Abstract
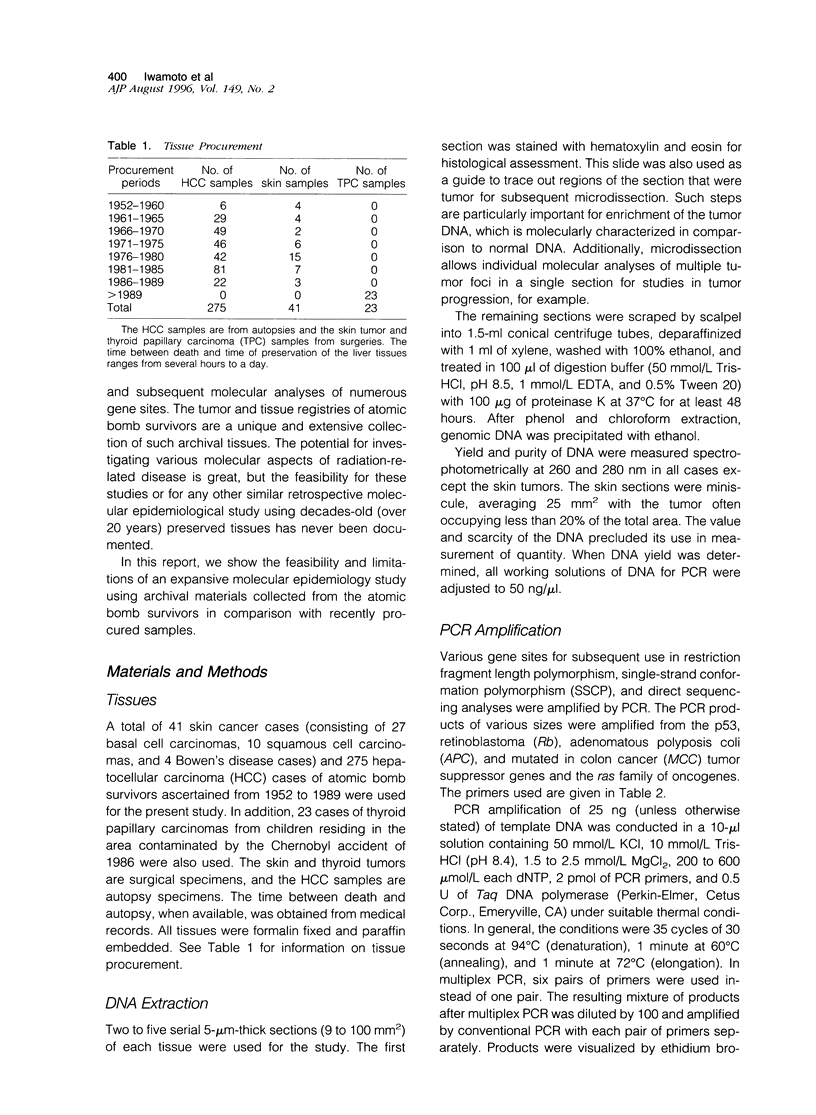
Archival tissues are a bountiful resource for various studies. Polymerase chain reaction permits the use of such tissues for molecular biological analyses of disease causation. However, a comprehensive study using a large number of decades-old samples (20 or more years) for molecular oncology/epidemiology has never been shown to be feasible. We have relied upon the unique tumor registry of atomic bomb survivors to show that such studies are possible using 275 hepatocellular carcinoma and 41 skin cancer cases. We used 23 relatively recent thyroid papillary carcinoma cases from persons living in the vicinity of the Chernobyl nuclear reactor accident for comparison. Degradation of DNA is severe in autopsy hepatocellular carcinoma samples but can be compensated for by decreasing the polymerase chain reaction product size. Increasing the amount of DNA that is used by a factor of 8 improved amplification efficiency from approximately 60 to 80%. Age of the samples was not as great a problem as was the source of procurement. The extracted DNA can be used for all types of assays that require polymerase chain reaction amplification, such as restriction fragment length polymorphism, single-strand conformation polymorphism, and direct sequencing.
