Reversible protein phosphorylation is a ubiquitous posttranslational modification in all eukaryotes. It is critically involved in the regulation of nearly all cellular processes and signaling pathways. Protein kinases, the enzymes that catalyze the phosphotransfer reaction, constitute one of the largest protein families, accounting for approximately 2% of the genes in any given eukaryotic genome (122). Few of these kinases are constitutively active; unregulated activity would be deleterious or lethal to cells in the cases of most protein kinases. Cells have thus developed a variety of finely tuned mechanisms to precisely control the activities of these enzymes.
We aim here to characterize the regulatory mechanisms governing the activities of protein kinases in Saccharomyces cerevisiae on a genome-wide scale. We do not attempt to review comprehensively the substrates, target sequences, or downstream effects of these kinases. Using yeast as a model system to analyze the regulation of protein kinases on a global scale has advantages. Yeast expresses a limited number of protein kinases relative to metazoans, and the regulation of most yeast kinases has been characterized to some extent and in some cases in exquisite detail. However, the relative simplicity of the yeast kinase collection also presents a limitation: entire families of protein kinases found in other eukaryotes (for example, the receptor- and Src-like tyrosine kinases present in metazoans) are not represented in yeast (122). Nonetheless, reviewing the regulatory paradigms of kinases in yeast is a feasible and illustrative task.
Using yeast as a model, the information reviewed herein suggests that organisms utilize a finite number of regulatory paradigms in controlling their complement of kinases. In fact, this is very much a story of recurrent themes, with similar modes of regulation arising in disparate kinase families. While a cadre of regulatory motifs can be found controlling the activities of constituent members of nearly all evolutionary families of protein kinases, distinct patterns are readily apparent. For example, activating interactions with partner proteins (e.g., calmodulin, Cdc42, and cyclins) and phosphorylation within and outside the activation loop are common regulatory paradigms. Knowledge of regulatory motifs common to specific protein kinase families can be instructive in guiding experiments intended to ascertain the regulation of related, uncharacterized kinases.
NAMES, FAMILIES, AND PHYLOGENY
The enzymes whose mechanisms of regulation are reviewed here are those phosphotransferases that catalyze the transfer of the gamma phosphate from a nucleoside triphosphate (usually ATP) to the hydroxyl groups present in the serine, threonine, and tyrosine side chains of proteins. The yeast genome encodes 117 protein kinases in the superfamily of eukaryotic protein kinases (ePKs) and an additional 10 atypical kinases. Four atypical protein kinases show sequence similarity to phosphoinositide kinases (Tor1, Tor2, Tec1, and Mec1) but are known to phosphorylate proteins; two are related to microbial histidine kinase two-component signal transducers (Sln2 and Ypd1), and though they fail to meet our definition of a protein kinase, their function in signal transduction merits inclusion in this review; two (Pkp1 and YGL059W) are in the pyruvate dehydrogenase kinase (PDHK) family (96); two (Rio1 and Rio2) are in the RIO (right open reading frame) family of protein kinases (100). An additional six yeast proteins that have been classified as kinases will not be reviewed, either because the annotation (13) is now known to be incorrect (as in the case of Twf1) or because there is no experimental evidence to indicate that these proteins catalyze a phosphotransfer reaction (Abc1, YLR253W, YPL109C, Tra1, and Taf1).
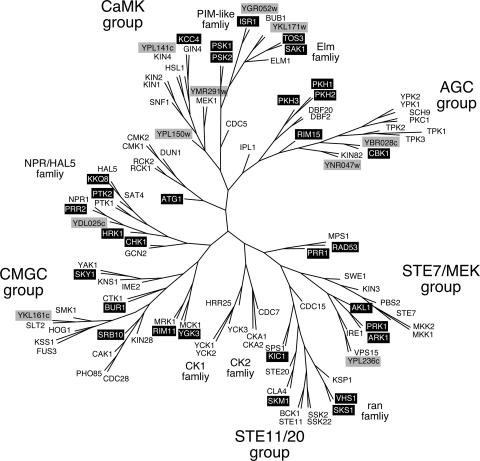
Hunter and Plowman (79) were the first to analyze the complete set of yeast protein kinases in Saccharomyces cerevisiae. In the 10 years since their publication, many of the hypothetical protein kinases that were encoded by uncharacterized open reading frames have been studied and named. Similarly, some kinases have had their names changed for a variety of reasons. In the Hunter and Plowman study, an unrooted dendrogram of 112 yeast kinase domains was constructed using a protein sequence parsimony method (79). We show here a revised version of this dendrogram that has been updated to contain the names currently used for these enzymes (Fig. 1). Thirty-one kinases (27%) have been named or renamed; only 10 remain as kinases encoded by uncharacterized open reading frames. Also in the intervening years, complete genomic sequence data from other eukaryotes have allowed comparison of kinase families across species (122). The study of Manning et al. (122) makes clear that S. cerevisiae completely lacks some families of protein kinases, notably the receptor- and Src-like tyrosine kinases, and contains others that appear in other fungi but are not present in metazoans. More sophisticated analyses of protein kinase phylogeny using hidden Markov models, PSI-BLAST, and homology-based gene predictions have been used to classify the groups, families, and subfamilies of eukaryotic kinases (122). We have used this more recent analysis as the source of our classification of the yeast kinases. Clearly, the present study has led to a reclassification of some of the yeast protein kinases. The discerning reader will note that the classification of some kinases in Tables 1 and 2 may not agree with their positions in the earlier dendrogram (Fig. 1). We do not attempt to resolve these few discrepancies. The focus of this review is not the classification of kinases but rather their mechanisms of regulation.
