Abstract
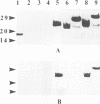
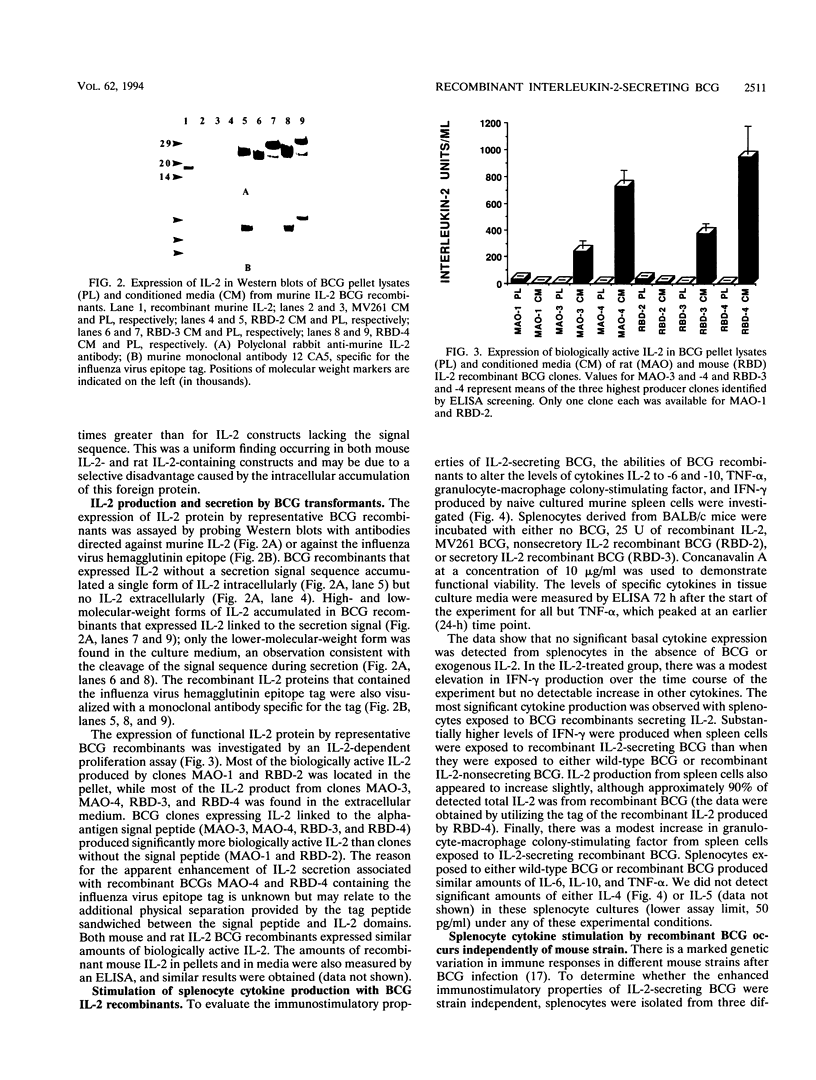
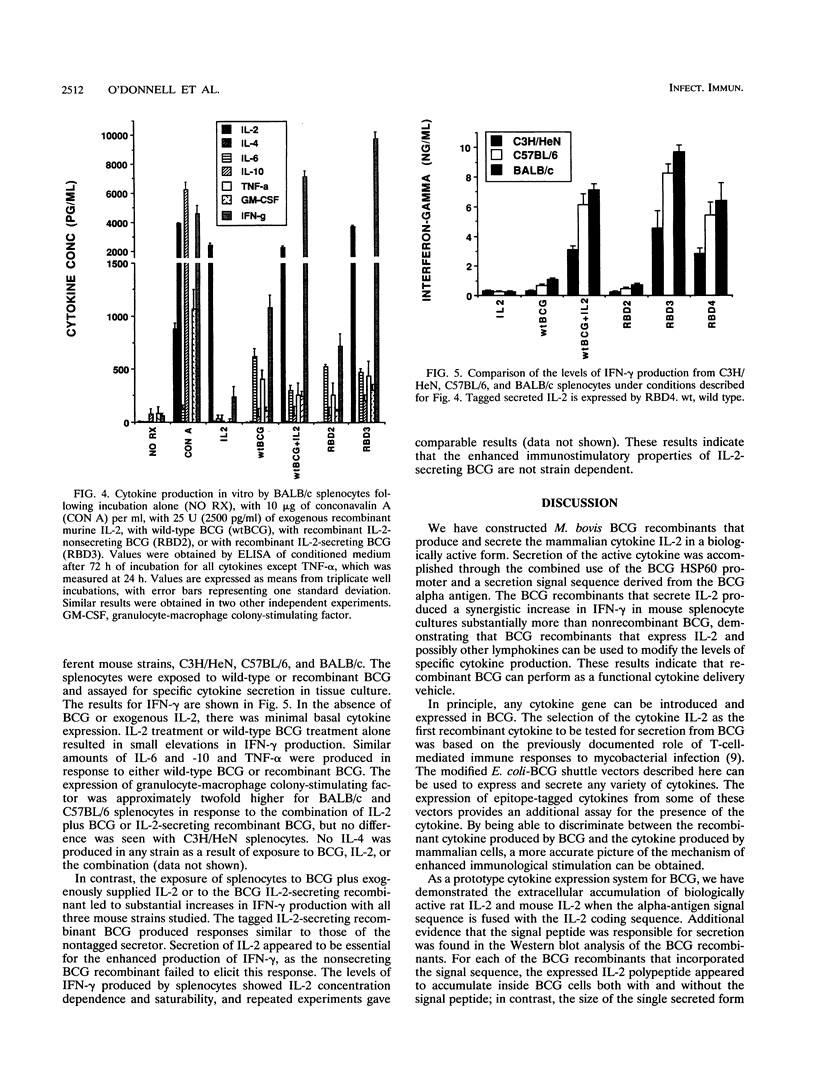
Mycobacterium bovis BCG was genetically engineered to express and secrete mouse interleukin-2 (IL-2) and rat IL-2. Genes encoding IL-2 were inserted into an Escherichia coli-BCG shuttle plasmid under the control of the BCG HSP60 promoter. To facilitate study of proteins produced in this system, the IL-2 gene product was expressed (i) alone, (ii) with the mycobacterial alpha-antigen secretion signal sequence at the amino terminus, (iii) with an influenza virus hemagglutinin epitope tag at the amino terminus, and (iv) with both the secretion signal sequence and the epitope tag. When expressed with the alpha-antigen signal sequence, biologically active IL-2 was secreted into the extracellular medium. Western blot (immunoblot) analysis of the intracellular IL-2 and extracellular IL-2 revealed that the secretion signal was appropriately cleaved from the recombinant lymphokine upon secretion. To assess the ability of recombinant BCG to stimulate cytokine production in a splenocyte population, mouse splenocytes were cultured together with wild-type or IL-2-producing BCG. IL-2-secreting BCG clones stimulated substantial increases in gamma interferon production, which could be reproduced by the addition of exogenous IL-2 to BCG. Levels of IL-6, IL-10, tumor necrosis factor alpha, and granulocyte-macrophage colony-stimulating factor were not significantly changed, while IL-4 and IL-5 remained undetectable (less than 50 pg/ml). The enhanced production of gamma interferon in response to IL-2-secreting BCG was strain independent. Recombinant BCG expressing mammalian cytokines provides a novel means to deliver cytokines and may augment the immunostimulatory properties of BCG in immunization and cancer therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Young R. A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991 Jun 6;351(6326):479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- Boom W. H., Wallis R. S., Chervenak K. A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991 Aug;59(8):2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Happ M. P., Dallas A., Reardon C., Kubo R., Shinnick T., Brennan P., O'Brien R. Recognition of heat shock proteins and gamma delta cell function. Immunol Today. 1990 Feb;11(2):40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- Carrier M. J., Chatfield S. N., Dougan G., Nowicka U. T., O'Callaghan D., Beesley J. E., Milano S., Cillari E., Liew F. Y. Expression of human IL-1 beta in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992 Feb 15;148(4):1176–1181. [PubMed] [Google Scholar]
