Abstract
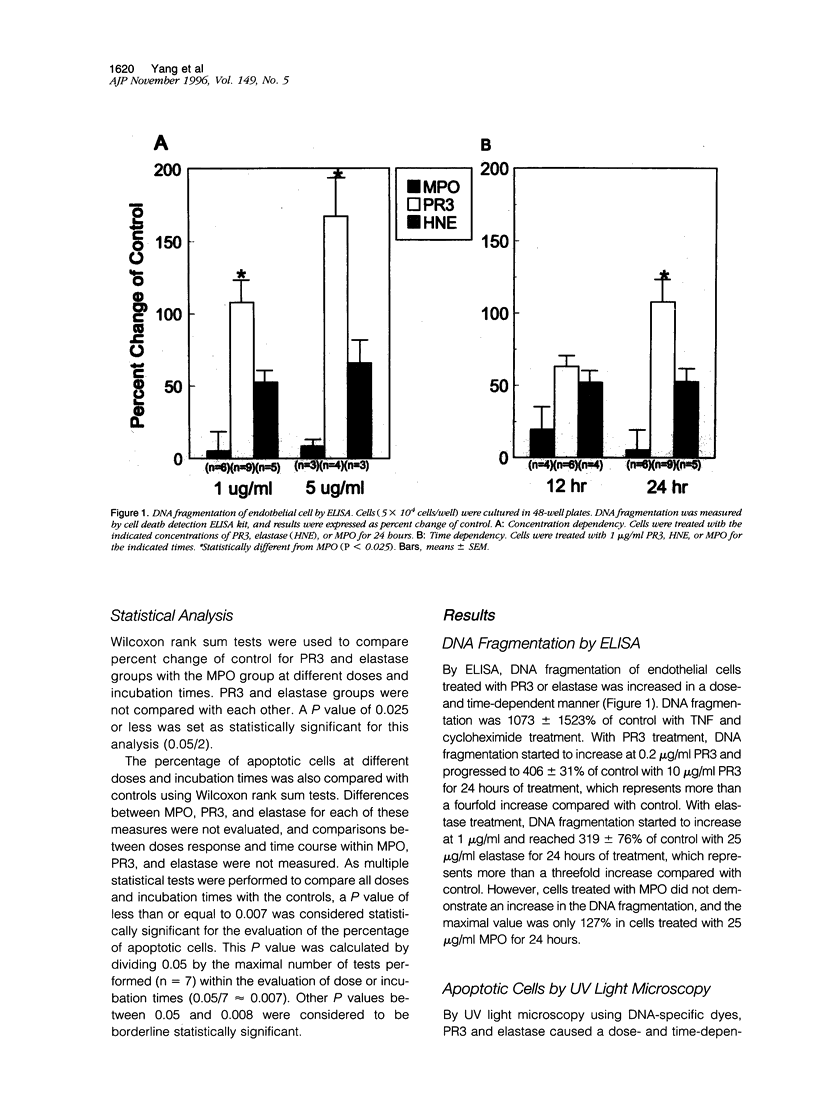
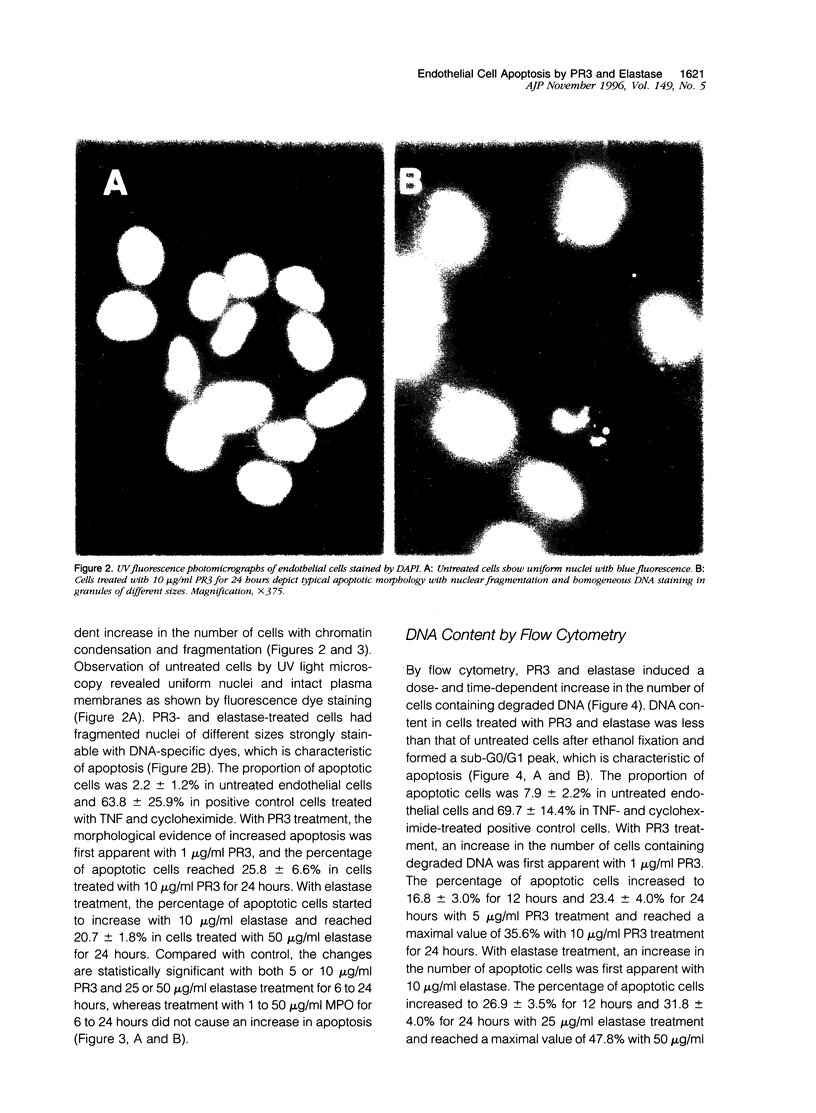
The pathogenesis of vasculitis associated with anti-neutrophil cytoplasmic antibodies is not established. The anti-neutrophil cytoplasmic antibody autoanigens proteinase 3 (PR3) and elastase induce detachment and cytolysis of endothelial cells in vitro. We investigated whether PR3 and elastase trigger endothelial cell apoptosis. Primary bovine pulmonary artery endothelial cells were treated with either PR3, elastase, or myeloperoxidase (MPO) and apoptosis assessed by four different methods. By the cell death detection enzyme-linked immunosorbent assay, DNA fragmentation increased to 208 +/- 84% or 153 +/- 27% of control with 1 micrograms/ml PR3 or elastase at 24 hours. By ultraviolet light microscopy, the percentage of apoptotic cells significantly increased (P < 0.05) with 5 or 10 micrograms/ml PR3 and 25 or 50 micrograms/ml elastase at 6, 12, or 24 hours. Values at the 24-hour time point are 15.3 +/- 6.4% or 25.8 +/- 6.6% for 5 or 10 micrograms/ml PR3 and 13.9 +/- 3.6% or 20.7 +/- 1.8% for 25 or 50 micrograms/ml elastase compared with 2.2 +/- 1.2% for control. Similarly, with flow cytometry, 5 or 10 micrograms/ml PR3 and 25 or 50 micrograms/ml elastase for 6, 12, or 24 hours demonstrated increasing apoptosis in a dose- and time-dependent manner with the highest values achieved at 24 hours (23.4 +/- 4.0% and 35.6% for 5 and 10 micrograms/ml PR3 and 31.8 +/- 4.0% and 47.8% for 25 and 50 micrograms/ml elastase compared with 7.9 +/- 2.2% in control). Typical DNA laddering was apparent from 6 to 24 hours at 5 or 10 micrograms/ml PR3 and 25 or 50 micrograms/ml elastase. Myeloperoxidase did not induce cell apoptosis. Release of PR3 and elastase by activated neutrophils during acute inflammation, including anti-neutrophil cytoplasmic antibody-associated vasculitis, may result in vascular damage by endothelial cell apoptosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C. Proteases and protease receptors in modulation of leukocyte effector functions. J Leukoc Biol. 1995 Aug;58(2):120–127. doi: 10.1002/jlb.58.2.120. [DOI] [PubMed] [Google Scholar]
- Ballieux B. E., Hiemstra P. S., Klar-Mohamad N., Hagen E. C., van Es L. A., van der Woude F. J., Daha M. R. Detachment and cytolysis of human endothelial cells by proteinase 3. Eur J Immunol. 1994 Dec;24(12):3211–3215. doi: 10.1002/eji.1830241245. [DOI] [PubMed] [Google Scholar]
