Abstract
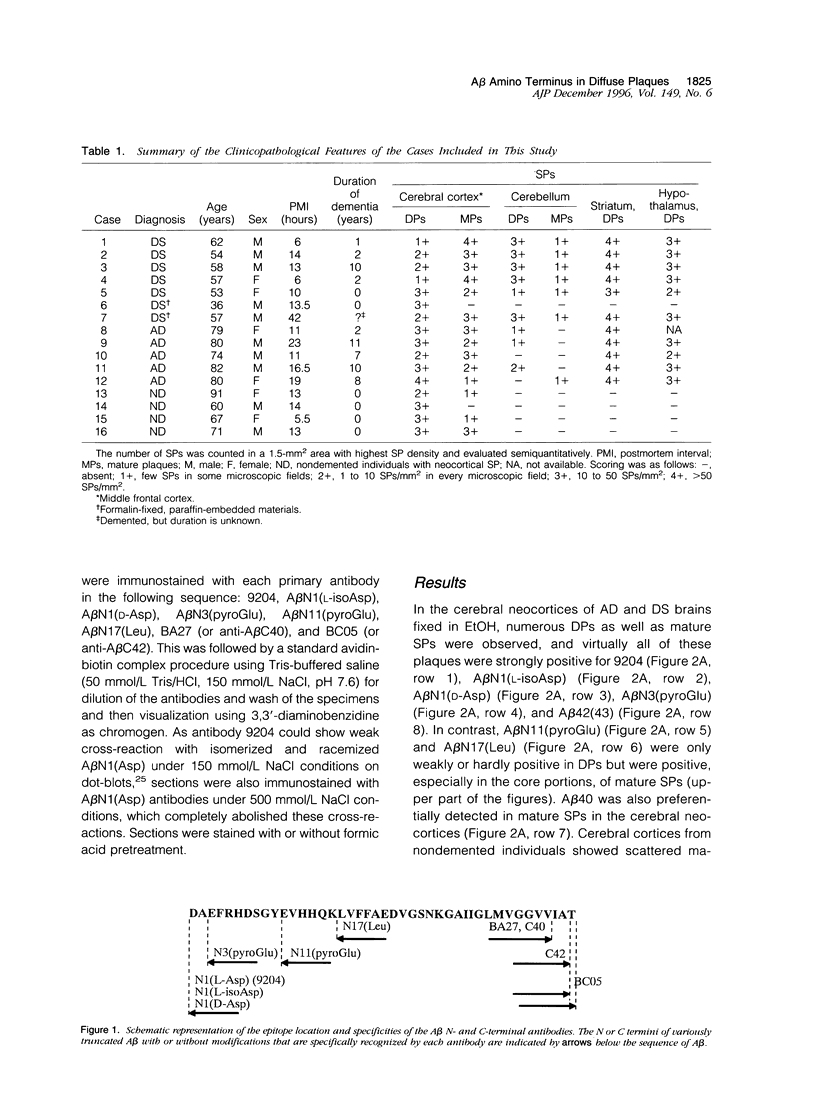
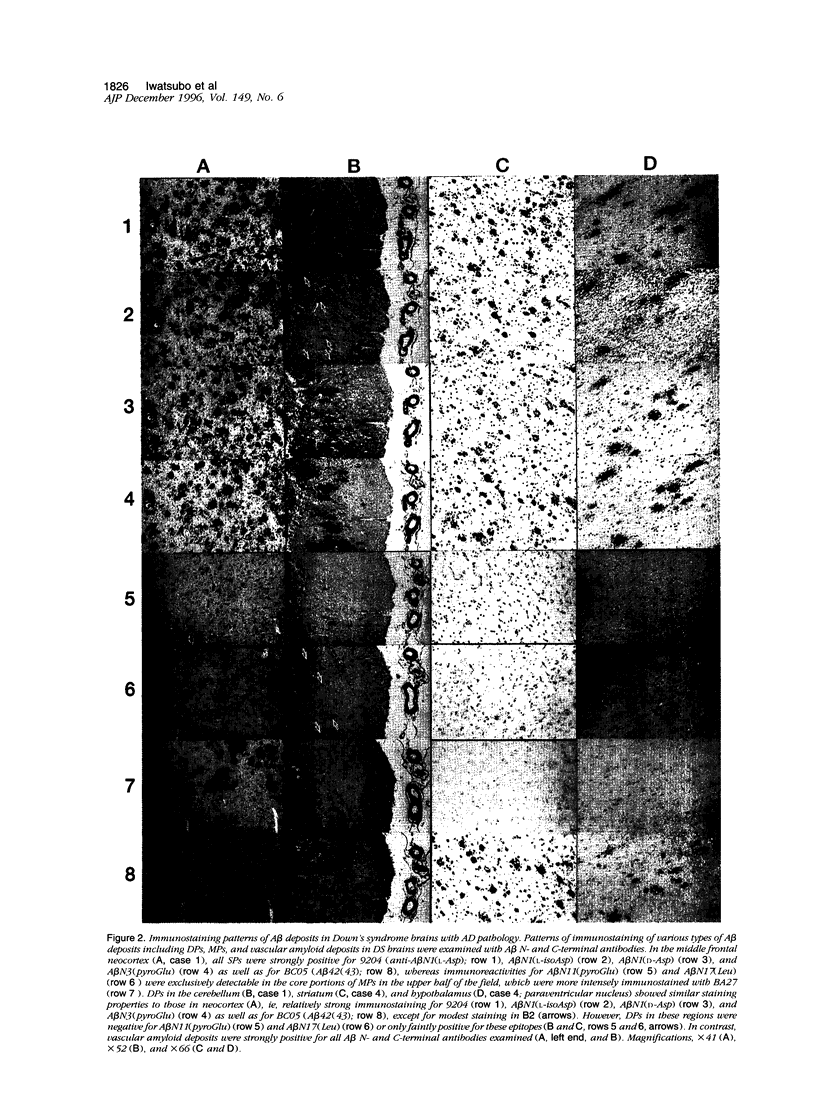
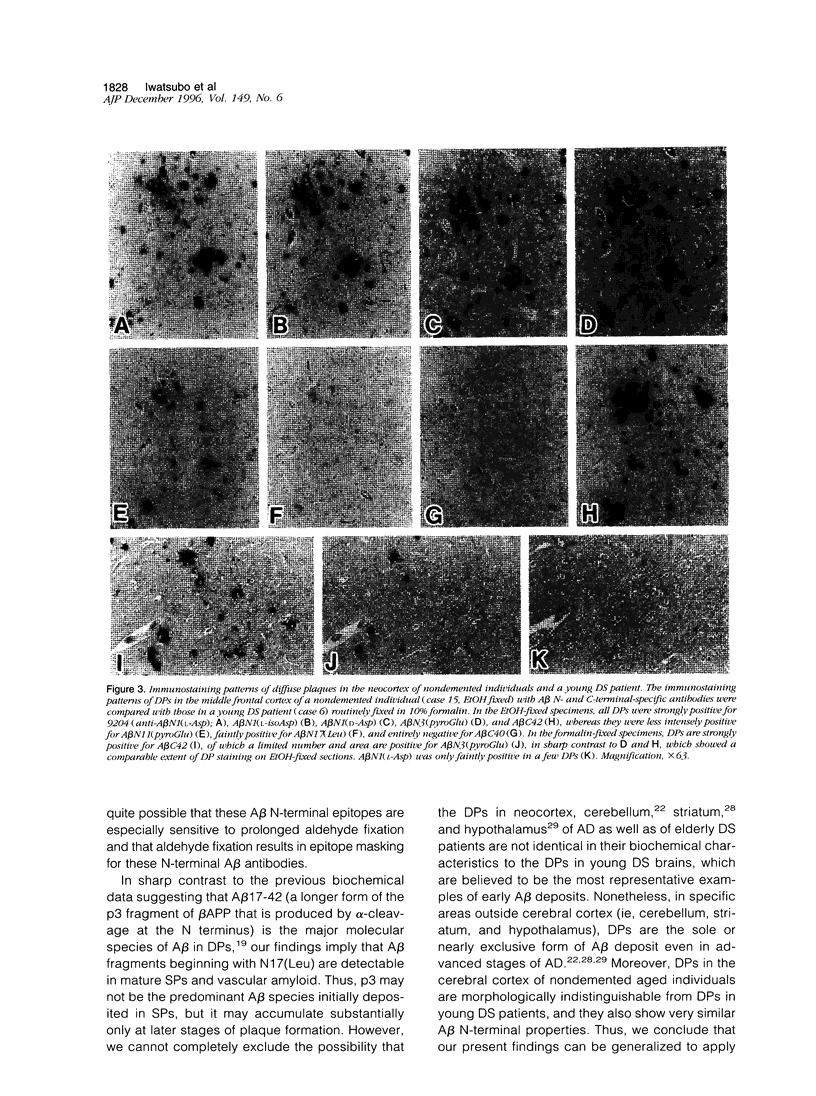
The amino- and carboxyl-terminal properties of the amyloid-beta (A beta) peptides deposited in diffuse plaques, one of the earliest forms of A beta deposition, were examined in the brains of patients with Down's syndrome and Alzheimer's disease and in aged individuals without dementia by immunocytochemistry. This was done using a panel of antibodies that specifically discriminate the terminal structures and modifications at the amino and carboxyl termini of A beta. Diffuse plaques found in the cerebral and cerebellar cortex, neostriatum, and hypothalamus of Down's syndrome, Alzheimer's disease, and nondemented brains were strongly immunoreactive for A beta N1(L-Asp), A beta N1(L-isoAsp), A beta N1(D-Asp), and A beta N3(pyroGlu) and weakly positive for A beta N11(pyroGlu) and A beta N17(Leu). Diffuse plaques also were positive for A beta 42(43) but negative for A beta 40, using carboxyl-terminal-specific anti-A beta antibodies. These results suggest that the amino termini of the A beta species that initially deposit in diffuse plaques begin with A beta N1(Asp) with or without structural modifications (isomerization and racemization), as well as with A beta N3(pyroGlu), and terminate preferentially at A beta 42(43) rather than A beta 40.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Lee V. M., Otvos L., Jr, Greenberg B. D., Lowery D. E., Sharma S. K., Schmidt M. L., Trojanowski J. Q. Defined neurofilament, tau, and beta-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2249–2253. doi: 10.1073/pnas.87.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Dovey H. F., Suomensaari-Chrysler S., Lieberburg I., Sinha S., Keim P. S. Cells with a familial Alzheimer's disease mutation produce authentic beta-peptide. Neuroreport. 1993 Aug;4(8):1039–1042. doi: 10.1097/00001756-199308000-00011. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Asami-Odaka A., Suzuki N., Shimada H., Ihara Y., Iwatsubo T. Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer's disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques. Am J Pathol. 1996 Jan;148(1):259–265. [PMC free article] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gowing E., Roher A. E., Woods A. S., Cotter R. J., Chaney M., Little S. P., Ball M. J. Chemical characterization of A beta 17-42 peptide, a component of diffuse amyloid deposits of Alzheimer disease. J Biol Chem. 1994 Apr 15;269(15):10987–10990. [PubMed] [Google Scholar]
- Iwatsubo T., Mann D. M., Odaka A., Suzuki N., Ihara Y. Amyloid beta protein (A beta) deposition: A beta 42(43) precedes A beta 40 in Down syndrome. Ann Neurol. 1995 Mar;37(3):294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994 Jul;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Lemere C. A., Blusztajn J. K., Yamaguchi H., Wisniewski T., Saido T. C., Selkoe D. J. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996 Feb;3(1):16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Woodruff-Pak D. S., Trojanowski J. Q. Amyloid plaques in cerebellar cortex and the integrity of Purkinje cell dendrites. Neurobiol Aging. 1994 Jan-Feb;15(1):1–9. doi: 10.1016/0197-4580(94)90139-2. [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Papayannopoulos I. A., Styles J., Bobin S. A., Lin Y. Y., Biemann K., Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993 Feb 15;301(1):41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Mori H., Takio K., Ogawara M., Selkoe D. J. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J Biol Chem. 1992 Aug 25;267(24):17082–17086. [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Wolkow C., Wang R., Cotter R. J., Reardon I. M., Zürcher-Neely H. A., Heinrikson R. L., Ball M. J. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J Biol Chem. 1993 Feb 15;268(5):3072–3083. [PubMed] [Google Scholar]