FIG. 1.
Unrooted dendrogram of 112 protein kinases of Saccharomyces cerevisiae. Kinases that have been named or renamed since this dendrogram was first published (79) are shown in white text over black rectangles. Kinases that remain uncharacterized are shown in black text over gray rectangles. The major groups and families of protein kinases are shown in larger text. Not shown in this dendrogram are Alk1, Alk2, Bud32, Iks1, and Scy1. (Modified from Fig. 1 of reference 79 with permission of the publisher.).
TABLE 1.
Mechanisms of kinase regulationa
| Gene | ORF | Group | Family | Subfamily | Mechanism(s) of regulationb (reference[s] and/or source) |
|---|---|---|---|---|---|
| Sch9 | YHR205W | AGC | AKT | A2 (180) | |
| Ypk1 | YKL126W | AGC | AKT | A2 (26, 37, 180) | |
| Ypk2 | YMR104C | AGC | AKT | A2 (26, 37, 180) | |
| Cbk1 | YNL161W | AGC | NDR | D1 (232) | |
| Rim15 | YFL033C | AGC | NDR | B2 (175, 212, 229); G (212, 229) | |
| Dbf2 | YGR092W | AGC | NDR | A2 (120); D1 (120); G (223) | |
| Dbf20 | YPR111W | AGC | NDR | U | |
| Pkh1 | YDR490C | AGC | PDK1 | E1 (112) | |
| Pkh2 | YOL100W | AGC | PDK1 | E1 (112) | |
| Pkh3 | YDR466W | AGC | PDK1 | U | |
| Tpk1 | YJL164C | AGC | PKA | D2 (87, 174); E1 (87) | |
| Tpk2 | YPL203W | AGC | PKA | D2 (87, 174); E1 (87) | |
| Tpk3 | YKL166C | AGC | PKA | D2 (87, 174); E1 (87) | |
| Pkc1 | YBL105C | AGC | PKC | A2 (80, 180); D1 (91); E1 (91); G (38) | |
| YBR028C | AGC | RSK | p70 | U | |
| Kin82 | YCR091W | AGC | RSK | U | |
| YNR047W | AGC | RSK | U | ||
| Cmk1 | YFR014C | CaMK | CaMK1 | D1 (152, 159); E1 (152, 159) | |
| Cmk2 | YOL016C | CaMK | CaMK1 | D1 (152, 159); E1 (152, 159) | |
| Rck1 | YGL158W | CaMK | CaMK1 | U | |
| Rck2 | YLR248W | CaMK | CaMK1 | B1 (17, 216); D2 (134); F (173, 211) | |
| Snf1 | YDR477W | CaMK | CaMKL | AMPK | A2 (76, 130, 144, 208); C2 (130); D1 (191); D2 (86, 104); G (74, 75, 221) |
| Chk1 | YBR274W | CaMK | CaMKL | CHK1 | B1 (3) |
| Gin4 | YDR507C | CaMK | CaMKL | NMR | A2 (K. Elbing and M. C. Schmidt, unpublished data); B1 (7); D1 (11, 41, 66, 142); G (155) |
| Hsl1 | YKL101W | CaMK | CaMKL | NMR | A2 (K. Elbing and M. C. Schmidt, unpublished data); B2 (31); D1 (11, 66); D2 (70); F (25); G (31, 138) |
| Kcc4 | YCL024W | CaMK | CaMKL | NMR | A2 (K. Elbing and M. C. Schmidt, unpublished data); D1 (11, 66, 154); G (154) |
| Kin1 | YDR122W | CaMK | CaMKL | Kin1 | D1 (45); D2 (45) |
| Kin2 | YLR096W | CaMK | CaMKL | Kin1 | D1 (45); D2 (45) |