- Berger S. P., Seelen M. A., Hiemstra P. S., Gerritsma J. S., Heemskerk E., van der Woude F. J., Daha M. R. Proteinase 3, the major autoantigen of Wegener's granulomatosis, enhances IL-8 production by endothelial cells in vitro. J Am Soc Nephrol. 1996 May;7(5):694–701. doi: 10.1681/ASN.V75694. [DOI] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Brouwer E., Huitema M. G., Mulder A. H., Heeringa P., van Goor H., Tervaert J. W., Weening J. J., Kallenberg C. G. Neutrophil activation in vitro and in vivo in Wegener's granulomatosis. Kidney Int. 1994 Apr;45(4):1120–1131. doi: 10.1038/ki.1994.149. [DOI] [PubMed] [Google Scholar]
- Campanelli D., Melchior M., Fu Y., Nakata M., Shuman H., Nathan C., Gabay J. E. Cloning of cDNA for proteinase 3: a serine protease, antibiotic, and autoantigen from human neutrophils. J Exp Med. 1990 Dec 1;172(6):1709–1715. doi: 10.1084/jem.172.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Davies D. J., Moran J. E., Niall J. F., Ryan G. B. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? 1982 Aug 28-Sep 4Br Med J (Clin Res Ed) 285(6342):606–606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bino G., Lassota P., Darzynkiewicz Z. The S-phase cytotoxicity of camptothecin. Exp Cell Res. 1991 Mar;193(1):27–35. doi: 10.1016/0014-4827(91)90534-2. [DOI] [PubMed] [Google Scholar]
- Ewert B. H., Jennette J. C., Falk R. J. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992 Feb;41(2):375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988 Jun 23;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Goldschmeding R., van der Schoot C. E., ten Bokkel Huinink D., Hack C. E., van den Ende M. E., Kallenberg C. G., von dem Borne A. E. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest. 1989 Nov;84(5):1577–1587. doi: 10.1172/JCI114335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck L. W., Blackburn W. D., Irwin M. H., Abrahamson D. R. Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. Am J Pathol. 1990 Jun;136(6):1267–1274. [PMC free article] [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Jennette J. C., Wilkman A. S., Falk R. J. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989 Nov;135(5):921–930. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Alpers C. E., Vissers M., Schulze M., Klebanoff S. J. The human neutrophil serine proteinases, elastase and cathepsin G, can mediate glomerular injury in vivo. J Exp Med. 1988 Sep 1;168(3):1169–1174. doi: 10.1084/jem.168.