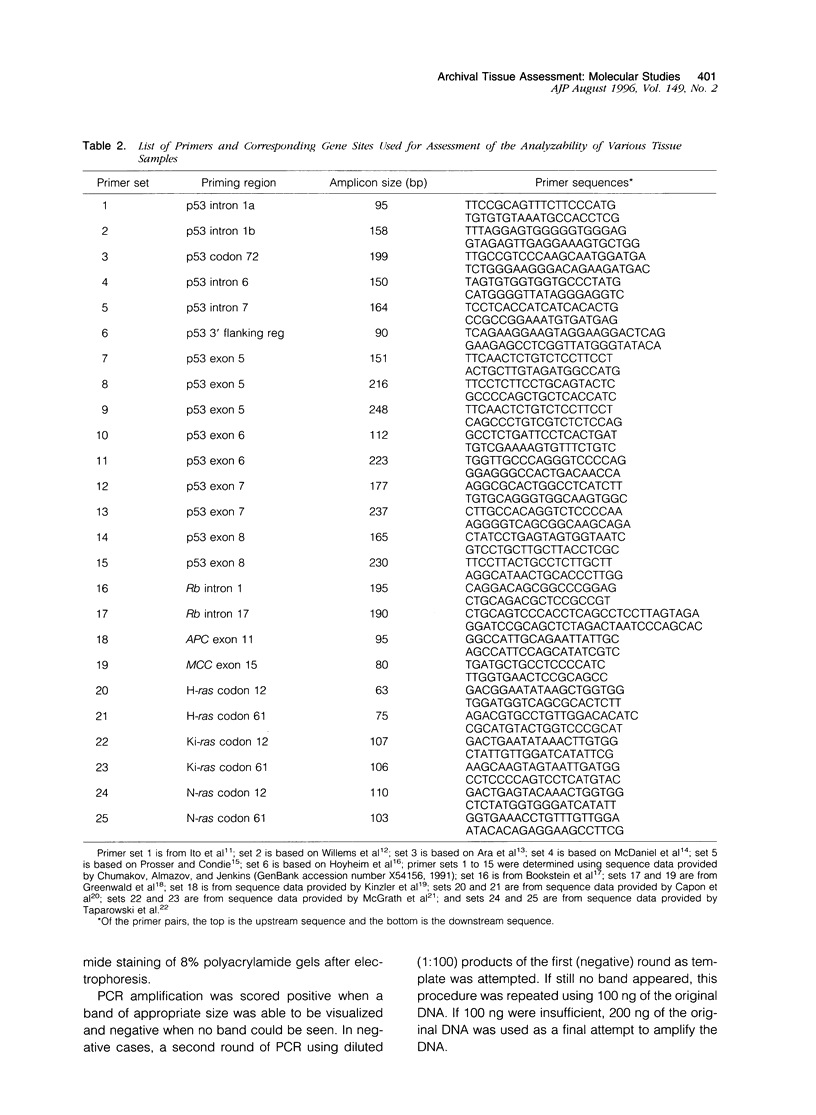
Full text
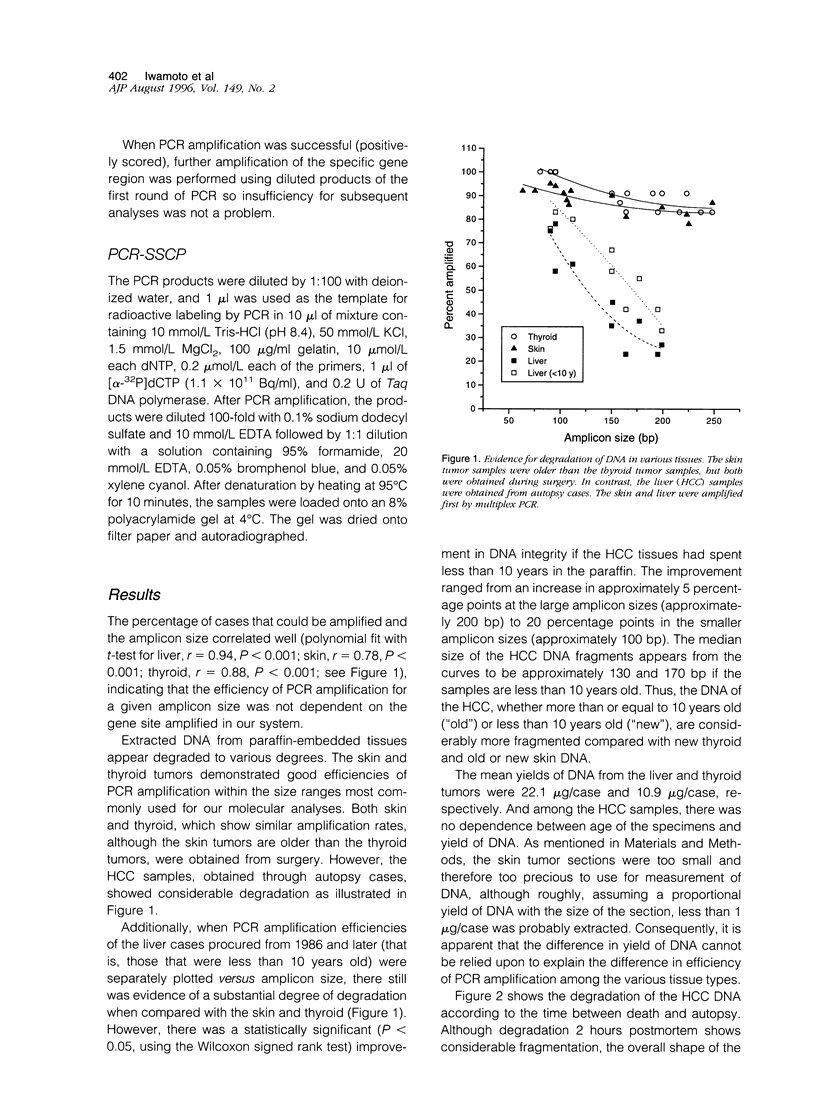
PDF
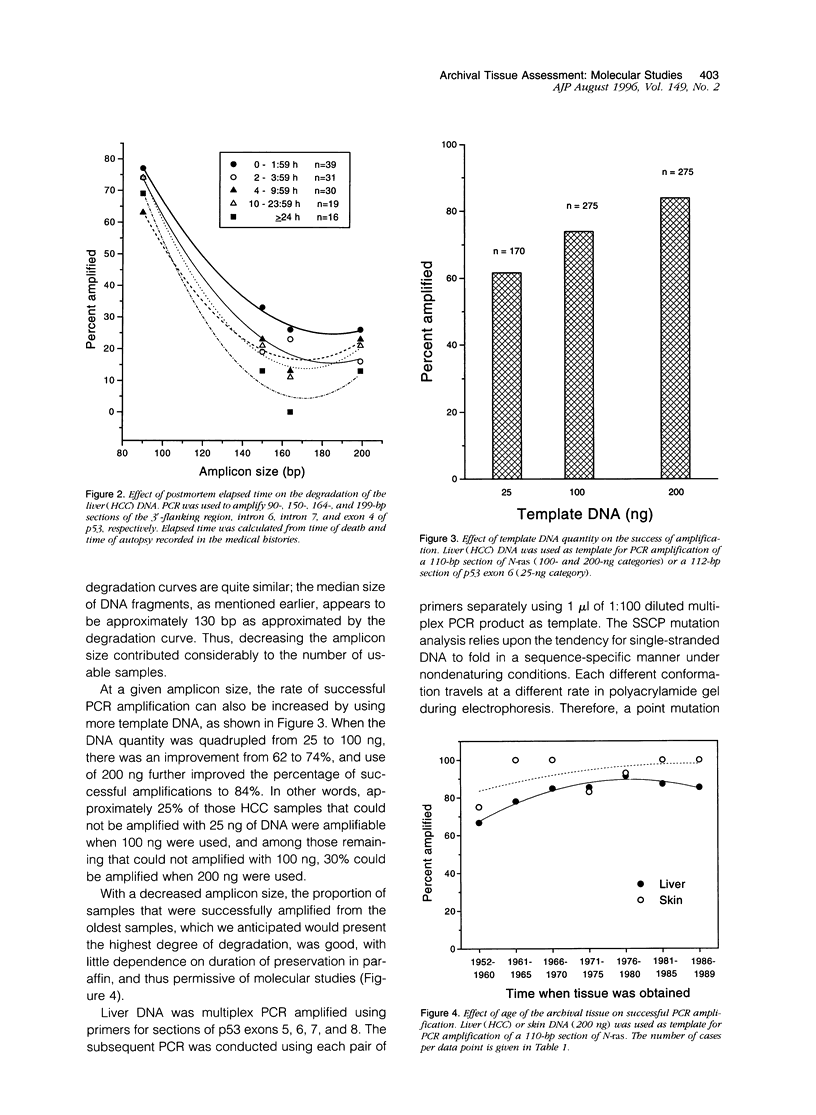



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ezra J., Johnson D. A., Rossi J., Cook N., Wu A. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem. 1991 Mar;39(3):351–354. doi: 10.1177/39.3.1704393. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Lai C. C., To H., Lee W. H. PCR-based detection of a polymorphic BamHI site in intron 1 of the human retinoblastoma (RB) gene. Nucleic Acids Res. 1990 Mar 25;18(6):1666–1666. doi: 10.1093/nar/18.6.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Greenwald B. D., Harpaz N., Yin J., Huang Y., Tong Y., Brown V. L., McDaniel T., Newkirk C., Resau J. H., Meltzer S. J. Loss of heterozygosity affecting the p53, Rb, and mcc/apc tumor suppressor gene loci in dysplastic and cancerous ulcerative colitis. Cancer Res. 1992 Feb 1;52(3):741–745. [PubMed] [Google Scholar]
- Hewett P. J., Firgaira F., Morley A. The influence of age of template DNA derived from archival tissue on the outcome of the polymerase chain reaction. Aust N Z J Surg. 1994 Aug;64(8):558–559. doi: 10.1111/j.1445-2197.1994.tb02286.x. [DOI] [PubMed] [Google Scholar]
- Høyheim B., Nakamura Y., White R. A BamHI-polymorphism is detected by a genomic p53-clone (pBHP53). Nucleic Acids Res. 1989 Nov 11;17(21):8898–8898. doi: 10.1093/nar/17.21.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impraim C. C., Saiki R. K., Erlich H. A., Teplitz R. L. Analysis of DNA extracted from formalin-fixed, paraffin-embedded tissues by enzymatic amplification and hybridization with sequence-specific oligonucleotides. Biochem Biophys Res Commun. 1987 Feb 13;142(3):710–716. doi: 10.1016/0006-291x(87)91472-0. [DOI] [PubMed] [Google Scholar]
