Abstract
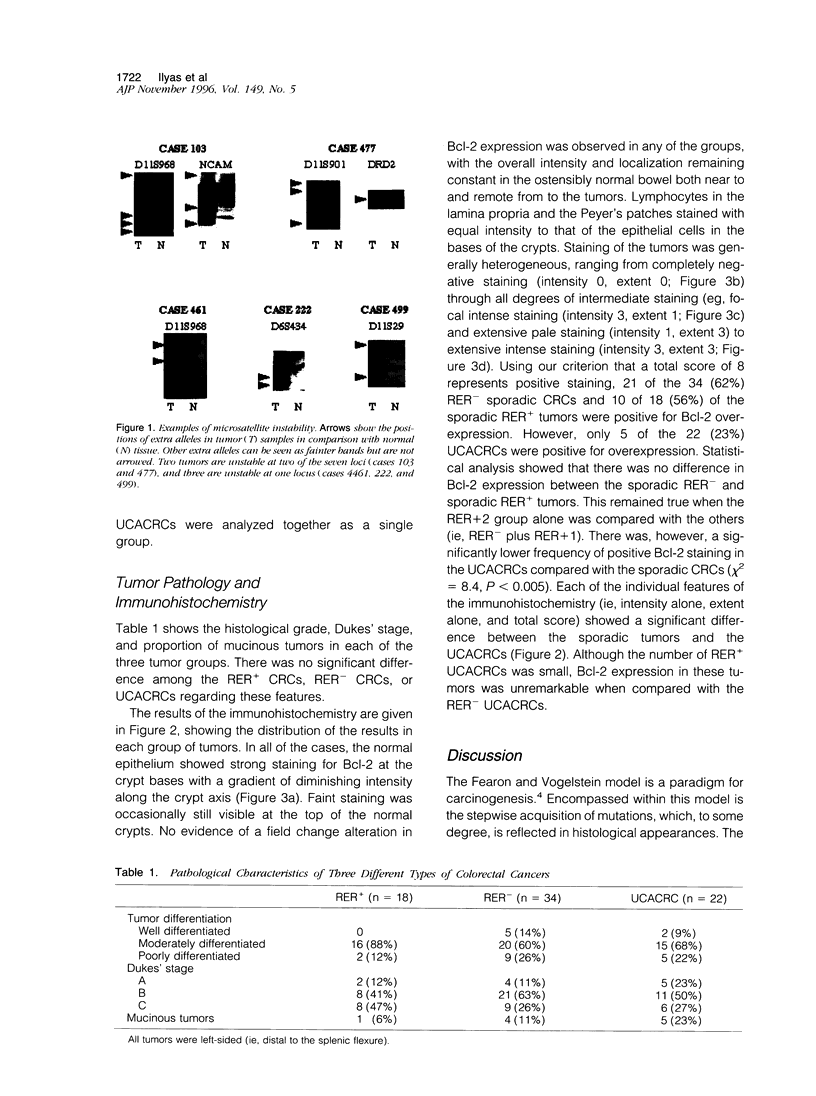
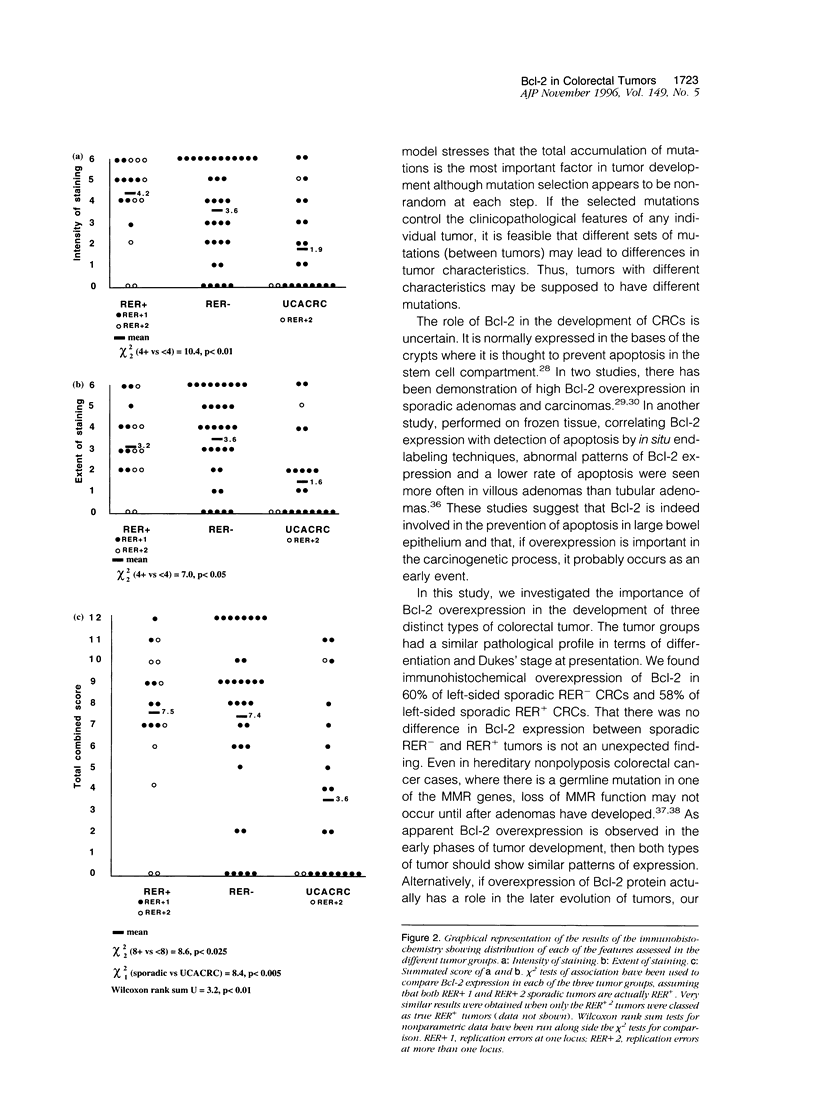
Colorectal cancers (CRCs) differ in their age at presentation, distribution, histological features, and prognosis. If tumor biology reflects genetic events, these tumors might be expected to show differences in their genetic pathways. In this study, we investigated the role of Bcl-2 in the development of three different tumor groups. Using markers at eight different microsatellite locl, we characterized one group of 34 left-sided sporadic CRCs as replication error negative (RER-) and another group of 18 left-sided sporadic CRCs as replication error positive (RER+). These tumors, together with a third group of 22 left-sided ulcerative-colitis-associated CRCs (UCACRCs), were then examined by immunohistochemistry for Bcl-2 overexpression. Of 34 of the RER- tumors, 21 (62%) and 10 of 18 (56%) of the RER+ tumors were positive for Bcl-2 overexpression. In contrast, only 5 of 22 (23%) of the UCACRCs showed similar overexpression. Our results show a significantly lower frequency of Bcl-2 overexpression in UCACRCs as compared with sporadic CRCs (P < 0.005) but no difference between sporadic left-sided RER+ and RER- CRCs. These data provide additional evidence that UCACRCs may develop along a pathway that is different from that of sporadic CRCs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Bedi A., Pasricha P. J., Akhtar A. J., Barber J. P., Bedi G. C., Giardiello F. M., Zehnbauer B. A., Hamilton S. R., Jones R. J. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995 May 1;55(9):1811–1816. [PubMed] [Google Scholar]
- Befrits R., Hammarberg C., Rubio C., Jaramillo E., Tribukait B. DNA aneuploidy and histologic dysplasia in long-standing ulcerative colitis. A 10-year follow-up study. Dis Colon Rectum. 1994 Apr;37(4):313–320. doi: 10.1007/BF02053590. [DOI] [PubMed] [Google Scholar]
- Bell S. M., Kelly S. A., Hoyle J. A., Lewis F. A., Taylor G. R., Thompson H., Dixon M. F., Quirke P. c-Ki-ras gene mutations in dysplasia and carcinomas complicating ulcerative colitis. Br J Cancer. 1991 Jul;64(1):174–178. doi: 10.1038/bjc.1991.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentnall T. A., Crispin D. A., Rabinovitch P. S., Haggitt R. C., Rubin C. E., Stevens A. C., Burmer G. C. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994 Aug;107(2):369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Bronner M. P., Culin C., Reed J. C., Furth E. E. The bcl-2 proto-oncogene and the gastrointestinal epithelial tumor progression model. Am J Pathol. 1995 Jan;146(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Carder P., Wyllie A. H., Purdie C. A., Morris R. G., White S., Piris J., Bird C. C. Stabilised p53 facilitates aneuploid clonal divergence in colorectal cancer. Oncogene. 1993 May;8(5):1397–1401. [PubMed] [Google Scholar]
- Colombel M., Symmans F., Gil S., O'Toole K. M., Chopin D., Benson M., Olsson C. A., Korsmeyer S., Buttyan R. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993 Aug;143(2):390–400. [PMC free article] [PubMed] [Google Scholar]