- Saido T. C., Iwatsubo T., Mann D. M., Shimada H., Ihara Y., Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron. 1995 Feb;14(2):457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Yamao-Harigaya W., Iwatsubo T., Kawashima S. Amino- and carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neurosci Lett. 1996 Sep 13;215(3):173–176. doi: 10.1016/0304-3940(96)12970-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Robinson K. A., Lee V. M., Trojanowski J. Q. Chemical and immunological heterogeneity of fibrillar amyloid in plaques of Alzheimer's disease and Down's syndrome brains revealed by confocal microscopy. Am J Pathol. 1995 Aug;147(2):503–515. [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer's disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest. 1991 May;64(5):693–702. [PubMed] [Google Scholar]
- Standaert D. G., Lee V. M., Greenberg B. D., Lowery D. E., Trojanowski J. Q. Molecular features of hypothalamic plaques in Alzheimer's disease. Am J Pathol. 1991 Sep;139(3):681–691. [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tamaoka A., Odaka A., Ishibashi Y., Usami M., Sahara N., Suzuki N., Nukina N., Mizusawa H., Shoji S., Kanazawa I. APP717 missense mutation affects the ratio of amyloid beta protein species (A beta 1-42/43 and a beta 1-40) in familial Alzheimer's disease brain. J Biol Chem. 1994 Dec 30;269(52):32721–32724. [PubMed] [Google Scholar]
- Trojanowski J. Q., Shin R. W., Schmidt M. L., Lee V. M. Relationship between plaques, tangles, and dystrophic processes in Alzheimer's disease. Neurobiol Aging. 1995 May-Jun;16(3):335–345. doi: 10.1016/0197-4580(94)00176-2. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Bancher C., Barcikowska M., Wen G. Y., Currie J. Spectrum of morphological appearance of amyloid deposits in Alzheimer's disease. Acta Neuropathol. 1989;78(4):337–347. doi: 10.1007/BF00688170. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Hirai S., Shoji M., Harigaya Y. Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol. 1989 Oct;135(4):593–597. [PMC free article] [PubMed] [Google Scholar]