- Champlin R., Hunter R. L. Studies on the composition of adjuvants which selectively enhance delayed-type hypersensitivity to lipid conjugated protein antigens. J Immunol. 1975 Jan;114(1 Pt 1):76–80. [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. The immunology of tuberculosis. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):42–49. doi: 10.1164/arrd.1982.125.3P2.42. [DOI] [PubMed] [Google Scholar]
- Colston M. J. BCG. Rebirth of a star performer. Nature. 1991 Jun 6;351(6326):442–443. doi: 10.1038/351442d0. [DOI] [PubMed] [Google Scholar]
- Croft M., Swain S. L. B cell response to T helper cell subsets. II. Both the stage of T cell differentiation and the cytokines secreted determine the extent and nature of helper activity. J Immunol. 1991 Dec 1;147(11):3679–3689. [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denich K., Börlin P., O'Hanley P. D., Howard M., Heath A. W. Expression of the murine interleukin-4 gene in an attenuated aroA strain of Salmonella typhimurium: persistence and immune response in BALB/c mice and susceptibility to macrophage killing. Infect Immun. 1993 Nov;61(11):4818–4827. doi: 10.1128/iai.61.11.4818-4827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Yoshikai Y., Matsuzaki G., Nomoto K. Early appearing gamma/delta-bearing T cells during infection with Calmétte Guérin bacillus. J Immunol. 1991 Apr 15;146(8):2754–2762. [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Barletta R. G., Udani R., Chan J., Kalkut G., Sosne G., Kieser T., Sarkis G. J., Hatfull G. F., Bloom B. R. Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science. 1993 May 7;260(5109):819–822. doi: 10.1126/science.8484123. [DOI] [PubMed] [Google Scholar]
- Kamijo R., Le J., Shapiro D., Havell E. A., Huang S., Aguet M., Bosland M., Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guérin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993 Oct 1;178(4):1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Lamm D. L. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992 Aug;19(3):573–580. [PubMed] [Google Scholar]
- Lotte A., Wasz-Höckert O., Poisson N., Dumitrescu N., Verron M., Couvet E. BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberc Res. 1984;21:107–193. [PubMed] [Google Scholar]
- Luelmo F. BCG vaccination. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):70–72. doi: 10.1164/arrd.1982.125.3P2.70. [DOI] [PubMed] [Google Scholar]
- Martin C. A., Dorf M. E. Interleukin-6 production by murine macrophage cell lines P388D1 and J774A.1: stimulation requirements and kinetics. Cell Immunol. 1990 Jul;128(2):555–568. doi: 10.1016/0008-8749(90)90048-v. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Terasaka K., Totsuka M., Kobayashi K., Yukitake H., Yamada T. Establishment of a foreign antigen secretion system in mycobacteria. Infect Immun. 1990 Dec;58(12):4049–4054. doi: 10.1128/iai.58.12.4049-4054.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight A. J., Mason D. W., Barclay A. N. Sequence of rat interleukin 2 and anomalous binding of a mouse interleukin 2 cDNA probe to rat MHC class II-associated invariant chain mRNA. Immunogenetics. 1989;30(2):145–147. doi: 10.1007/BF02421547. [DOI] [PubMed] [Google Scholar]