- Ito T., Seyama T., Hayashi T., Mizuno T., Iwamoto K. S., Tsuyama N., Dohi K., Nakamura N., Akiyama M. HaeIII polymorphism in intron 1 of the human p53 gene. Hum Genet. 1994 Feb;93(2):222–222. doi: 10.1007/BF00210619. [DOI] [PubMed] [Google Scholar]
- Ito T., Seyama T., Mizuno T., Tsuyama N., Hayashi T., Hayashi Y., Dohi K., Nakamura N., Akiyama M. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 1992 Mar 1;52(5):1369–1371. [PubMed] [Google Scholar]
- Larsen S., Rygaard K., Asnaes S., Spang-Thomsen M. Northern and Southern blot analysis of human RNA and DNA in autopsy material. APMIS. 1992 Jun;100(6):498–502. doi: 10.1111/j.1699-0463.1992.tb00902.x. [DOI] [PubMed] [Google Scholar]
- Ludes B., Pfitzinger H., Mangin P. DNA fingerprinting from tissues after variable postmortem periods. J Forensic Sci. 1993 May;38(3):686–690. [PubMed] [Google Scholar]
- McDaniel T., Carbone D., Takahashi T., Chumakov P., Chang E. H., Pirollo K. F., Yin J., Huang Y., Meltzer S. J. The MspI polymorphism in intron 6 of p53 (TP53) detected by digestion of PCR products. Nucleic Acids Res. 1991 Sep 11;19(17):4796–4796. doi: 10.1093/nar/19.17.4796-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Smith D. H., Chen E. Y., Seeburg P. H., Goeddel D. V., Levinson A. D. Structure and organization of the human Ki-ras proto-oncogene and a related processed pseudogene. Nature. 