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg C. G., Brouwer E., Mulder A. H., Stegeman C. A., Weening J. J., Tervaert J. W. ANCA--pathophysiology revisited. Clin Exp Immunol. 1995 Apr;100(1):1–3. doi: 10.1111/j.1365-2249.1995.tb03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg C. G., Brouwer E., Weening J. J., Tervaert J. W. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994 Jul;46(1):1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- Kao R. C., Wehner N. G., Skubitz K. M., Gray B. H., Hoidal J. R. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988 Dec;82(6):1963–1973. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key N. S., Platt J. L., Vercellotti G. M. Vascular endothelial cell proteoglycans are susceptible to cleavage by neutrophils. Arterioscler Thromb. 1992 Jul;12(7):836–842. doi: 10.1161/01.atv.12.7.836. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Kinsella M. G., Wight T. N. Degradation of endothelial cell matrix heparan sulfate proteoglycan by elastase and the myeloperoxidase-H2O2-chloride system. Am J Pathol. 1993 Sep;143(3):907–917. [PMC free article] [PubMed] [Google Scholar]
- Lüdemann J., Utecht B., Gross W. L. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990 Jan 1;171(1):357–362. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthinuss J., Andrade-Gordon P., Seiberg M. A secreted serine protease can induce apoptosis in Pam212 keratinocytes. Cell Growth Differ. 1995 Jul;6(7):807–816. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowka C., Csernok E., Gross W. L., Feucht H. E., Bechtel U., Thoenes G. H. Distribution of the granulocyte serine proteinases proteinase 3 and elastase in human glomerulonephritis. Am J Kidney Dis. 1995 Feb;25(2):253–261. doi: 10.1016/0272-6386(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Henkart P. A. Cytotoxic lymphocyte granzymes trigger a target cell internal disintegration pathway leading to cytolysis and DNA breakdown. J Immunol. 1994 Feb 1;152(3):1057–1063. [PubMed] [Google Scholar]
- Okrent D. G., Lichtenstein A. K., Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis. 1990 Jan;141(1):179–185. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- Polunovsky V. A., Wendt C. H., Ingbar D. H., Peterson M. S., Bitterman P. B. Induction of endothelial cell apoptosis by TNF alpha: modulation by inhibitors of protein synthesis. Exp Cell Res. 1994 Oct;214(2):584–594. doi: 10.1006/excr.1994.1296. [DOI] [PubMed] [Google Scholar]