- Morales A., Nickel J. C. Immunotherapy for superficial bladder cancer. A developmental and clinical overview. Urol Clin North Am. 1992 Aug;19(3):549–556. [PubMed] [Google Scholar]
- Morton D. L., Foshag L. J., Hoon D. S., Nizze J. A., Famatiga E., Wanek L. A., Chang C., Davtyan D. G., Gupta R. K., Elashoff R. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg. 1992 Oct;216(4):463–482. doi: 10.1097/00000658-199210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray P. J., Young R. A. Stress and immunological recognition in host-pathogen interactions. J Bacteriol. 1992 Jul;174(13):4193–4196. doi: 10.1128/jb.174.13.4193-4196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott S., James K., Hargreave T. B., Chisholm G. D., Smyth J. F. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol. 1992 Jun;147(6):1636–1642. doi: 10.1016/s0022-5347(17)37668-1. [DOI] [PubMed] [Google Scholar]
- Ramshaw I., Ruby J., Ramsay A., Ada G., Karupiah G. Expression of cytokines by recombinant vaccinia viruses: a model for studying cytokines in virus infections in vivo. Immunol Rev. 1992 Jun;127:157–182. doi: 10.1111/j.1600-065x.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Schmitt D., Hayashi Y., Pollard R. B., Suzuki F. Induction of interleukin 3 and tumor resistance by SSM, a cancer immunotherapeutic agent extracted from Mycobacterium tuberculosis. Cancer Res. 1990 Jul 1;50(13):4032–4037. [PubMed] [Google Scholar]
- Scharton T. M., Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993 Aug 1;178(2):567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Lugosi L., Jekkel A., Melton R. E., Kieser T., Bloom B. R., Jacobs W. R., Jr Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6987–6991. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J. M. Rate of antigen catabolism and immunogenicity of [131-I]BGG in mice. I. Action of mycobacterial adjuvants. Immunology. 1970 Sep;19(3):449–456. [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Terasaka K., Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Yamada T. Complete nucleotide sequence of immunogenic protein MPB70 from Mycobacterium bovis BCG. FEMS Microbiol Lett. 1989 Apr;49(2-3):273–276. doi: 10.1016/0378-1097(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Walker K. B., Butler R., Colston M. J. Role of Th-1 lymphocytes in the development of protective immunity against Mycobacterium leprae. Analysis of lymphocyte function by polymerase chain reaction detection of cytokine messenger RNA. J Immunol. 1992 Mar 15;148(6):1885–1889. [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. Introduction of "natural" killer' cells by BCG. Nature. 1976 Aug 12;262(5569):584–586. doi: 10.1038/262584a0. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]