Abstract
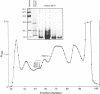
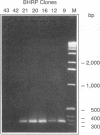
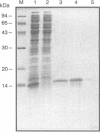
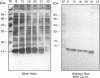
The decline in prevalence of leprosy is not necessarily matched by a fall in incidence, emphasizing the need for new antigens to measure disease transmission and reservoirs of infection. Mycobacterium leprae obtained from armadillo tissues was disrupted and subjected to differential centrifugation to arrive at preparations of cell wall, cytoplasmic membrane, and cytosol. By committing 0.3 g of M. leprae to the task, it was possible to isolate from the cytosol and fully define the major cytosolic protein. Amino-terminus sequencing and chemical and enzymatic cleavage, followed by more sequencing and fast atom bombardment-mass spectrometry of fragments, allowed description of the entire amino acid sequence of a protein of 10,675-Da molecular mass. The sequence derived by chemical means is identical to that deduced previously from DNA analysis of the gene of a 10-kDa protein, a GroES analog. The work represents the first complete chemical definition of an M. leprae protein. PCR amplification of the 10-kDa protein gene, when cloned into Escherichia coli with a pTRP expression vector, allowed production of the recombinant protein. Chemical analysis of the expressed protein demonstrated that it exactly reflected the native protein. The recombinant major cytosolic protein appears to be a promising reagent for skin testing, still probably the most appropriate and pragmatic means of measuring incidence of leprosy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Harboe M., Rook G. A. Characterization of the secreted antigens of Mycobacterium bovis BCG: comparison of the 46-kilodalton dimeric protein with proteins MPB64 and MPB70. Infect Immun. 1987 Dec;55(12):3213–3214. doi: 10.1128/iai.55.12.3213-3214.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. Cloning and sequence analysis of the 10 kDa antigen gene of Mycobacterium tuberculosis. J Gen Microbiol. 1989 Apr;135(4):931–939. doi: 10.1099/00221287-135-4-931. [DOI] [PubMed] [Google Scholar]
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Chandrasekhar G. N., Tilly K., Woolford C., Hendrix R., Georgopoulos C. Purification and properties of the groES morphogenetic protein of Escherichia coli. J Biol Chem. 1986 Sep 15;261(26):12414–12419. [PubMed] [Google Scholar]
- Chatterjee D., Lowell K., Rivoire B., McNeil M. R., Brennan P. J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem. 1992 Mar 25;267(9):6234–6239. [PubMed] [Google Scholar]
- Cherayil B. J., Young R. A. A 28-kDa protein from Mycobacterium leprae is a target of the human antibody response in lepromatous leprosy. J Immunol. 1988 Dec 15;141(12):4370–4375. [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Turneer M., Weckx M., Nyabenda J., Van Vooren J. P., Falmagne P., Wiker H. G., Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987 Jan;55(1):245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Stoker N. G., Lee S. P., Jackson M., Grant K. A., Jouy N. F., Lucas S. B., Hasan R., Hussain R., McAdam K. P. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect Immun. 1989 Jul;57(7):1979–1983. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T. M., Booth R. J., Love S. G., Gibson J. J., Harding D. R., Watson J. D. Characterization of an antibody-binding epitope from the 18-kDa protein on Mycobacterium leprae. J Immunol. 1989 Mar 1;142(5):1691–1695. [PubMed] [Google Scholar]
- Eiglmeier K., Honoré N., Woods S. A., Caudron B., Cole S. T. Use of an ordered cosmid library to deduce the genomic organization of Mycobacterium leprae. Mol Microbiol. 1993 Jan;7(2):197–206. doi: 10.1111/j.1365-2958.1993.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Garsia R. J., Hellqvist L., Booth R. J., Radford A. J., Britton W. J., Astbury L., Trent R. J., Basten A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect Immun. 1989 Jan;57(1):204–212. doi: 10.1128/iai.57.1.204-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Annu Rev Microbiol. 1987;41:645–675. doi: 10.1146/annurev.mi.41.100187.003241. [DOI] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragerov A., Nudler E., Komissarova N., Gaitanaris G. A., Gottesman M. E., Nikiforov V. Cooperation of GroEL/GroES and DnaK/DnaJ heat shock proteins in preventing protein misfolding in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10341–10344. doi: 10.1073/pnas.89.21.10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hawke D. H., Harris D. C., Shively J. E. Microsequence analysis of peptides and proteins. V. Design and performance of a novel gas-liquid-solid phase instrument. Anal Biochem. 1985 Jun;147(2):315–330. doi: 10.1016/0003-2697(85)90278-7. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Hunter S. W., Rivoire B., Mehra V., Bloom B. R., Brennan P. J. The major native proteins of the leprosy bacillus. J Biol Chem. 1990 Aug 25;265(24):14065–14068. [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Kabelitz D. Gamma/delta T lymphocytes and heat shock proteins. Curr Top Microbiol Immunol. 1991;167:191–207. doi: 10.1007/978-3-642-75875-1_11. [DOI] [PubMed] [Google Scholar]
