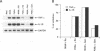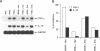Abstract
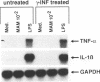
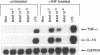
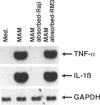
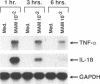
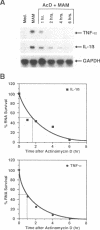
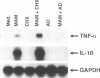
Soluble factors produced by Mycoplasma arthritidis play an important role in the pathology of arthritis in rodents, which closely resembles human rheumatoid arthritis. At least one of the products of these microorganisms, M. arthritidis-T cell mitogen (MAM), has biological activities in common with superantigens. These superantigens activate T cells in a V beta-restricted fashion, and this response is strictly dependent on the presence of major histocompatibility complex (MHC) class II-positive cells. In the present study, we have examined the ability of MAM to induce proinflammatory monokine (interleukin 1 beta [IL-1 beta] and tumor necrosis factor alpha [TNF-alpha]) gene expression in the THP-1 monocytic cell line. Treatment of these cells (which express a very low level of HLA-DR molecules) with gamma interferon (INF-gamma) induced HLA-DR, -DQ, and -DP molecules and enabled them to respond to MAM in a dose-dependent manner, resulting in an increase in the level of steady-state mRNA for IL-1 beta and TNF-alpha. Stimulation of the U937 monocytic cell line (MHC class II-negative even after INF-gamma treatment) with MAM did not induce either IL-1 beta or TNF-alpha transcription. Moreover, MAM adsorption on Raji (MHC class II-positive) cells resulted in the loss of its cytokine-inducing activity to induce monokine gene expression. These findings demonstrate clearly that MAM induces monokine gene expression following interaction with MHC class II molecules. Pretreatment of INF-gamma-treated THP-1 cells with the transcription inhibitor actinomycin D prevented the induction of monokine mRNA, whereas cycloheximide superinduced mRNA after stimulation with MAM. Finally, our results, obtained with protein tyrosine kinase inhibitors and antiphosphotyrosine Western blotting (immunoblotting), indicate that protein tyrosine kinase is involved in MAM-induced IL-1 beta and TNF-alpha gene expression in the THP-1 monocytic cell line. The capacity of MAM to induce proinflammatory cytokine transcription in monocytes via MHC class II molecules can be one pathway of MAM contribution to autoimmune diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Atkin C. L., Cole B. C., Sullivan G. J., Washburn L. R., Wiley B. B. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. V. A small basic protein from culture supernatants is a potent T cell mitogen. J Immunol. 1986 Sep 1;137(5):1581–1589. [PubMed] [Google Scholar]
- Bakouche O., Moreau J. L., Lachman L. B. Secretion of IL-1: role of protein kinase C. J Immunol. 1992 Jan 1;148(1):84–91. [PubMed] [Google Scholar]
- Bauer A., Giese M., Kirchner H. Role of interleukin 1 in mycoplasma mitogen-induced proliferation of human T cells. Immunobiology. 1989 Mar;179(1):124–130. doi: 10.1016/S0171-2985(89)80011-7. [DOI] [PubMed] [Google Scholar]
- Calman A. F., Peterlin B. M. Mutant human B cell lines deficient in class II major histocompatibility complex transcription. J Immunol. 1987 Oct 1;139(7):2489–2495. [PubMed] [Google Scholar]
- Cambier J. C., Newell M. K., Justement L. B., McGuire J. C., Leach K. L., Chen Z. Z. Ia binding ligands and cAMP stimulate nuclear translocation of PKC in B lymphocytes. Nature. 1987 Jun 18;327(6123):629–632. doi: 10.1038/327629a0. [DOI] [PubMed] [Google Scholar]
- Chatila T., Geha R. S. Signal transduction by microbial superantigens via MHC class II molecules. Immunol Rev. 1993 Feb;131:43–59. doi: 10.1111/j.1600-065x.1993.tb01529.x. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Atkin C. L. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol Today. 1991 Aug;12(8):271–276. doi: 10.1016/0167-5699(91)90125-D. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Balderas R. A., Ahmed E. A., Kono D., Theofilopoulos A. N. Genomic composition and allelic polymorphisms influence V beta usage by the Mycoplasma arthritidis superantigen. J Immunol. 1993 Apr 15;150(8 Pt 1):3291–3299. [PubMed] [Google Scholar]
- Cole B. C., David C. S., Lynch D. H., Kartchner D. R. The use of transfected fibroblasts and transgenic mice establishes that stimulation of T cells by the Mycoplasma arthritidis mitogen is mediated by E alpha. J Immunol. 1990 Jan 15;144(2):420–424. [PubMed] [Google Scholar]
