Abstract
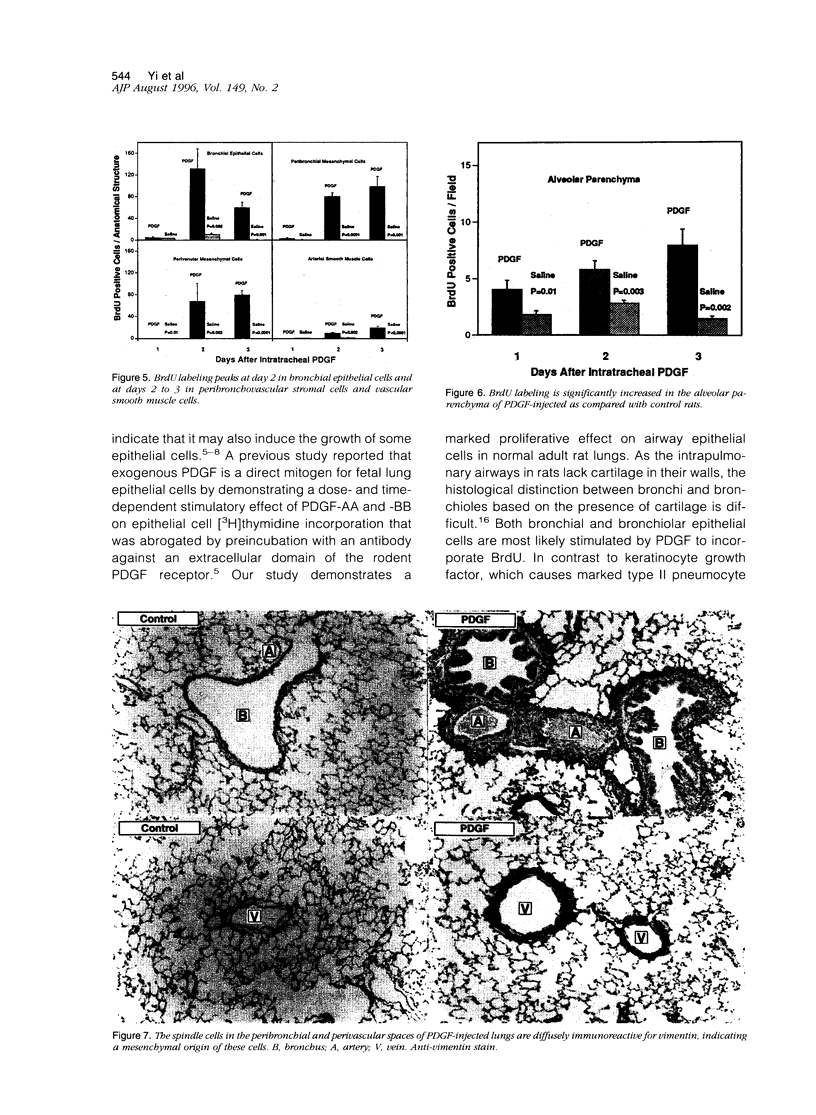
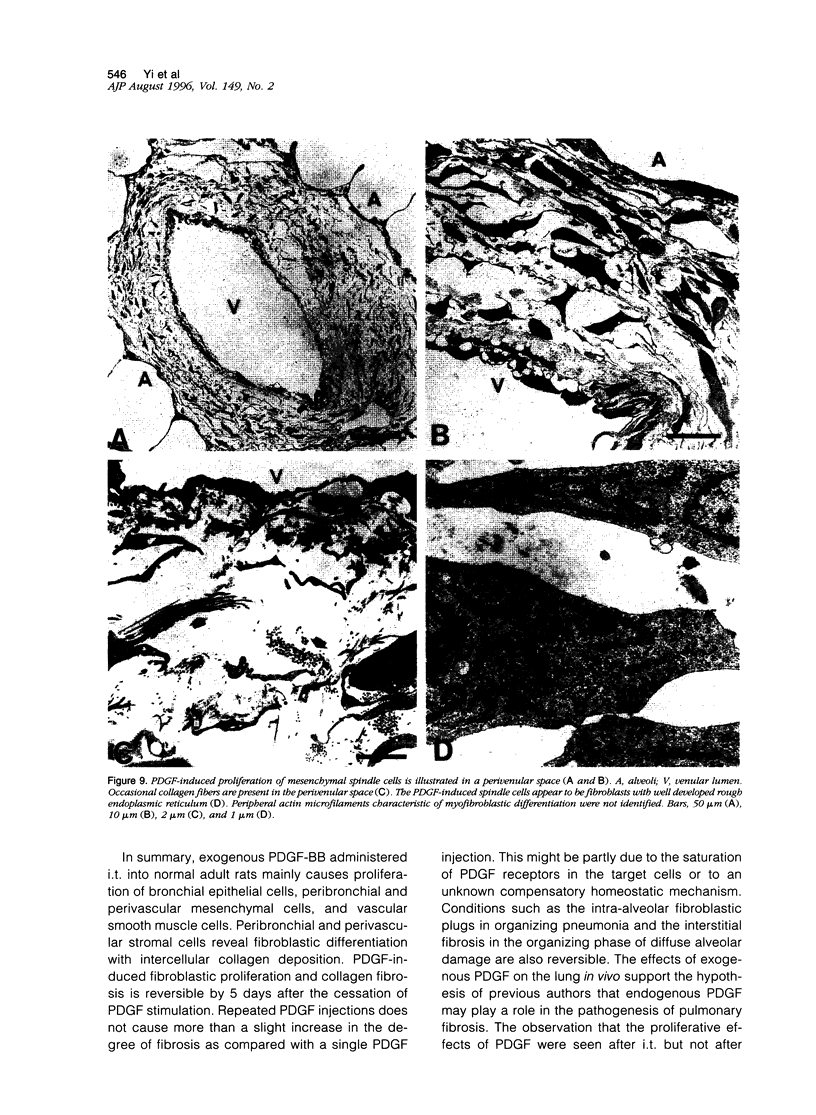
Platelet-derived growth factor (PDGF) is postulated to play a role in the pathophysiology of pulmonary fibrosis. Recombinant human PDGF-BB administered as a single intratracheal injection in rats causes an increase in peribronchial and perivascular stromal cells on days 2 and 3 after injection as evaluated by hematoxylin and eosin histology and 5-bromodeoxyuridine incorporation. Proliferation of bronchial epithelial cells and arterial smooth muscle cells, although not evident by routine histological examination alone, is detected on days 2 and 3 by increased 5-bromodeoxyuridine incorporation. A mild increase in 5-bromodeoxyuridine labeling is observed in peripheral alveolar parenchyma after injection of PDGF. The proliferative peribronchial and perivascular mesenchymal cells appear by light microscopic and ultrastructural criteria to be fibroblasts that are immunoreactive for vimentin but negative for alpha-smooth muscle actin and desmin. Daily intratracheal injection of PDGF-BB for 3 days causes a slightly more pronounced peribronchial and perivascular spindle cell proliferation accompanied by collagen deposition as evaluated by Masson's trichrome stain. PDGF-induced increases in cellularity and collagen resolve within 5 days after the last PDGF injection. In conclusion, intratracheal injection of PDGF-BB causes transient proliferation of pulmonary mesenchymal and epithelial cells accompanied by collagen deposition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Ehsani N., Palmer H., Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991 Sep-Oct;11(5):1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Galanopoulos T., Neville-Golden J., O'Hara C. J. Malignant epithelial cells in primary human lung carcinomas coexpress in vivo platelet-derived growth factor (PDGF) and PDGF receptor mRNAs and their protein products. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3942–3946. doi: 10.1073/pnas.89.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh J. C. Gene amplification in human lung cancer. The myc family genes and other proto-oncogenes and growth factor genes. Am Rev Respir Dis. 1990 Dec;142(6 Pt 2):S20–S26. doi: 10.1164/ajrccm/142.6_Pt_2.S20. [DOI] [PubMed] [Google Scholar]