- Connell W. R., Talbot I. C., Harpaz N., Britto N., Wilkinson K. H., Kamm M. A., Lennard-Jones J. E. Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis. Gut. 1994 Oct;35(10):1419–1423. doi: 10.1136/gut.35.10.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOULDS L. The natural history of cancer. J Chronic Dis. 1958 Jul;8(1):2–37. doi: 10.1016/0021-9681(58)90039-0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fishel R., Kolodner R. D. Identification of mismatch repair genes and their role in the development of cancer. Curr Opin Genet Dev. 1995 Jun;5(3):382–395. doi: 10.1016/0959-437x(95)80055-7. [DOI] [PubMed] [Google Scholar]
- Fozard J. B., Quirke P., Dixon M. F., Giles G. R., Bird C. C. DNA aneuploidy in ulcerative colitis. Gut. 1986 Dec;27(12):1414–1418. doi: 10.1136/gut.27.12.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald B. D., Harpaz N., Yin J., Huang Y., Tong Y., Brown V. L., McDaniel T., Newkirk C., Resau J. H., Meltzer S. J. Loss of heterozygosity affecting the p53, Rb, and mcc/apc tumor suppressor gene loci in dysplastic and cancerous ulcerative colitis. Cancer Res. 1992 Feb 1;52(3):741–745. [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Gyde S. N., Prior P., Allan R. N., Stevens A., Jewell D. P., Truelove S. C., Lofberg R., Brostrom O., Hellers G. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988 Feb;29(2):206–217. doi: 10.1136/gut.29.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague A., Moorghen M., Hicks D., Chapman M., Paraskeva C. BCL-2 expression in human colorectal adenomas and carcinomas. Oncogene. 1994 Nov;9(11):3367–3370. [PubMed] [Google Scholar]
- Hauge X. Y., Grandy D. K., Eubanks J. H., Evans G. A., Civelli O., Litt M. Detection and characterization of additional DNA polymorphisms in the dopamine D2 receptor gene. Genomics. 1991 Jul;10(3):527–530. doi: 10.1016/0888-7543(91)90431-d. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Ilyas M., Jalal H., Linton C., Rooney N. The use of the polymerase chain reaction in the diagnosis of B-cell lymphomas from formalin-fixed paraffin-embedded tissue. Histopathology. 1995 Apr;26(4):333–338. doi: 10.1111/j.1365-2559.1995.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R. Colorectal adenomas in surgical specimens from subjects with hereditary non-polyposis colorectal cancer. Histopathology. 1995 Sep;27(3):263–267. doi: 10.1111/j.1365-2559.1995.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Stewart S. M., Stewart J., Lane M. R. Hereditary non-polyposis colorectal cancer--morphologies, genes and mutations. Mutat Res. 1994 Oct 1;310(1):125–133. doi: 10.1016/0027-5107(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Kim H., Jen J., Vogelstein B., Hamilton S. R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994 Jul;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Levin B. Inflammatory bowel disease and colon cancer. Cancer. 1992 Sep 1;70(5 Suppl):1313–1316. doi: 10.1002/1097-0142(19920901)70:3+<1313::aid-cncr2820701518>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Lu Q. L., Poulsom R., Wong L., Hanby A. M. Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol. 1993 Apr;169(4):431–437. doi: 10.1002/path.1711690408. [DOI] [PubMed] [Google Scholar]
