Abstract
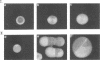
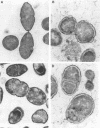
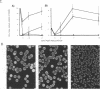
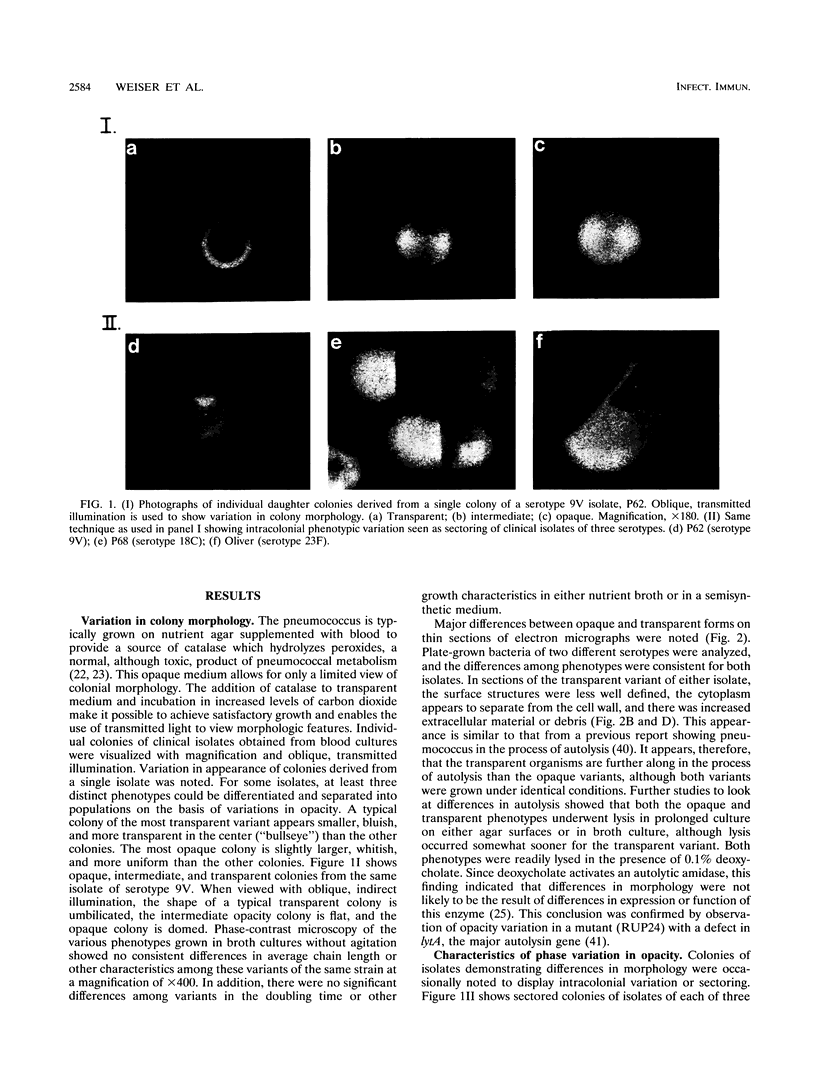
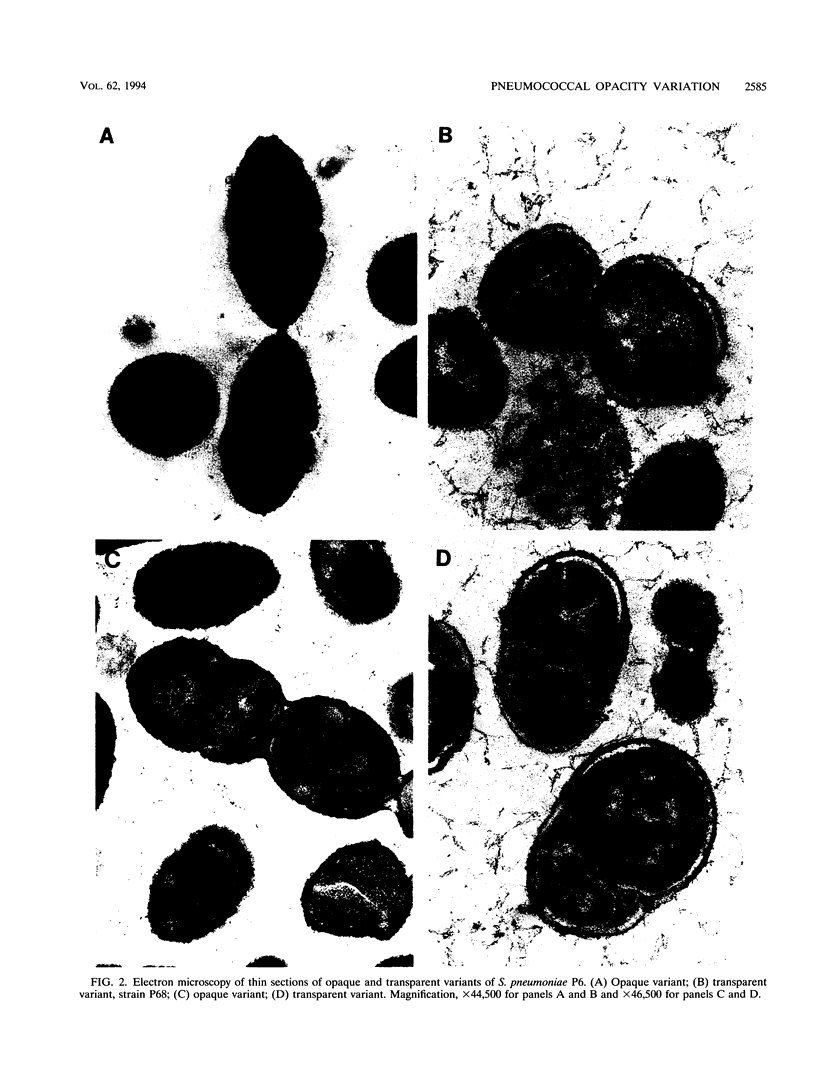
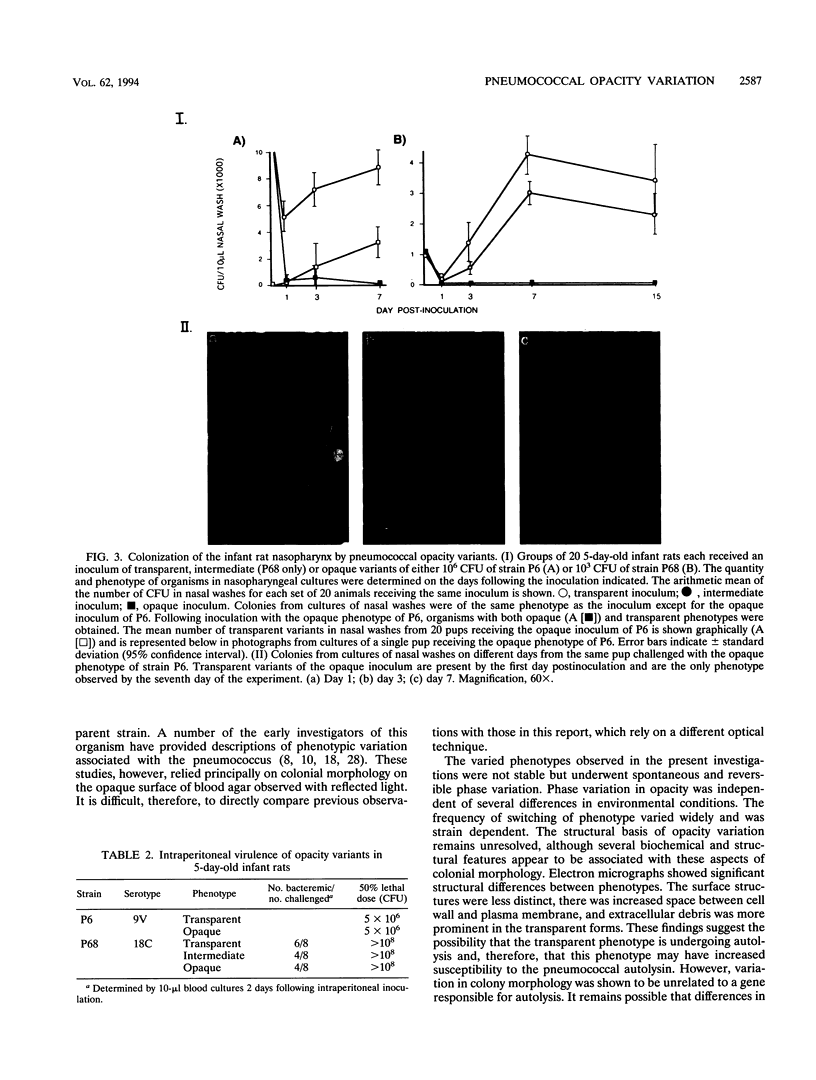
When colonies of encapsulated isolates of Streptococcus pneumoniae are viewed with oblique, transmitted light on a transparent surface, they are heterogeneous in appearance because of variation in opacity. There is spontaneous phase variation among at least three discernible phenotypes at frequencies from 10(-3) to 10(-6). The ability to detect differences in opacity varies according to serotype, but variation is independent of capsule expression. Electron microscopy shows no difference in chain length but suggests that autolysis occurs earlier in the growth of the transparent variant. There was no identifiable difference in membrane protein profiles of opaque and transparent variants of the same strain. In an infant rat model of nasopharyngeal carriage, there was no significant colonization by opaque variants. Efficient and stable colonization by the transparent variants was observed, suggesting a selective advantage for this phenotype in the nasopharynx. In contrast, there was no difference in the incidence of bacteremia or in the 50% lethal dose among the variants following their intraperitoneal inoculation. These results suggest that phase variation which is marked by differences in colonial morphology may provide insight into the interaction of the pneumococcus with its host.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R. Morphologic variation in pneumococcus. II. Control of pneumococcal morphology through transformation reactions. J Exp Med. 1953 Jul;98(1):35–40. doi: 10.1084/jem.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Dahmén J., Frejd T., Leffler H., Magnusson G., Noori G., Edén C. S. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med. 1983 Aug 1;158(2):559–570. doi: 10.1084/jem.158.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Eriksson B., Falsen E., Fogh A., Hanson L. A., Nylén O., Peterson H., Svanborg Edén C. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981 Apr;32(1):311–317. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R., Collins P. Importance of carbon dioxide in the isolation of pneumococci. J Bacteriol. 1966 Nov;92(5):1281–1284. doi: 10.1128/jb.92.5.1281-1284.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986 Jul;18 (Suppl A):35–45. doi: 10.1093/jac/18.supplement_a.35. [DOI] [PubMed] [Google Scholar]
- Finland M. Increased resistance in the pneumococcus. N Engl J Med. 1971 Jan 28;284(4):212–214. doi: 10.1056/NEJM197101282840411. [DOI] [PubMed] [Google Scholar]
- Geelen S., Bhattacharyya C., Tuomanen E. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect Immun. 1993 Apr;61(4):1538–1543. doi: 10.1128/iai.61.4.1538-1543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990 Apr;3(2):171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsch E. M., Knepper B., Kuroki T., Heuer I., Meyer T. F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993 Feb;12(2):641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- McCarty M. The nature of the opaque colony variation in group A streptococci. J Hyg (Lond) 1966 Jun;64(2):185–190. doi: 10.1017/s0022172400040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Musher D. M., Groover J. E., Rowland J. M., Watson D. A., Struewing J. B., Baughn R. E., Mufson M. A. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin Infect Dis. 1993 Jul;17(1):66–73. doi: 10.1093/clinids/17.1.66. [DOI] [PubMed] [Google Scholar]
- Paul J. R. Pneumococcus Variants: I. Intermediate Forms and the Influence of Environment in Their Production During In-vitro S to R and R to S Transitions. J Bacteriol. 1934 Jul;28(1):45–67. doi: 10.1128/jb.28.1.45-67.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce B. J., Yin Y. B., Masure H. R. Genetic identification of exported proteins in Streptococcus pneumoniae. Mol Microbiol. 1993 Sep;9(5):1037–1050. doi: 10.1111/j.1365-2958.1993.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Pincus S. H., Cole R. L., Kamanga-Sollo E., Fischer S. H. Interaction of group B streptococcal opacity variants with the host defense system. Infect Immun. 