- Brandt-Rauf P. W., Smith S., Hemminki K., Koskinen H., Vainio H., Niman H., Ford J. Serum oncoproteins and growth factors in asbestosis and silicosis patients. Int J Cancer. 1992 Apr 1;50(6):881–885. doi: 10.1002/ijc.2910500610. [DOI] [PubMed] [Google Scholar]
- Bravo M., Vàsquez R., Rubio H., Salazar M., Pardo A., Selman M. Production of platelet-derived growth factor by human lung cancer. Respir Med. 1991 Nov;85(6):479–485. doi: 10.1016/s0954-6111(06)80265-9. [DOI] [PubMed] [Google Scholar]
- Caniggia I., Liu J., Han R., Buch S., Funa K., Tanswell K., Post M. Fetal lung epithelial cells express receptors for platelet-derived growth factor. Am J Respir Cell Mol Biol. 1993 Jul;9(1):54–63. doi: 10.1165/ajrcmb/9.1.54. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Au Y. P., Clowes A. W., Reidy M. A. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990 Nov-Dec;10(6):1082–1087. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- Forsberg K., Bergh J., Westermark B. Expression of functional PDGF beta receptors in a human large-cell lung-carcinoma cell line. Int J Cancer. 1993 Feb 20;53(4):556–560. doi: 10.1002/ijc.2910530405. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Ishizaki M., Masuda Y., Kimura G., Kawanami O., Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol. 1987 Jan;126(1):171–182. [PMC free article] [PubMed] [Google Scholar]
- Hart C. E., Bailey M., Curtis D. A., Osborn S., Raines E., Ross R., Forstrom J. W. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990 Jan 9;29(1):166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Boldt J., King T. E., Jr, Crouch E., Vartio T., McDonald J. A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989 Dec;140(6):1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- Leslie K., King T. E., Jr, Low R. Smooth muscle actin is expressed by air space fibroblast-like cells in idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Chest. 1991 Mar;99(3 Suppl):47S–48S. doi: 10.1378/chest.99.3_supplement.47s. [DOI] [PubMed] [Google Scholar]
- Lindner V., Giachelli C. M., Schwartz S. M., Reidy M. A. A subpopulation of smooth muscle cells in injured rat arteries expresses platelet-derived growth factor-B chain mRNA. Circ Res. 1995 Jun;76(6):951–957. doi: 10.1161/01.res.76.6.951. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Platelet-derived growth factor ligand and receptor expression by large vessel endothelium in vivo. Am J Pathol. 1995 Jun;146(6):1488–1497. [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A. Idiopathic pulmonary fibrosis. A paradigm for lung injury and repair. Chest. 1991 Mar;99(3 Suppl):87S–93S. doi: 10.1378/chest.99.3_supplement.87s. [DOI] [PubMed] [Google Scholar]