- Rao N. V., Wehner N. G., Marshall B. C., Gray W. R., Gray B. H., Hoidal J. R. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem. 1991 May 25;266(15):9540–9548. [PubMed] [Google Scholar]
- Re F., Zanetti A., Sironi M., Polentarutti N., Lanfrancone L., Dejana E., Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994 Oct;127(2):537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. O., Pottinger B. E., Gaskin G., Pusey C. D., Pearson J. D. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol. 1992 Aug;141(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Shi L., Kam C. M., Powers J. C., Aebersold R., Greenberg A. H. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992 Dec 1;176(6):1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M. J., Trapani J. A. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995 Apr;16(4):202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- Ueda N., Shah S. V. Endonuclease-induced DNA damage and cell death in oxidant injury to renal tubular epithelial cells. J Clin Invest. 1992 Dec;90(6):2593–2597. doi: 10.1172/JCI116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Ginsburg I., Schuger L., Gibbs D. F., Bromberg J., Johnson K. J., Ryan U. S., Ward P. A. Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol. 1989 Sep;135(3):435–438. [PMC free article] [PubMed] [Google Scholar]
- Westlin W. F., Gimbrone M. A., Jr Neutrophil-mediated damage to human vascular endothelium. Role of cytokine activation. Am J Pathol. 1993 Jan;142(1):117–128. [PMC free article] [PubMed] [Google Scholar]
- Williams M. S., Henkart P. A. Apoptotic cell death induced by intracellular proteolysis. J Immunol. 1994 Nov 1;153(9):4247–4255. [PubMed] [Google Scholar]
- Yang J. J., Jennette J. C., Falk R. J. Immune complex glomerulonephritis is induced in rats immunized with heterologous myeloperoxidase. Clin Exp Immunol. 1994 Sep;97(3):466–473. doi: 10.1111/j.1365-2249.1994.tb06111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. H., Jones S. J., Lockwood C. M. Bactericidal/permeability-increasing protein (BPI) is an important antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in vasculitis. Clin Exp Immunol. 1995 Jan;99(1):49–56. doi: 10.1111/j.1365-2249.1995.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude F. J., Rasmussen N., Lobatto S., Wiik A., Permin H., van Es L. A., van der Giessen M., van der Hem G. K., The T. H. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985 Feb 23;1(8426):425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]




