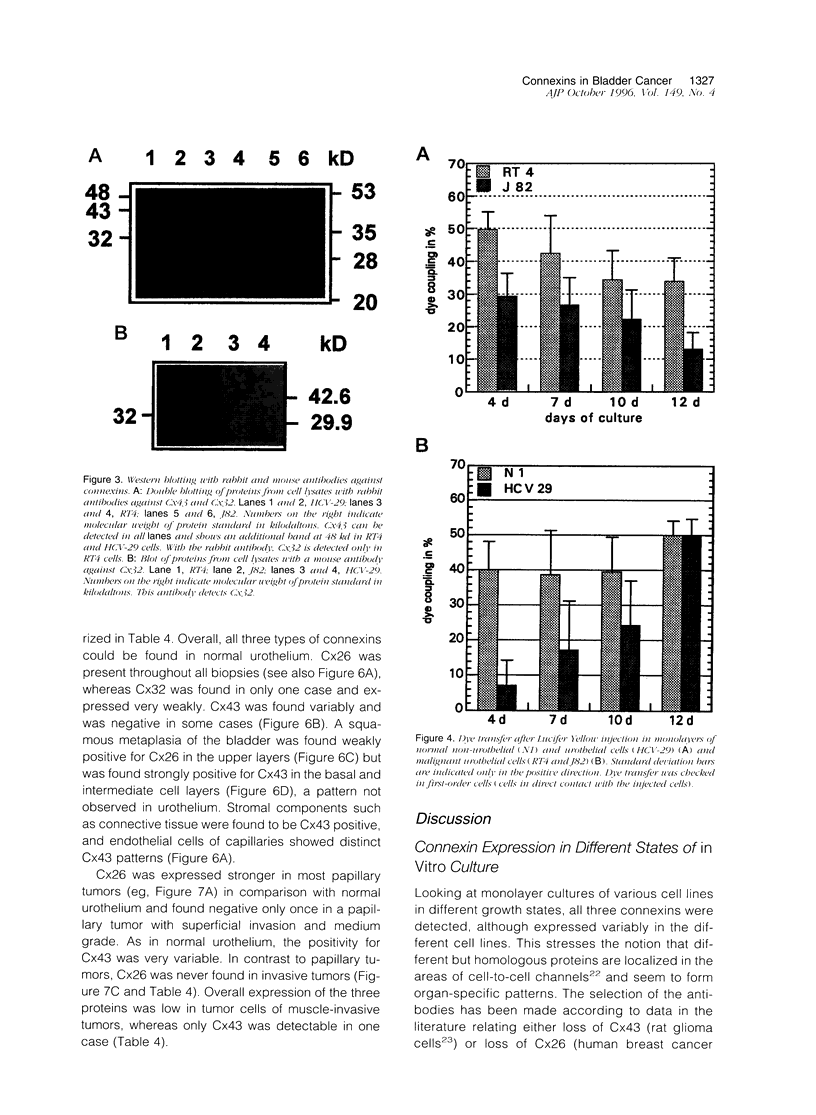


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asamoto M., Oyamada M., el Aoumari A., Gros D., Yamasaki H. Molecular mechanisms of TPA-mediated inhibition of gap-junctional intercellular communication: evidence for action on the assembly or function but not the expression of connexin 43 in rat liver epithelial cells. Mol Carcinog. 1991;4(4):322–327. doi: 10.1002/mc.2940040411. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Russell T. R. Cyclic AMP effects on cell-to-cell junctional membrane permeability during adipocyte differentiation of 3T3-L1 fibroblasts. J Cell Biol. 1985 Jan;100(1):265–269. doi: 10.1083/jcb.100.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Beyer E. C. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- Dermietzel R., Spray D. C. Gap junctions in the brain: where, what type, how many and why? Trends Neurosci. 1993 May;16(5):186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- Elfgang C., Eckert R., Lichtenberg-Fraté H., Butterweck A., Traub O., Klein R. A., Hülser D. F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995 May;129(3):805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg-Newton J., Simpson I., Loewenstein W. R. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979 Jul 27;205(4404):404–407. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Goldberg G. S., Lau A. F. Dynamics of connexin43 phosphorylation in pp60v-src-transformed cells. Biochem J. 1993 Nov 1;295(Pt 3):735–742. doi: 10.1042/bj2950735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman H. B., Liebert M., Lee I. W., Lee S. W. Decreased connexin expression and intercellular communication in human bladder cancer cells. Cancer Res. 1994 Jun 1;54(11):3062–3065. [PubMed] [Google Scholar]
- Holder J. W., Elmore E., Barrett J. C. Gap junction function and cancer. Cancer Res. 1993 Aug 1;53(15):3475–3485. [PubMed] [Google Scholar]
- Janssen-Timmen U., Traub O., Dermietzel R., Rabes H. M., Willecke K. Reduced number of gap junctions in rat hepatocarcinomas detected by monoclonal antibody. Carcinogenesis. 1986 Sep;7(9):1475–1482. doi: 10.1093/carcin/7.9.1475. [DOI] [PubMed] [Google Scholar]
- Knuechel R., Keng P., Hofstaedter F., Langmuir V., Sutherland R. M., Penney D. P. Differentiation patterns in two- and three-dimensional culture systems of human squamous carcinoma cell lines. Am J Pathol. 1990 Sep;137(3):725–736. [PMC free article] [PubMed] [Google Scholar]
- Krutovskikh V. A., Oyamada M., Yamasaki H. Sequential changes of gap-junctional intercellular communications during multistage rat liver carcinogenesis: direct measurement of communication in vivo. Carcinogenesis. 1991 Sep;12(9):1701–1706. doi: 10.1093/carcin/12.9.1701. [DOI] [PubMed] [Google Scholar]
- Krutovskikh V., Mazzoleni G., Mironov N., Omori Y., Aguelon A. M., Mesnil M., Berger F., Partensky C., Yamasaki H. Altered homologous and heterologous gap-junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer. 1994 Jan 2;56(1):87–94. doi: 10.1002/ijc.2910560116. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Paul D., Keyomarsi K., Sager R. Transcriptional downregulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. J Cell Biol. 1992 Sep;118(5):1213–1221. doi: 10.1083/jcb.118.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Sager R. Positive selection of candidate tumor-suppressor genes by subtractive hybridization. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2825–2829. doi: 10.1073/pnas.88.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbridge J. N., Knüchel R., Knapp A. M., Sutherland R. M. Importance of tyrosine phosphatases in the effects of cell-cell contact and microenvironments on EGF-stimulated tyrosine phosphorylation. J Cell Physiol. 1992 Jun;151(3):433–442. doi: 10.1002/jcp.1041510302. [DOI] [PubMed] [Google Scholar]