- Nagaoka I., Trapnell B. C., Crystal R. G. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest. 1990 Jun;85(6):2023–2027. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Tarpley J. E., Tseng J., Bready J., Chang D., Kenney W. C., Rudolph R., Robson M. C., Vande Berg J., Reid P. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995 Sep;96(3):1336–1350. doi: 10.1172/JCI118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom W. N., Travis W. D., Brody A. R. Cellular and molecular basis of the asbestos-related diseases. Am Rev Respir Dis. 1991 Feb;143(2):408–422. doi: 10.1164/ajrccm/143.2.408. [DOI] [PubMed] [Google Scholar]
- Sundberg C., Ljungström M., Lindmark G., Gerdin B., Rubin K. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol. 1993 Nov;143(5):1377–1388. [PMC free article] [PubMed] [Google Scholar]
- Taverna D., Groner B., Hynes N. E. Epidermal growth factor receptor, platelet-derived growth factor receptor, and c-erbB-2 receptor activation all promote growth but have distinctive effects upon mouse mammary epithelial cell differentiation. Cell Growth Differ. 1991 Mar;2(3):145–154. [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Longmuir K., Yin S., Biltz R., Morris C. F., Housley R. M., Pierce G. F. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994 Mar;93(3):1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaud J. M., Marie B., Klein N., Plénat F., Pech M., Borrelly J., Martinet N., Duprez A., Martinet Y. The role of platelet-derived growth factor production by tumor-associated macrophages in tumor stroma formation in lung cancer. Cancer Res. 1994 Oct 15;54(20):5455–5463. [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sakuma J., Hayashi S., Abe K., Saito I., Harada S., Sakatani M., Yamamoto S., Matsumoto N., Kaneda Y. A histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor beta 1, or platelet-derived growth factor B gene. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9570–9574. doi: 10.1073/pnas.92.21.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Rekhter M. D., Gordon D., Phan S. H. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994 Jul;145(1):114–125. [PMC free article] [PubMed] [Google Scholar]