Abstract
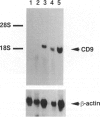
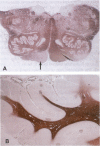
CD9 is a member of the newly identified tetra-membrane-spanning protein family. We show here that CD9 is a constituent of myelin in the central and peripheral nervous systems. Expression of CD9 was detected in human cerebral white matter and sciatic nerve by Northern and Western blotting. Myelin in the central and peripheral nervous systems was strongly stained with a monoclonal antibody against human CD9 antigen in paraffin-embedded sections. CD9 was detected in adult nervous tissue but not in developing brain at less than 20 weeks of gestation. Immunohistochemical studies indicated that expression of CD9 is correlated with myelination and is somewhat delayed compared with expression of myelin basic protein, a major component protein of myelin. In the central nervous system, CD9 was detected along the outermost membrane of compact myelin but not inside compact myelin or the periaxonal region. Although the membrane-anchored form of heparin-binding epidermal-growth-factor-like growth factor (proHB-EGF), which is identical to the diphtheria toxin receptor, forms a complex with CD9 in some human and monkey cell lines, proHB-EGF was not detected in myelin immunocytochemically. The distribution of CD9 in the outer surface of myelin and its relatively late developmental appearance suggest that CD9 may interact with the extracellular matrix or cell adhesion molecules and participate in the maintenance of the entire myelin sheath.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGARWAL S. C., PRYCE D. M. Experimental diphtheritic paralysis in rats. J Pathol Bacteriol. 1959 Jul;78:171–177. [PubMed] [Google Scholar]
- Allt G., Cavanagh J. B. Ultrastructural changes in the region of the node of ranvier in the rat caused by diphtheria toxin. Brain. 1969;92(2):459–468. doi: 10.1093/brain/92.2.459. [DOI] [PubMed] [Google Scholar]
- Amiguet P., Gardinier M. V., Zanetta J. P., Matthieu J. M. Purification and partial structural and functional characterization of mouse myelin/oligodendrocyte glycoprotein. J Neurochem. 1992 May;58(5):1676–1682. doi: 10.1111/j.1471-4159.1992.tb10040.x. [DOI] [PubMed] [Google Scholar]
- Boucheix C., Benoit P. CD9 antigen: will platelet physiology help to explain the function of a surface molecule during hemopoietic differentiation? Nouv Rev Fr Hematol. 1988;30(4):201–202. [PubMed] [Google Scholar]
- Boucheix C., Benoit P., Frachet P., Billard M., Worthington R. E., Gagnon J., Uzan G. Molecular cloning of the CD9 antigen. A new family of cell surface proteins. J Biol Chem. 1991 Jan 5;266(1):117–122. [PubMed] [Google Scholar]
- Burger D., Steck A. J., Bernard C. C., Kerlero De Rosbo N. Human myelin/oligodendrocyte glycoprotein: a new member of the L2/HNK-1 family. Schweiz Arch Neurol Psychiatr (1985) 1993;144(3):227–228. [PubMed] [Google Scholar]
- Campagnoni A. T. Molecular biology of myelin proteins from the central nervous system. J Neurochem. 1988 Jul;51(1):1–14. doi: 10.1111/j.1471-4159.1988.tb04827.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinier M. V., Matthieu J. M. Cloning and cDNA sequence analysis of myelin/oligodendrocyte glycoprotein: a novel member of the immunoglobulin gene superfamily. Schweiz Arch Neurol Psychiatr (1985) 1993;144(3):201–207. [PubMed] [Google Scholar]
- Higashiyama S., Iwamoto R., Goishi K., Raab G., Taniguchi N., Klagsbrun M., Mekada E. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995 Mar;128(5):929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsí V., Vlcek C. Novel structurally distinct family of leucocyte surface glycoproteins including CD9, CD37, CD53 and CD63. FEBS Lett. 1991 Aug 19;288(1-2):1–4. doi: 10.1016/0014-5793(91)80988-f. [DOI] [PubMed] [Google Scholar]
- Ikeyama S., Koyama M., Yamaoko M., Sasada R., Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993 May 1;177(5):1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto R., Senoh H., Okada Y., Uchida T., Mekada E. An antibody that inhibits the binding of diphtheria toxin to cells revealed the association of a 27-kDa membrane protein with the diphtheria toxin receptor. J Biol Chem. 1991 Oct 25;266(30):20463–20469. [PubMed] [Google Scholar]
- Jacobs J. M. Experimental diphtheritic neuropathy in the rat. Br J Exp Pathol. 1967 Apr;48(2):204–216. [PMC free article] [PubMed] [Google Scholar]
- Jennings L. K., Fox C. F., Kouns W. C., McKay C. P., Ballou L. R., Schultz H. E. The activation of human platelets mediated by anti-human platelet p24/CD9 monoclonal antibodies. J Biol Chem. 1990 Mar 5;265(7):3815–3822. [PubMed] [Google Scholar]
