Abstract
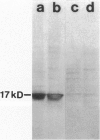
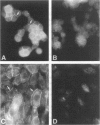
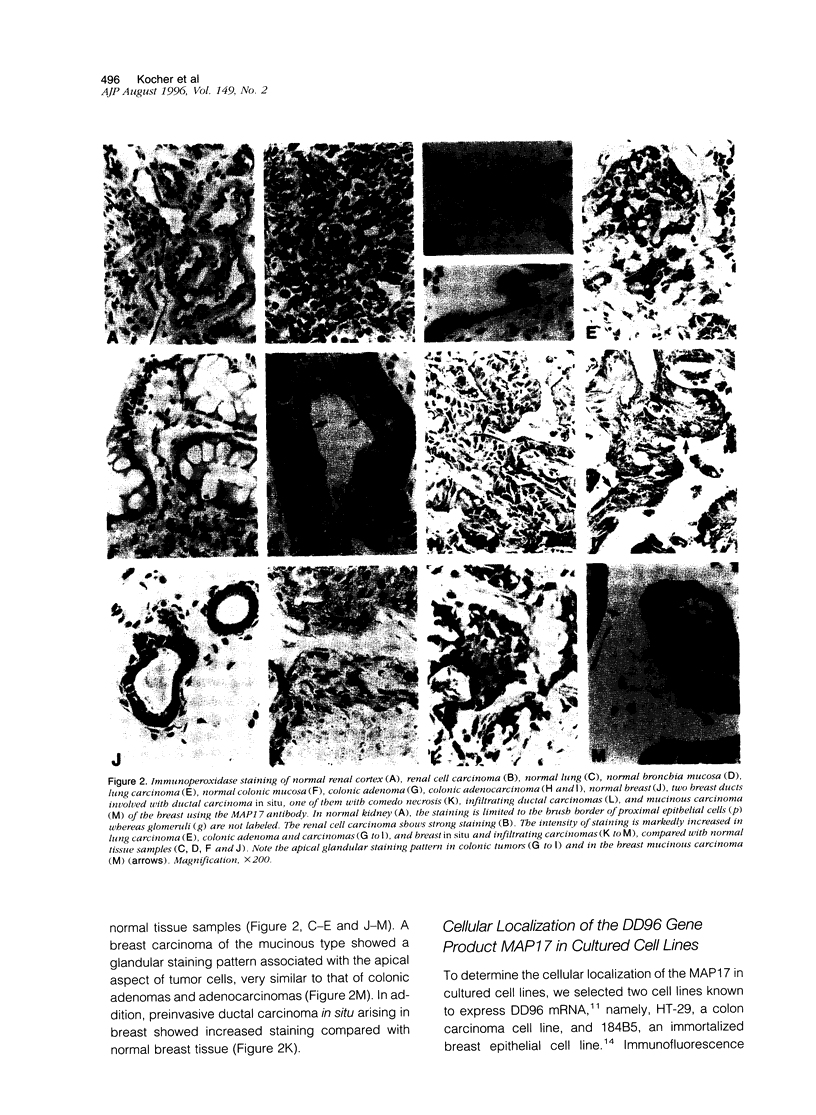
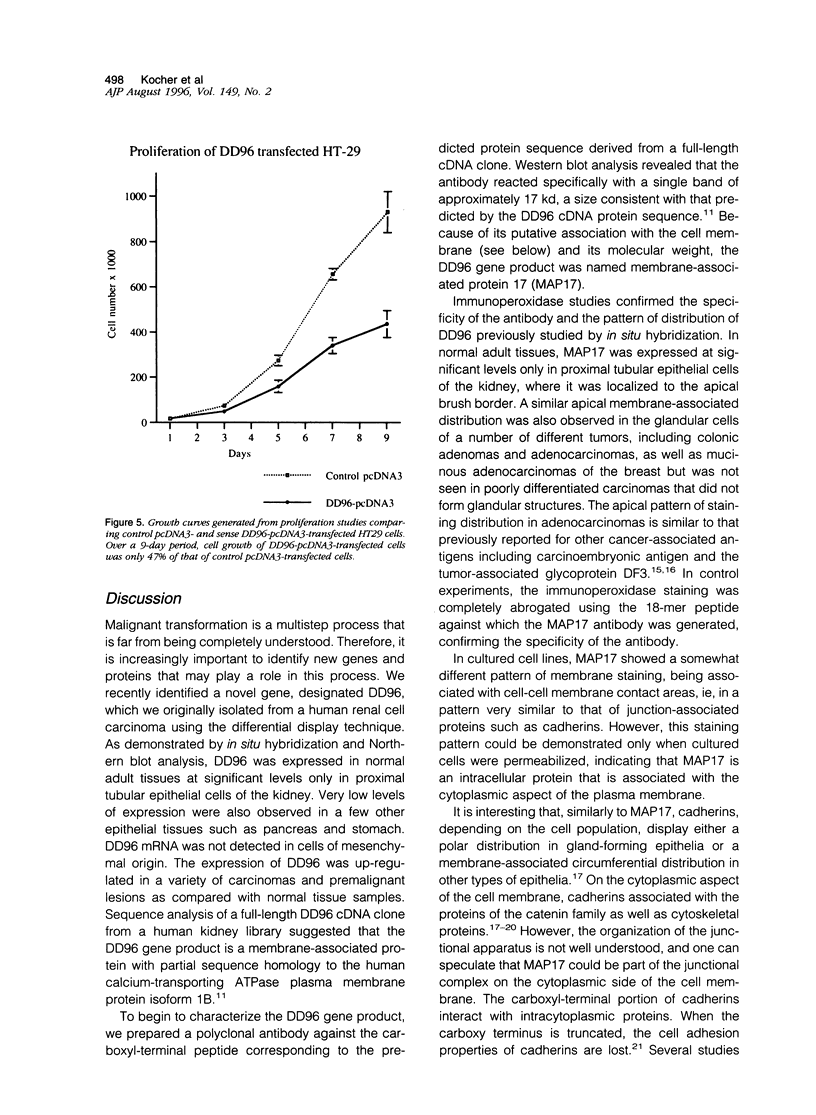
Using the differential display technique, we have recently reported the identification of a novel gene originally designated DD96. As determined by Northern blot and in situ hybridization, DD96 was expressed at significant levels only in a single epithelial cell population, the proximal tubular epithelial cells of the kidney. However, it was diffusely expressed in various carcinomas originating from kidney, colon, lung, and breast. Using a specific polyclonal antibody, we have not determined that the DD96 protein product is a 17-kd membrane-associated protein, which we have therefore redesignated MAP17. In normal tissues, MAP17 is expressed in significant amounts only in the kidney, where it was localized to the brush border of proximal tubular epithelial cells. However, MAP17 is expressed abundantly in carcinomas arising from kidney, colon, lung, and breast, in some cases with a membrane-associated apical glandular distribution. In tissue culture, MAP17 was localized to the cell membrane in areas of cell-cell contact, ie, the distribution of cell-function-associated proteins. Transfection of a full-length wild-type DD96 cDNA clone into a colon carcinoma cell line, HT-29, markedly decreased cell proliferation in vitro and tumor growth in vivo. Although the precise function of MAP17 remains to be determined, our findings suggest that this protein may play an important role in tumor biology.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. W., Jr, Jessup J. M., Goldman H., Hayes D. F., Kufe D. W., O'Hara C. J., Steele G. D., Jr Localization of tumor-associated glycoprotein DF3 in normal, inflammatory, and neoplastic lesions of the colon. Cancer. 1993 Dec 1;72(11):3185–3190. doi: 10.1002/1097-0142(19931201)72:11<3185::aid-cncr2820721109>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Chassin D., Bénifla J. L., Delattre C., Fernandez H., Ginisty D., Janneau J. L., Prade M., Contesso G., Caillou B., Tournaire M. Identification of genes overexpressed in tumors through preferential expression screening in trophoblasts. Cancer Res. 1994 Oct 1;54(19):5217–5223. [PubMed] [Google Scholar]
- Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995 Sep;147(3):545–560. [PMC free article] [PubMed] [Google Scholar]
- Cowin P. Unraveling the cytoplasmic interactions of the cadherin superfamily. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10759–10761. doi: 10.1073/pnas.91.23.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Cheresh P., Brown L. F., Lee S. W. Identification of a novel gene, selectively up-regulated in human carcinomas, using the differential display technique. Clin Cancer Res. 1995 Oct;1(10):1209–1215. [PubMed] [Google Scholar]