1993 Sep;61(9):3761–3768. doi: 10.1128/iai.61.9.3761-3768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. H., Cole R. L., Wessels M. R., Corwin M. D., Kamanga-Sollo E., Hayes S. F., Cieplak W., Jr, Swanson J. Group B streptococcal opacity variants. J Bacteriol. 1992 Jun;174(11):3739–3749. doi: 10.1128/jb.174.11.3739-3749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman G., Bornstein D. L., Austrian R. Capsulation of pneumococcus with soluble cell wall-like polysaccharide. II. Nonidentity of cell wall and soluble cell wall-like polysaccharides derived from the same and from different pneumococcal strains. J Exp Med. 1971 Sep 1;134(3 Pt 1):600–617. doi: 10.1084/jem.134.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Lopez R., Tomasz A. Cell surface-located deoxyribonucleic acid receptors in transformable pneumococci. J Bacteriol. 1975 Jun;122(3):1339–1350. doi: 10.1128/jb.122.3.1339-1350.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Guild W. R. A plasmid in Streptococcus pneumoniae. J Bacteriol. 1979 Feb;137(2):735–739. doi: 10.1128/jb.137.2.735-739.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., McCarty M. Electron microscopic studies on opaque colony variants of group A streptococci. J Bacteriol. 1969 Oct;100(1):505–511. doi: 10.1128/jb.100.1.505-511.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Moreillon P., Pozzi G. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J Bacteriol. 1988 Dec;170(12):5931–5934. doi: 10.1128/jb.170.12.5931-5934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Everson J. S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981 Mar;31(3):965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser J. N. Relationship between colony morphology and the life cycle of Haemophilus influenzae: the contribution of lipopolysaccharide phase variation to pathogenesis. J Infect Dis. 1993 Sep;168(3):672–680. doi: 10.1093/infdis/168.3.672. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Williams A., Moxon E. R. Phase-variable lipopolysaccharide structures enhance the invasive capacity of Haemophilus influenzae. Infect Immun. 1990 Oct;58(10):3455–3457. doi: 10.1128/iai.58.10.3455-3457.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]