Abstract
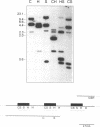
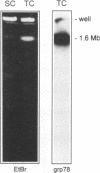
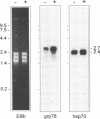
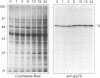
The protozoan Trypanosoma cruzi is the etiologic agent of Chagas' disease, an illness responsible for morbidity and death among millions of Latin Americans. Mice also develop this disease when infected with T. cruzi and are a useful model organism for the study of parasite-specific immune responses. To identify immunogenic T. cruzi antigens, serum from an infected mouse was used to isolate clones from a T. cruzi epimastigote cDNA expression library. One of these clones was found to encode the 78-kDa glucose-regulated protein (grp78), the endoplasmic reticular member of the 70-kDa heat shock protein (hsp70) family. Like the mammalian and yeast grp78s, the T. cruzi protein contains an endoplasmic reticular leader peptide and a carboxyl-terminal endoplasmic reticular retention sequence. T. cruzi grp78 is encoded by a tandemly arranged family of three genes located on a chromosome of 1.6 Mb. The effects on grp78 expression of heat shock and tunicamycin treatment, the latter of which specifically stimulates mammalian grp78, were investigated. While the level of the grp78 protein remained constant under all circumstances, grp78 mRNA was unaffected by heat shock but induced fivefold by tunicamycin. Finally, we found that grp78 is the most immunogenic of the T. cruzi heat shock proteins we have characterized, reacting strongly in immunoblots with sera from infected mice.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangs J. D., Uyetake L., Brickman M. J., Balber A. E., Boothroyd J. C. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993 Aug;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gambill B. D., Nelson R. J. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993 Jun;57(2):402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragon E. A., Sias S. R., Kato E. A., Gabe J. D. The genome of Trypanosoma cruzi contains a constitutively expressed, tandemly arranged multicopy gene homologous to a major heat shock protein. Mol Cell Biol. 1987 Mar;7(3):1271–1275. doi: 10.1128/mcb.7.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond I. A., Lee A. S., Resendez E., Jr, Steinhardt R. A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987 Sep 15;262(26):12801–12805. [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Engman D. M., Dragon E. A., Donelson J. E. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J Immunol. 1990 May 15;144(10):3987–3991. [PubMed] [Google Scholar]
- Engman D. M., Fehr S. C., Donelson J. E. Specific functional domains of mitochondrial hsp70s suggested by sequence comparison of the trypanosome and yeast proteins. Mol Biochem Parasitol. 1992 Mar;51(1):153–155. doi: 10.1016/0166-6851(92)90210-b. [DOI] [PubMed] [Google Scholar]
- Engman D. M., Kirchhoff L. V., Donelson J. E. Molecular cloning of mtp70, a mitochondrial member of the hsp70 family. Mol Cell Biol. 1989 Nov;9(11):5163–5168. doi: 10.1128/mcb.9.11.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman D. M., Krause K. H., Blumin J. H., Kim K. S., Kirchhoff L. V., Donelson J. E. A novel flagellar Ca2+-binding protein in trypanosomes. J Biol Chem. 1989 Nov 5;264(31):18627–18631. [PubMed] [Google Scholar]
- Engman D. M., Reddy L. V., Donelson J. E., Kirchhoff L. V. Trypanosoma cruzi exhibits inter- and intra-strain heterogeneity in molecular karyotype and chromosomal gene location. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):115–123. doi: 10.1016/0166-6851(87)90041-7. [DOI] [PubMed] [Google Scholar]
- Engman D. M., Sias S. R., Gabe J. D., Donelson J. E., Dragon E. A. Comparison of HSP70 genes from two strains of Trypanosoma cruzi. Mol Biochem Parasitol. 1989 Dec;37(2):285–287. doi: 10.1016/0166-6851(89)90161-8. [DOI] [PubMed] [Google Scholar]