- Modrich P. Mechanisms and biological effects of mismatch repair. Annu Rev Genet. 1991;25:229–253. doi: 10.1146/annurev.ge.25.120191.001305. [DOI] [PubMed] [Google Scholar]
- Peltomäki P. Microsatellite instability and hereditary non-polyposis colon cancer. J Pathol. 1995 Aug;176(4):329–330. doi: 10.1002/path.1711760402. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Turley H., Kuzu I., Tungekar M. F., Dunnill M. S., Pierce C. B., Harris A., Gatter K. C., Mason D. Y. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993 Sep 2;329(10):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- Redston M. S., Papadopoulos N., Caldas C., Kinzler K. W., Kern S. E. Common occurrence of APC and K-ras gene mutations in the spectrum of colitis-associated neoplasias. Gastroenterology. 1995 Feb;108(2):383–392. doi: 10.1016/0016-5085(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Rubin C. E., Haggitt R. C., Burmer G. C., Brentnall T. A., Stevens A. C., Levine D. S., Dean P. J., Kimmey M., Perera D. R., Rabinovitch P. S. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992 Nov;103(5):1611–1620. doi: 10.1016/0016-5085(92)91185-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Harpaz N., Tarmin L., Yin J., Jiang H. Y., Bell J. D., Hontanosas M., Groisman G. M., Abraham J. M., Meltzer S. J. Microsatellite instability in ulcerative colitis-associated colorectal dysplasias and cancers. Cancer Res. 1994 Sep 15;54(18):4841–4844. [PubMed] [Google Scholar]
- Tarmin L., Yin J., Harpaz N., Kozam M., Noordzij J., Antonio L. B., Jiang H. Y., Chan O., Cymes K., Meltzer S. J. Adenomatous polyposis coli gene mutations in ulcerative colitis-associated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res. 1995 May 15;55(10):2035–2038. [PubMed] [Google Scholar]
- Telatar M., Concannon P., Tolun A. Dinucleotide repeat polymorphism at the NCAM locus. Hum Mol Genet. 1994 May;3(5):842–842. doi: 10.1093/hmg/3.5.842. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Warnich L., Groenewald I., Theart L., Retief A. E. Highly informative dinucleotide repeat polymorphism at the D11S29 locus on chromosome 11q23. Hum Genet. 1992 May;89(3):357–359. doi: 10.1007/BF00220560. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Weinberg R. A. The integration of molecular genetics into cancer management. Cancer. 1992 Sep 15;70(6 Suppl):1653–1658. doi: 10.1002/1097-0142(19920915)70:4+<1653::aid-cncr2820701603>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Yin J., Harpaz N., Tong Y., Huang Y., Laurin J., Greenwald B. D., Hontanosas M., Newkirk C., Meltzer S. J. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology. 1993 Jun;104(6):1633–1639. doi: 10.1016/0016-5085(93)90639-t. [DOI] [PubMed] [Google Scholar]