Abstract
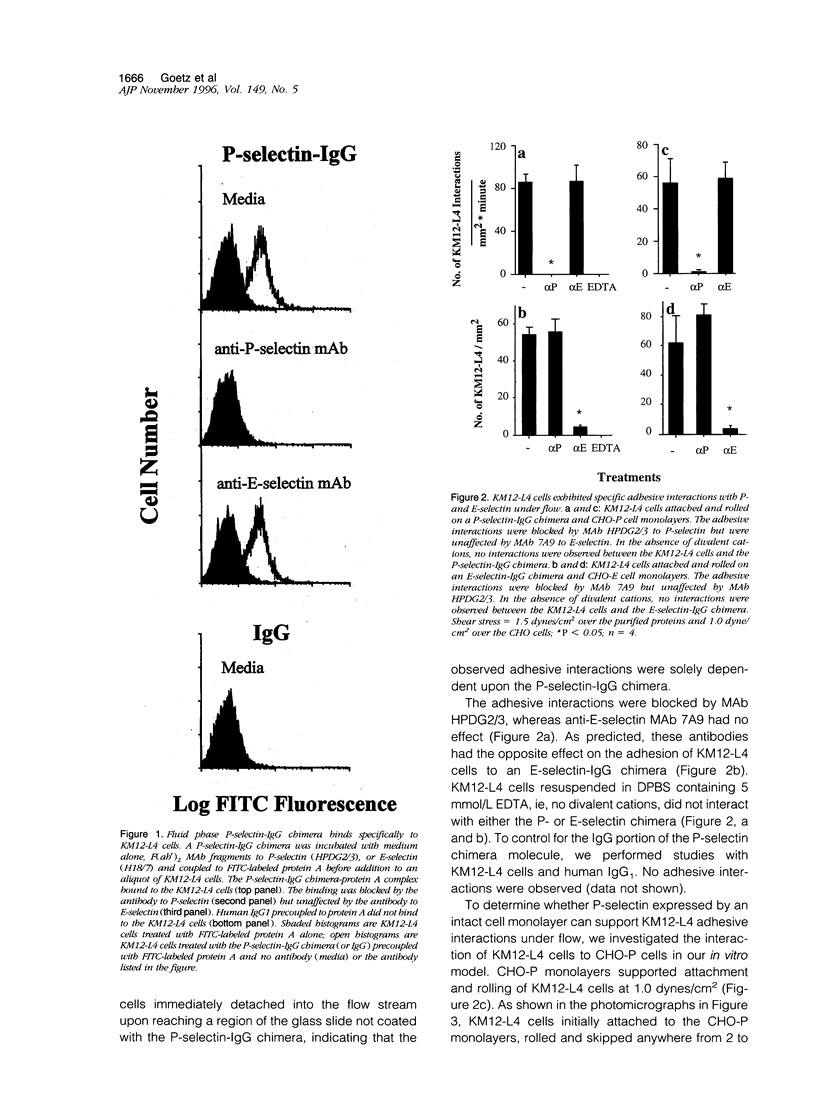
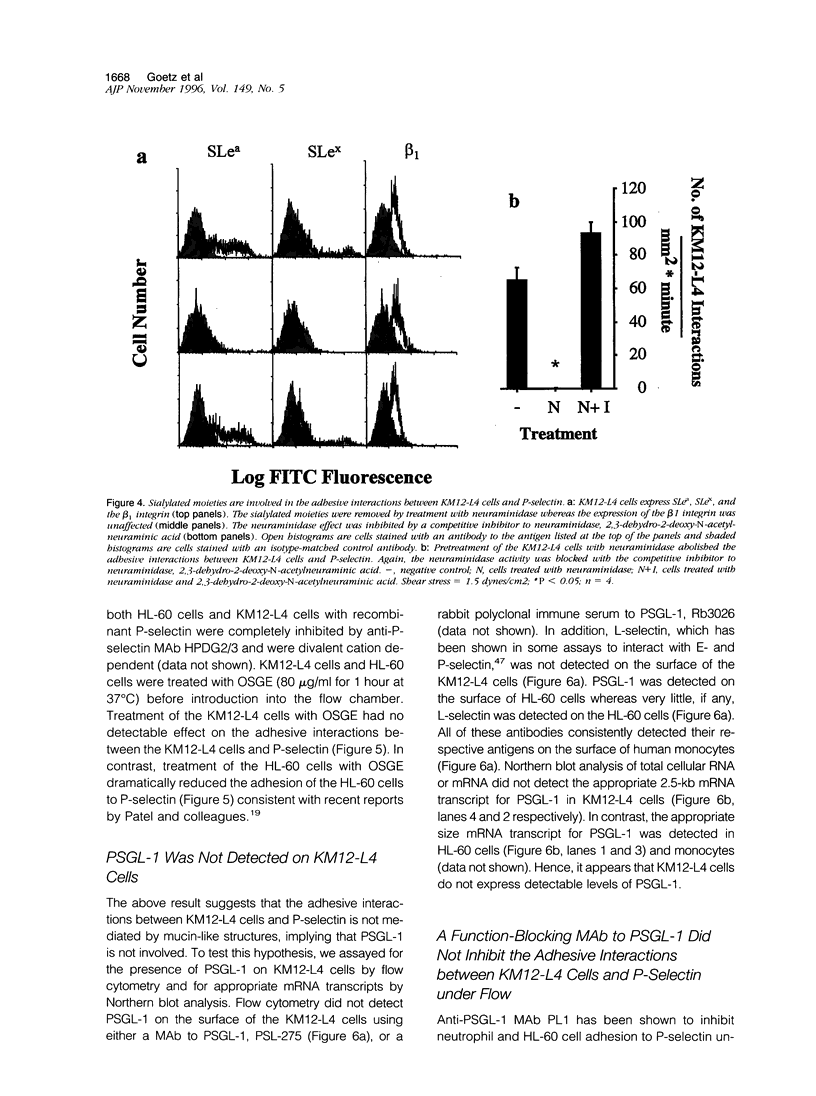
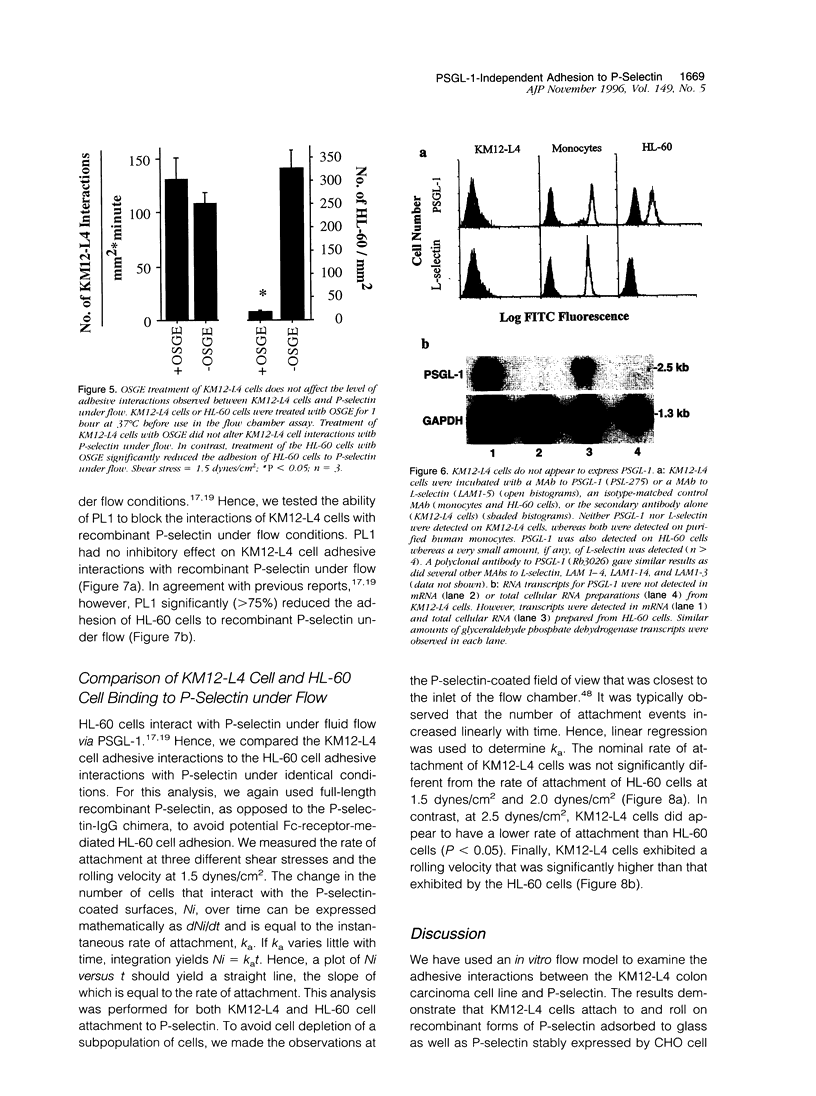
It has been postulated that endothelial cell adhesion molecules involved in leukocyte recruitment play a role in metastasis. Using an in vitro flow model, we studied the adhesion of the human colon carcinoma cell line KM12-L4 to P-selectin, an inducible endothelial-expressed adhesion molecule involved in leukocyte recruitment. Recombinant forms of P-selectin and Chinese hamster ovary cells stably expressing P-selectin supported attachment and rolling of KM12-L4 cells at 1 to 2 dynes/cm2. The adhesive interactions to P-selectin were abolished by pretreatment of the KM12-L4 cells with neuraminidase but were unaltered by pretreatment of the KM12-L4 cells with O-sialoglycoprotein endopeptidase, an enzyme that cleaves mucin type glycoproteins such as P-selectin glycoprotein ligand-1 (PSGL-1). PSGL-1 is the only counter-receptor for P-selectin known to mediate myeloid cell adhesion to P-selectin under flow. Flow cytometric and Northern blot analyses revealed that KM12-L4 cells did not express PSGL-1 and monoclonal antibody PL1, a function-blocking monoclonal antibody to PSGL-1, had no inhibitory effect on KM12-L4 adhesion to P-selectin under flow. Compared with HL-60 cells, which express PSGL-1, the KM12-L4 cells exhibited a slightly lower rate of attachment to P-selectin and rolled at a significantly higher velocity. In summary, KM12-L4 human colon carcinoma cells interact with P-selectin, under flow, through a PSGL-1-independent adhesion pathway.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbassi O., Kishimoto T. K., McIntire L. V., Anderson D. C., Smith C. W. E-selectin supports neutrophil rolling in vitro under conditions of flow. J Clin Invest. 1993 Dec;92(6):2719–2730. doi: 10.1172/JCI116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R., Hammer D. A., Springer T. A. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995 Apr 6;374(6522):539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Dietsch M. T., Wan H., Hellström K. E., Hellström I. Granule membrane protein 140 (GMP140) binds to carcinomas and carcinoma-derived cell lines. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2292–2296. doi: 10.1073/pnas.89.6.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Kolanus W., Walz G., Fredman P., Seed B. CD62/P-selectin recognition of myeloid and tumor cell sulfatides. Cell. 1991 Oct 4;67(1):35–44. doi: 10.1016/0092-8674(91)90570-o. [DOI] [PubMed] [Google Scholar]