- Masters J. R., Hepburn P. J., Walker L., Highman W. J., Trejdosiewicz L. K., Povey S., Parkar M., Hill B. T., Riddle P. R., Franks L. M. Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines. Cancer Res. 1986 Jul;46(7):3630–3636. [PubMed] [Google Scholar]
- McKay I. A., Taylor-Papadimitriou J. The non-selective junctional communication phenotype of normal and transformed human epidermal keratinocytes in vitro. Exp Cell Res. 1982 Sep;141(1):171–180. doi: 10.1016/0014-4827(82)90079-9. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Cunningham B. A., Edelman G. M., Goodenough D. A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990 Nov;111(5 Pt 1):2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991 Dec;115(5):1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993 Sep 24;74(6):1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Naus C. C., Bechberger J. F., Caveney S., Wilson J. X. Expression of gap junction genes in astrocytes and C6 glioma cells. Neurosci Lett. 1991 May 13;126(1):33–36. doi: 10.1016/0304-3940(91)90364-y. [DOI] [PubMed] [Google Scholar]
- Nicholson B., Dermietzel R., Teplow D., Traub O., Willecke K., Revel J. P. Two homologous protein components of hepatic gap junctions. Nature. 1987 Oct 22;329(6141):732–734. doi: 10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- Rose B., Mehta P. P., Loewenstein W. R. Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis. 1993 May;14(5):1073–1075. doi: 10.1093/carcin/14.5.1073. [DOI] [PubMed] [Google Scholar]
- Schuster U., Büttner R., Hofstädter F., Knüchel R. A heterologous in vitro coculture system to study interaction between human bladder cancer cells and fibroblasts. J Urol. 1994 Jun;151(6):1707–1711. doi: 10.1016/s0022-5347(17)35349-1. [DOI] [PubMed] [Google Scholar]
- Stutenkemper R., Geisse S., Schwarz H. J., Look J., Traub O., Nicholson B. J., Willecke K. The hepatocyte-specific phenotype of murine liver cells correlates with high expression of connexin32 and connexin26 but very low expression of connexin43. Exp Cell Res. 1992 Jul;201(1):43–54. doi: 10.1016/0014-4827(92)90346-a. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Importance of critical metabolites and cellular interactions in the biology of microregions of tumors. Cancer. 1986 Oct 15;58(8):1668–1680. doi: 10.1002/1097-0142(19861015)58:8<1668::aid-cncr2820580816>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Neveu M. J., Daley J., Horan P. K., Sager R. Specificity of gap junction communication among human mammary cells and connexin transfectants in culture. J Cell Biol. 1993 Jul;122(1):157–167. doi: 10.1083/jcb.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgenbus K. K., Kirkpatrick C. J., Knuechel R., Willecke K., Traub O. Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. Int J Cancer. 1992 Jun 19;51(4):522–529. doi: 10.1002/ijc.2910510404. [DOI] [PubMed] [Google Scholar]