- Crow M. K., Zagon G., Chu Z., Ravina B., Tumang J. R., Cole B. C., Friedman S. M. Human B cell differentiation induced by microbial superantigens: unselected peripheral blood lymphocytes secrete polyclonal immunoglobulin in response to Mycoplasma arthritidis mitogen. Autoimmunity. 1992;14(1):23–32. doi: 10.3109/08916939309077353. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Emery P., Panayi G. S., Welsh K. I., Cole B. C. Rheumatoid factors and HLA-DR4 in RA. J Rheumatol. 1985 Apr;12(2):217–222. [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W., Clark B. D., Webb A. C., Auron P. E. Human pro-IL-1 beta gene expression in monocytic cells is regulated by two distinct pathways. J Immunol. 1988 Apr 1;140(7):2267–2273. [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry M., Caon A. C., Naccache P. H. Modulation of the activity and subcellular distribution of protein tyrosine kinases in human neutrophils by phorbol esters. FASEB J. 1993 May;7(8):687–693. doi: 10.1096/fasebj.7.8.7684713. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Brehm G., Nicklas W., Beck R., Herbst F. Biochemical characterization of the T-cell mitogen derived from Mycoplasma arthritidis. Scand J Immunol. 1986 Sep;24(3):245–249. doi: 10.1111/j.1365-3083.1986.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T-cell repertoire for antigen and MHC. Immunol Today. 1988 Oct;9(10):308–315. doi: 10.1016/0167-5699(88)91324-2. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Matthes M., Schrezenmeier H., Homfeld J., Fleischer S., Malissen B., Kirchner H., Fleischer B. Clonal analysis of human T cell activation by the Mycoplasma arthritidis mitogen (MAS). Eur J Immunol. 1988 Nov;18(11):1733–1737. doi: 10.1002/eji.1830181112. [DOI] [PubMed] [Google Scholar]
- Mourad W., Mehindate K., Schall T. J., McColl S. R. Engagement of major histocompatibility complex class II molecules by superantigen induces inflammatory cytokine gene expression in human rheumatoid fibroblast-like synoviocytes. J Exp Med. 1992 Feb 1;175(2):613–616. doi: 10.1084/jem.175.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad W., Scholl P., Diaz A., Geha R., Chatila T. The staphylococcal toxic shock syndrome toxin 1 triggers B cell proliferation and differentiation via major histocompatibility complex-unrestricted cognate T/B cell interaction. J Exp Med. 1989 Dec 1;170(6):2011–2022. doi: 10.1084/jem.170.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad W., al-Daccak R., Chatila T., Geha R. S. Staphylococcal superantigens as inducers of signal transduction in MHC class II-positive cells. Semin Immunol. 1993 Feb;5(1):47–55. doi: 10.1006/smim.1993.1007. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Gilbert C., Caon A. C., Gaudry M., Huang C. K., Bonak V. A., Umezawa K., McColl S. R. Selective inhibition of human neutrophil functional responsiveness by erbstatin, an inhibitor of tyrosine protein kinase. Blood. 1990 Nov 15;76(10):2098–2104. [PubMed] [Google Scholar]
- Nouri A. M., Panayi G. S., Goodman S. M. Cytokines and the chronic inflammation of rheumatic disease. I. The presence of interleukin-1 in synovial fluids. Clin Exp Immunol. 1984 Feb;55(2):295–302. [PMC free article] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Posnett D. N. Do superantigens play a role in autoimmunity? Semin Immunol. 1993 Feb;5(1):65–72. doi: 10.1006/smim.1993.1009. [DOI] [PubMed] [Google Scholar]
- Rathanaswami P., Hachicha M., Sadick M., Schall T. J., McColl S. R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993 Mar 15;268(8):5834–5839. [PubMed] [Google Scholar]
- Rink L., Kirchner H. Mycoplasma arthritidis-derived superantigen. Chem Immunol. 1992;55:137–145. [PubMed] [Google Scholar]
- Rink L., Kruse A., Nicklas W., Hoyer J., Kirchner H. Induction of cytokines in human peripheral blood and spleen cells by the Mycoplasma arthritidis-derived superantigen. Lymphokine Cytokine Res. 1992 Apr;11(2):105–108. [PubMed] [Google Scholar]
- Saxne T., Palladino M. A., Jr, Heinegård D., Talal N., Wollheim F. A. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988 Aug;31(8):1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Scholl P. R., Trede N., Chatila T. A., Geha R. S. Role of protein tyrosine phosphorylation in monokine induction by the staphylococcal superantigen toxic shock syndrome toxin-1. J Immunol. 1992 Apr 1;148(7):2237–2241. [PubMed] [Google Scholar]
- See R. H., Chow A. W. Staphylococcal toxic shock syndrome toxin 1-induced tumor necrosis factor alpha and interleukin-1 beta secretion by human peripheral blood monocytes and T lymphocytes is differentially suppressed by protein kinase inhibitors. Infect Immun. 1992 Aug;60(8):3456–3459. doi: 10.1128/iai.60.8.3456-3459.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumang J. R., Posnett D. N., Cole B. C., Crow M. K., Friedman S. M. Helper T cell-dependent human B cell differentiation mediated by a mycoplasmal superantigen bridge. J Exp Med. 1990 Jun 1;171(6):2153–2158. doi: 10.1084/jem.171.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Band H., Bonneville F., Yunis E. J. Differential expression of MHC class II antigens in myelomonocytic leukemia cell lines. Blood. 1989 Mar;73(4):931–937. [PubMed] [Google Scholar]