- Baumheter S., Singer M. S., Henzel W., Hemmerich S., Renz M., Rosen S. D., Lasky L. A. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993 Oct 15;262(5132):436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Giavazzi R., Foppolo M., Dossi R., Remuzzi A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest. 1993 Dec;92(6):3038–3044. doi: 10.1172/JCI116928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B. Ras C-terminal processing enzymes--new drug targets? Cell. 1991 Apr 5;65(1):1–4. doi: 10.1016/0092-8674(91)90352-y. [DOI] [PubMed] [Google Scholar]
- Goetz D. J., Brandley B. K., Hammer D. A. An E-selectin-IgG chimera supports sialylated moiety dependent adhesion of colon carcinoma cells under fluid flow. Ann Biomed Eng. 1996 Jan-Feb;24(1):87–98. doi: 10.1007/BF02770998. [DOI] [PubMed] [Google Scholar]
- Hammer D. A., Apte S. M. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J. 1992 Jul;63(1):35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai N., Shitara K., Furuya A., Yoshida H., Dohi T., Nudelman E., Hakomori S., Satoh S. Detailed characterization of reactivities of anti-gastric cancer monoclonal antibodies to carbohydrate antigen. Anticancer Res. 1990 Nov-Dec;10(6):1579–1586. [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., McIntire L. V. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990 Jan 1;75(1):227–237. [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Neutrophils roll on E-selectin. J Immunol. 1993 Dec 1;151(11):6338–6346. [PubMed] [Google Scholar]
- Lipkind G. M., Fozzard H. A. A structural model of the tetrodotoxin and saxitoxin binding site of the Na+ channel. Biophys J. 1994 Jan;66(1):1–13. doi: 10.1016/S0006-3495(94)80746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Ding H., Lichtman A. H. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor alpha-activated vascular endothelium under flow. J Exp Med. 1995 Mar 1;181(3):1179–1186. doi: 10.1084/jem.181.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Kansas G. S., Ding H., Pizcueta P., Schleiffenbaum B. E., Tedder T. F., Gimbrone M. A., Jr Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol. 1994 Jun;125(6):1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Lawler J. Integrins as dynamic regulators of vascular function. FASEB J. 1994 Sep;8(12):929–938. doi: 10.1096/fasebj.8.12.7522194. [DOI] [PubMed] [Google Scholar]