1983 Aug 11;304(5926):501–506. doi: 10.1038/304501a0. [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Prosser J., Condie A. Biallelic ApaI polymorphism of the human p53 gene (TP53). Nucleic Acids Res. 1991 Sep 11;19(17):4799–4799. doi: 10.1093/nar/19.17.4799-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Arnheim N. Analysis of DNA sequences in forty-year-old paraffin-embedded thin-tissue sections: a bridge between molecular biology and classical histology. Cancer Res. 1988 Aug 15;48(16):4564–4566. [PubMed] [Google Scholar]
- Taparowsky E., Shimizu K., Goldfarb M., Wigler M. Structure and activation of the human N-ras gene. Cell. 1983 Sep;34(2):581–586. doi: 10.1016/0092-8674(83)90390-2. [DOI] [PubMed] [Google Scholar]
- Thompson D. E., Mabuchi K., Ron E., Soda M., Tokunaga M., Ochikubo S., Sugimoto S., Ikeda T., Terasaki M., Izumi S. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958-1987. Radiat Res. 1994 Feb;137(2 Suppl):S17–S67. [PubMed] [Google Scholar]
- Willems P. M., Meyerink J. P., van de Locht L. T., Smetsers T. F., de Vries N., Mensink E. J. PCR detection of a BglII polymorphism in intron I of the human p53 gene (TP53). Nucleic Acids Res. 1992 Mar 11;20(5):1172–1172. doi: 10.1093/nar/20.5.1172-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z., Bertheau P., Emmert-Buck M. R., Liotta L. A., Gnarra J., Linehan W. M., Lubensky I. A. A microdissection technique for archival DNA analysis of specific cell populations in lesions < 1 mm in size. Am J Pathol. 1995 Mar;146(3):620–625. [PMC free article] [PubMed] [Google Scholar]