- Kocher O., Kennedy S. P., Madri J. A. Alternative splicing of endothelial cell fibronectin mRNA in the IIICS region. Functional significance. Am J Pathol. 1990 Dec;137(6):1509–1524. [PMC free article] [PubMed] [Google Scholar]
- Kuhajda F. P., Offutt L. E., Mendelsohn G. The distribution of carcinoembryonic antigen in breast carcinoma. Diagnostic and prognostic implications. Cancer. 1983 Oct 1;52(7):1257–1264. doi: 10.1002/1097-0142(19831001)52:7<1257::aid-cncr2820520721>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Paul D., Keyomarsi K., Sager R. Transcriptional downregulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. J Cell Biol. 1992 Sep;118(5):1213–1221. doi: 10.1083/jcb.118.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Sager R. Positive selection of candidate tumor-suppressor genes by subtractive hybridization. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2825–2829. doi: 10.1073/pnas.88.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Keyomarsi K., Sager R., Pardee A. B. Differential display and cloning of messenger RNAs from human breast cancer versus mammary epithelial cells. Cancer Res. 1992 Dec 15;52(24):6966–6968. [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Ishihara S., Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J Cell Biol. 1994 Oct;127(1):235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page D. L., Simpson J. F. Are catenins and cadherins relevant to tumor biology? Good fences make good neighbors. Lab Invest. 1995 May;72(5):491–493. [PubMed] [Google Scholar]
- Rimm D. L., Sinard J. H., Morrow J. S. Reduced alpha-catenin and E-cadherin expression in breast cancer. Lab Invest. 1995 May;72(5):506–512. [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Neveu M., Liang P., Sotiropoulou G. Identification by differential display of alpha 6 integrin as a candidate tumor suppressor gene. FASEB J. 1993 Jul;7(10):964–970. doi: 10.1096/fasebj.7.10.8344495. [DOI] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Watabe M., Nagafuchi A., Tsukita S., Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994 Oct;127(1):247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The integration of molecular genetics into cancer management. Cancer. 1992 Sep 15;70(6 Suppl):1653–1658. doi: 10.1002/1097-0142(19920915)70:4+<1653::aid-cncr2820701603>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zou Z., Anisowicz A., Hendrix M. J., Thor A., Neveu M., Sheng S., Rafidi K., Seftor E., Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994 Jan 28;263(5146):526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]