- Majuri M. L., Mattila P., Renkonen R. Recombinant E-selectin-protein mediates tumor cell adhesion via sialyl-Le(a) and sialyl-Le(x). Biochem Biophys Res Commun. 1992 Feb 14;182(3):1376–1382. doi: 10.1016/0006-291x(92)91885-t. [DOI] [PubMed] [Google Scholar]
- Mannori G., Crottet P., Cecconi O., Hanasaki K., Aruffo A., Nelson R. M., Varki A., Bevilacqua M. P. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995 Oct 1;55(19):4425–4431. [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Eaton S. F., Lyons D. E., Lichenstein H. S., Cummings R. D., McEver R. P. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem. 1994 Sep 16;269(37):23318–23327. [PubMed] [Google Scholar]
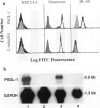
- Moore K. L., Patel K. D., Bruehl R. E., Li F., Johnson D. A., Lichenstein H. S., Cummings R. D., Bainton D. F., McEver R. P. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995 Feb;128(4):661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992 Jul;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Varki A., McEver R. P. GMP-140 binds to a glycoprotein receptor on human neutrophils: evidence for a lectin-like interaction. J Cell Biol. 1991 Feb;112(3):491–499. doi: 10.1083/jcb.112.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K., Walker S. M., Jessup J. M., Fidler I. J. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988 Apr 1;48(7):1943–1948. [PubMed] [Google Scholar]
- Norgard K. E., Moore K. L., Diaz S., Stults N. L., Ushiyama S., McEver R. P., Cummings R. D., Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993 Jun 15;268(17):12764–12774. [PubMed] [Google Scholar]
- Patel K. D., Moore K. L., Nollert M. U., McEver R. P. Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest. 1995 Oct;96(4):1887–1896. doi: 10.1172/JCI118234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Phillips M. L., Wayner E., Nudelman E., Singhal A. K., Hakomori S., Paulson J. C. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyani T., Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995 Oct 20;83(2):333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- Read M. A., Neish A. S., Luscinskas F. W., Palombella V. J., Maniatis T., Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995 May;2(5):493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Sako D., Comess K. M., Barone K. M., Camphausen R. T., Cumming D. A., Shaw G. D. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995 Oct 20;83(2):323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Sammar M., Aigner S., Hubbe M., Schirrmacher V., Schachner M., Vestweber D., Altevogt P. Heat-stable antigen (CD24) as ligand for mouse P-selectin. Int Immunol. 1994 Jul;6(7):1027–1036. doi: 10.1093/intimm/6.7.1027. [DOI] [PubMed] [Google Scholar]