- Kaprielian Z., Patterson P. H. Surface and cytoskeletal markers of rostrocaudal position in the mammalian nervous system. J Neurosci. 1993 Jun;13(6):2495–2508. doi: 10.1523/JNEUROSCI.13-06-02495.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey J. H., LeBien T. W., Abramson C. S., Newman R., Sutherland R., Greaves M. P-24: a human leukemia-associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody. J Exp Med. 1981 Mar 1;153(3):726–731. doi: 10.1084/jem.153.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada Y., Peiper S. C., Melvin S. L., Metzger D. W., Tarnowski B. H., Green A. A. A monoclonal antibody (SJ-9A4) to P24 present on common alls, neuroblastomas and platelets - I. Characterization and development of a unique radioimmunometric assay. Leuk Res. 1983;7(4):487–498. doi: 10.1016/0145-2126(83)90044-9. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Fowler A. V., Takahashi Y. cDNA cloning and amino acid sequence of bovine brain 2',3'-cyclic-nucleotide 3'-phosphodiesterase. J Biol Chem. 1987 Mar 5;262(7):3256–3261. [PubMed] [Google Scholar]
- Lanza F., Wolf D., Fox C. F., Kieffer N., Seyer J. M., Fried V. A., Coughlin S. R., Phillips D. R., Jennings L. K. cDNA cloning and expression of platelet p24/CD9. Evidence for a new family of multiple membrane-spanning proteins. J Biol Chem. 1991 Jun 5;266(16):10638–10645. [PubMed] [Google Scholar]
- Lemke G., Axel R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell. 1985 Mar;40(3):501–508. doi: 10.1016/0092-8674(85)90198-9. [DOI] [PubMed] [Google Scholar]
- Linington C., Bradl M., Lassmann H., Brunner C., Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988 Mar;130(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- Martini R., Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol. 1986 Dec;103(6 Pt 1):2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Alonso J. M., Hernando N., Ghosh S., Coca-Prados M. Molecular cloning of the bovine CD9 antigen from ocular ciliary epithelial cells. J Biochem. 1992 Jul;112(1):63–67. doi: 10.1093/oxfordjournals.jbchem.a123866. [DOI] [PubMed] [Google Scholar]
- Masellis-Smith A., Shaw A. R. CD9-regulated adhesion. Anti-CD9 monoclonal antibody induce pre-B cell adhesion to bone marrow fibroblasts through de novo recognition of fibronectin. J Immunol. 1994 Mar 15;152(6):2768–2777. [PubMed] [Google Scholar]
- Mitamura T., Higashiyama S., Taniguchi N., Klagsbrun M., Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995 Jan 20;270(3):1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- Mitamura T., Iwamoto R., Umata T., Yomo T., Urabe I., Tsuneoka M., Mekada E. The 27-kD diphtheria toxin receptor-associated protein (DRAP27) from vero cells is the monkey homologue of human CD9 antigen: expression of DRAP27 elevates the number of diphtheria toxin receptors on toxin-sensitive cells. J Cell Biol. 1992 Sep;118(6):1389–1399. doi: 10.1083/jcb.118.6.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M., Koyama M., Seno M., Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med. 1991 Dec 1;174(6):1347–1354. doi: 10.1084/jem.174.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Iwamoto R., Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin alpha 3 beta 1 at cell-cell contact sites. J Cell Biol. 1995 Jun;129(6):1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Ohta Y. Immunohistochemical study of human placental stromal cells. Hum Pathol. 1990 Sep;21(9):936–940. doi: 10.1016/0046-8177(90)90178-8. [DOI] [PubMed] [Google Scholar]
- Olee T., Powers J. M., Brostoff S. W. A T cell epitope for experimental allergic neuritis. J Neuroimmunol. 1988 Aug;19(1-2):167–173. doi: 10.1016/0165-5728(88)90046-x. [DOI] [PubMed] [Google Scholar]
- Owens G. C., Boyd C. J., Bunge R. P., Salzer J. L. Expression of recombinant myelin-associated glycoprotein in primary Schwann cells promotes the initial investment of axons by myelinating Schwann cells. J Cell Biol. 1990 Sep;111(3):1171–1182. doi: 10.1083/jcb.111.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Harper A. A., Moynihan M., Brockes J. P. Diphtheria toxin and related proteins: effect of route of injection on toxicity and the determination of cytotoxicity for various cultured cells. J Infect Dis. 1982 Jan;145(1):94–102. doi: 10.1093/infdis/145.1.94. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Prayoonwiwat N., Howe C., Sanborn K. Proteolipid protein gene expression in demyelination and remyelination of the central nervous system: a model for multiple sclerosis. J Neuropathol Exp Neurol. 1994 Mar;53(2):136–143. doi: 10.1097/00005072-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Billard M., Plaisance S., Prenant M., Boucheix C. Molecular cloning of the mouse equivalent of CD9 antigen. Thromb Res. 1993 Sep 1;71(5):377–383. doi: 10.1016/0049-3848(93)90162-h. [DOI] [PubMed] [Google Scholar]
- Si Z., Hersey P. Expression of the neuroglandular antigen and analogues in melanoma. CD9 expression appears inversely related to metastatic potential of melanoma. Int J Cancer. 1993 Apr 22;54(1):37–43. doi: 10.1002/ijc.2910540107. [DOI] [PubMed] [Google Scholar]
- Slupsky J. R., Seehafer J. G., Tang S. C., Masellis-Smith A., Shaw A. R. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb-IIIa complex. J Biol Chem. 1989 Jul 25;264(21):12289–12293. [PubMed] [Google Scholar]
- Tole S., Patterson P. H. Distribution of CD9 in the developing and mature rat nervous system. Dev Dyn. 1993 Jun;197(2):94–106. doi: 10.1002/aja.1001970203. [DOI] [PubMed] [Google Scholar]
- WELLER R. O. DIPHTHERITIC NEUROPATHY IN THE CHICKEN: AN ELECTRON-MICROSCOPE STUDY. J Pathol Bacteriol. 1965 Apr;89:591–598. doi: 10.1002/path.1700890218. [DOI] [PubMed] [Google Scholar]