- Giambiagi-de Marval M., Gottesdiener K., Rondinelli E., Van der Ploeg L. H. Predicted amino acid sequence and genomic organization of Trypanosoma cruzi hsp 60 genes. Mol Biochem Parasitol. 1993 Mar;58(1):25–31. doi: 10.1016/0166-6851(93)90087-e. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Lerner T. J., Huecas M., Sosa-Pineda B., Nogueira N., Lizardi P. M. Apparent generation of a segmented mRNA from two separate tandem gene families in Trypanosoma cruzi. Nucleic Acids Res. 1985 Aug 26;13(16):5789–5804. doi: 10.1093/nar/13.16.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Hieny S., Shiver G. M., Snary D., Sher A. Cryptic epitope explains the failure of a monoclonal antibody to bind to certain isolates of Trypanosoma cruzi. J Immunol. 1984 Nov;133(5):2731–2735. [PubMed] [Google Scholar]
- Kirchhoff L. V., Neva F. A. Chagas' disease in Latin American immigrants. JAMA. 1985 Dec 6;254(21):3058–3060. [PubMed] [Google Scholar]
- Kumar N., Zheng H. Nucleotide sequence of a Plasmodium falciparum stress protein with similarity to mammalian 78-kDa glucose-regulated protein. Mol Biochem Parasitol. 1992 Dec;56(2):353–356. doi: 10.1016/0166-6851(92)90187-o. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- McCormick T. S., Rowland E. C. Trypanosoma cruzi: cross-reactive anti-heart autoantibodies produced during infection in mice. Exp Parasitol. 1989 Nov;69(4):393–401. doi: 10.1016/0014-4894(89)90088-x. [DOI] [PubMed] [Google Scholar]
- Muhich M. L., Boothroyd J. C. Polycistronic transcripts in trypanosomes and their accumulation during heat shock: evidence for a precursor role in mRNA synthesis. Mol Cell Biol. 1988 Sep;8(9):3837–3846. doi: 10.1128/mcb.8.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nadeau K., Sullivan M. A., Bradley M., Engman D. M., Walsh C. T. 83-kilodalton heat shock proteins of trypanosomes are potent peptide-stimulated ATPases. Protein Sci. 1992 Aug;1(8):970–979. doi: 10.1002/pro.5560010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport G., Culpepper J., Agabian N. Parasite heat-shock proteins. Parasitol Today. 1988 Nov;4(11):306–312. doi: 10.1016/0169-4758(88)90111-1. [DOI] [PubMed] [Google Scholar]
- Normington K., Kohno K., Kozutsumi Y., Gething M. J., Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989 Jun 30;57(7):1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Olson C. L., Nadeau K. C., Sullivan M. A., Winquist A. G., Donelson J. E., Walsh C. T., Engman D. M. Molecular and biochemical comparison of the 70-kDa heat shock proteins of Trypanosoma cruzi. J Biol Chem. 1994 Feb 4;269(5):3868–3874. [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sorger P. K. Heat shock factor and the heat shock response. Cell. 1991 May 3;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Ting J., Lee A. S. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988 May;7(4):275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. P., Misra L. M., Rose M. D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990 Jun;110(6):1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., So M. Genomic variation of Trypanosoma cruzi: involvement of multicopy genes. Infect Immun. 1990 Oct;58(10):3217–3224. doi: 10.1128/iai.58.10.3217-3224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder J. A., Eder P. S., Engman D. M., Brentano S. T., Walder R. Y., Knutzon D. S., Dorfman D. M., Donelson J. E. The 35-nucleotide spliced leader sequence is common to all trypanosome messenger RNA's. Science. 1986 Aug 1;233(4763):569–571. doi: 10.1126/science.3523758. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Wu F. S., Park Y. C., Roufa D., Martonosi A. Selective stimulation of the synthesis of an 80,000-dalton protein by calcium ionophores. J Biol Chem. 1981 Jun 10;256(11):5309–5312. [PubMed] [Google Scholar]
- Xiao H., Perisic O., Lis J. T. Cooperative binding of Drosophila heat shock factor to arrays of a conserved 5 bp unit. Cell. 1991 Feb 8;64(3):585–593. doi: 10.1016/0092-8674(91)90242-q. [DOI] [PubMed] [Google Scholar]
- Zwierzynski T. A., Widmer G., Buck G. A. In vitro 3' end processing and poly(A) tailing of RNA in Trypanosoma cruzi. Nucleic Acids Res. 1989 Jun 26;17(12):4647–4660. doi: 10.1093/nar/17.12.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]