- Sawada R., Lowe J. B., Fukuda M. E-selectin-dependent adhesion efficiency of colonic carcinoma cells is increased by genetic manipulation of their cell surface lysosomal membrane glycoprotein-1 expression levels. J Biol Chem. 1993 Jun 15;268(17):12675–12681. [PubMed] [Google Scholar]
- Spertini O., Kansas G. S., Reimann K. A., Mackay C. R., Tedder T. F. Function and evolutionary conservation of distinct epitopes on the leukocyte adhesion molecule-1 (TQ-1, Leu-8) that regulate leukocyte migration. J Immunol. 1991 Aug 1;147(3):942–949. [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Kansas G. S., Munro J. M., Griffin J. D., Gimbrone M. A., Jr, Tedder T. F. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991 Oct 15;147(8):2565–2573. [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stone J. P., Wagner D. D. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest. 1993 Aug;92(2):804–813. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. R., Abdullah K. M., Cyopick P., Mellors A. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. J Immunol. 1992 Mar 1;148(5):1458–1464. [PubMed] [Google Scholar]
- Südhof T. C., De Camilli P., Niemann H., Jahn R. Membrane fusion machinery: insights from synaptic proteins. Cell. 1993 Oct 8;75(1):1–4. [PubMed] [Google Scholar]
- Tedder T. F., Steeber D. A., Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995 Jun 1;181(6):2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A., Kleinman H. K., Grant D. S., Morales D., Mercurio A. M., Byers S. W. E-selectin-mediated dynamic interactions of breast- and colon-cancer cells with endothelial-cell monolayers. Int J Cancer. 1995 Jan 27;60(3):426–431. doi: 10.1002/ijc.2910600326. [DOI] [PubMed] [Google Scholar]
- Vachino G., Chang X. J., Veldman G. M., Kumar R., Sako D., Fouser L. A., Berndt M. C., Cumming D. A. P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. J Biol Chem. 1995 Sep 15;270(37):21966–21974. doi: 10.1074/jbc.270.37.21966. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Moore K. L., Smith D. F., Varki A., McEver R. P., Cummings R. D. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J Cell Biol. 1991 Oct;115(2):557–564. doi: 10.1083/jcb.115.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Hasslen S. R., Nelson R. D., Erlandsen S. L., Butcher E. C. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995 Sep 22;82(6):989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]