- Laal S., Sharma Y. D., Prasad H. K., Murtaza A., Singh S., Tangri S., Misra R. S., Nath I. Recombinant fusion protein identified by lepromatous sera mimics native Mycobacterium leprae in T-cell responses across the leprosy spectrum. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1054–1058. doi: 10.1073/pnas.88.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloyd G. M., Draper P. Separation of Mycobacterium leprae from contamination with armadillo-liver-derived "pigment" particles. Int J Lepr Other Mycobact Dis. 1986 Dec;54(4):578–583. [PubMed] [Google Scholar]
- McNeil M. R., Brennan P. J. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res Microbiol. 1991 May;142(4):451–463. doi: 10.1016/0923-2508(91)90120-y. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Bajardi A. C., Grisso C. L., Sieling P. A., Alland D., Convit J., Fan X. D., Hunter S. W., Brennan P. J. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J Exp Med. 1992 Jan 1;175(1):275–284. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nerland A. H., Mustafa A. S., Sweetser D., Godal T., Young R. A. A protein antigen of Mycobacterium leprae is related to a family of small heat shock proteins. J Bacteriol. 1988 Dec;170(12):5919–5921. doi: 10.1128/jb.170.12.5919-5921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen S. K., Lopez Bravo L., Sundaresan T. K. Estimated number of leprosy cases in the world. Bull World Health Organ. 1992;70(1):7–10. [PMC free article] [PubMed] [Google Scholar]
- Patarroyo M. E., Parra C. A., Pinilla C., del Portillo P., Torres M. L., Clavijo P., Salazar L. M., Jimenez C. Immunogenic synthetic peptides against mycobacteria of potential immunodiagnostic and immunoprophylactic value. Lepr Rev. 1986 Dec;57 (Suppl 2):163–168. doi: 10.5935/0305-7518.19860068. [DOI] [PubMed] [Google Scholar]
- Pessolani M. C., Brennan P. J. Mycobacterium leprae produces extracellular homologs of the antigen 85 complex. Infect Immun. 1992 Nov;60(11):4452–4459. doi: 10.1128/iai.60.11.4452-4459.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessolani M. C., Rumjanek F. D., Marques M. A., de Melo F. S., Sarno E. N. Serological response of patients with leprosy to a 28- to 30-kilodalton protein doublet from early cultures of Mycobacterium bovis BCG. J Clin Microbiol. 1989 Oct;27(10):2184–2189. doi: 10.1128/jcm.27.10.2184-2189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke de Wit T. F., Bekelie S., Osland A., Miko T. L., Hermans P. W., van Soolingen D., Drijfhout J. W., Schöningh R., Janson A. A., Thole J. E. Mycobacteria contain two groEL genes: the second Mycobacterium leprae groEL gene is arranged in an operon with groES. Mol Microbiol. 1992 Jul;6(14):1995–2007. doi: 10.1111/j.1365-2958.1992.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Sela S., Thole J. E., Ottenhoff T. H., Clark-Curtiss J. E. Identification of Mycobacterium leprae antigens from a cosmid library: characterization of a 15-kilodalton antigen that is recognized by both the humoral and cellular immune systems in leprosy patients. Infect Immun. 1991 Nov;59(11):4117–4124. doi: 10.1128/iai.59.11.4117-4124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellman V. L., Pettijohn D. E. Introduction of proteins into living bacterial cells: distribution of labeled HU protein in Escherichia coli. J Bacteriol. 1991 May;173(10):3047–3059. doi: 10.1128/jb.173.10.3047-3059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Plikaytis B. B., Hyche A. D., Van Landingham R. M., Walker L. L. The Mycobacterium tuberculosis BCG-a protein has homology with the Escherichia coli GroES protein. Nucleic Acids Res. 1989 Feb 11;17(3):1254–1254. doi: 10.1093/nar/17.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Miller P., Ronk M. Microsequence analysis of peptides and proteins. VI. A continuous flow reactor for sample concentration and sequence analysis. Anal Biochem. 1987 Jun;163(2):517–529. doi: 10.1016/0003-2697(87)90257-0. [DOI] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Jenner P. J., Jeyakumar L. H., Colston M. J. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990 Jun;58(6):1937–1942. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Whipple D. L., Le Febvre R. B., Andrews R. E., Jr, Thiermann A. B. Isolation and analysis of restriction endonuclease digestive patterns of chromosomal DNA from Mycobacterium paratuberculosis and other Mycobacterium species. J Clin Microbiol. 1987 Aug;25(8):1511–1515. doi: 10.1128/jcm.25.8.1511-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Terasaka K., Yamada T. Immunogenic protein MPB57 from Mycobacterium bovis BCG: molecular cloning, nucleotide sequence and expression. FEBS Lett. 1988 Nov 21;240(1-2):115–117. doi: 10.1016/0014-5793(88)80350-8. [DOI] [PubMed] [Google Scholar]
- Young D. B., Fohn M. J., Khanolkar S. R., Buchanan T. M. Monoclonal antibodies to a 28,000 mol. wt protein antigen of Mycobacterium leprae. Clin Exp Immunol. 1985 Jun;60(3):546–552. [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]
- de Wit M. Y., Klatser P. R. Purification and characterization of a 36 kDa antigen of Mycobacterium leprae. J Gen Microbiol. 1988 Jun;134(6):1541–1548. doi: 10.1099/00221287-134-6-1541. [DOI] [PubMed] [Google Scholar]