| Kin4 | YOR233W | CaMK | CaMKL | Kin4 | G (35) |
| YPL141C | CaMK | CaMKL | Kin4 | U | |
| YPL150W | CaMK | CaMKL | MARK | U | |
| Psk1 | YAL017W | CaMK | CaMKL | PASK | D1 (185) |
| Psk2 | YOL045W | CaMK | CaMKL | PASK | D1 (185) |
| YKL171W | CaMK | Unique | U | ||
| Mek1 | YOR351C | CaMK | Unique | D1 (148, 225) | |
| Prr1 | YKL116C | CaMK | Unique | U | |
| YMR291W | CaMK | Unique | U | ||
| Dun1 | YDL101C | CaMK | RAD53 | A2 (28); B1 (12); D1 (12, 103) | |
| Rad53 | YPL153C | CaMK | RAD53 | A1 (117); B1 (103) | |
| Hrr25 | YPL204W | CK1 | CK1 | CK1-D | D1 (162); G (219) |
| Yck2 | YNL154C | CK1 | CK1 | CK1-G | C1 (60); G (9, 219) |
| Yck3 | YER123W | CK1 | CK1 | CK1-G | G (206) |
| Yck1 | YHR135C | CK1 | CK1 | CK1-G | C1 (60); D1 (139); G (219) |
| Cdc28 | YBR160W | CMGC | CDK | CDC2 | A2 (49, 183); B2 (19); C1 (110, 184); C2 (29); D1 (39, 63, 65, 207); D2 (135, 151); G (136) |
| Pho85 | YPL031C | CMGC | CDK | CDK5 | B1 (147); D1 (51, 77, 132, 133, 228); D2 (78, 192) |
| Kin28 | YDL108W | CMGC | CDK | CDK7 | A2 (50, 94); D1 (93, 210) |
| Srb10 | YPL042C | CMGC | CDK | CDK8 | D1 (98, 108) |
| Ctk1 | YKL139W | CMGC | CDK | CRK7 | A2 (157); D1 (73, 203) |
| Cak1 | YFL029C | CMGC | CDK | F (54, 224); H (89, 209) | |
| Bur1 | YPR161C | CMGC | CDK | A2 (242); D1 (241) | |
| Cka1 | YIL035C | CMGC | CK2 | D1 (15, 97) | |
| Cka2 | YOR061W | CMGC | CK2 | D1 (97) | |
| Kns1 | YLL019C | CMGC | CLK | H (101) | |
| Yak1 | YJL141C | CMGC | DYRK | YAK | A1 (92); B2 (125, 245); F (200); G (125, 140) |
| Mck1 | YNL307C | CMGC | GSK | A1 (109) | |
| Mrk1 | YDL079C | CMGC | GSK | U | |
| Rim11 | YMR139W | CMGC | GSK | A1 (247) | |
| Ygk3 | YOL128C | CMGC | GSK | U | |
| Fus3 | YBL016W | CMGC | MAPK | ERK | A2 (47, 62); C2 (246); D1 (30, 57, 95, 124); G (18, 30, 169) |
| Hog1 | YLR113W | CMGC | MAPK | ERK | A2 (23); C2 (123, 128, 230, 239, 243); G (55) |
| Kss1 | YGR040W | CMGC | MAPK | ERK | A2 (62, 116) |
| Slt2 | YHR030C | CMGC | MAPK | ERK | A2 (127); C2 (32, 56, 69, 129); D1 (137); G (220) |
| Smk1 | YPR054W | CMGC | MAPK | ERK | A2 (187); F (163) |
| YKL161C | CMGC | MAPK | ERK | U | |
| Ime2 | YJL106W | CMGC | RCK | A1 (189); A2 (188); D2 (43); F (22, 24, 68, 168) | |
| Sky1 | YMR216C | CMGC | SRPK | H (150) | |
| Ipl1 | YPL209C | Other | AUR | A1 (52); G (16) | |
| Bub1 | YGR188C | Other | BUB | D1 (179); G (64, 179) | |
| Bud32 | YGR262C | Other | Bud32 | A1 (53) | |
| Elm1 | YKL048C | Other | CaMKK | ELM | D1 (183); G (21) |
| Sak1 | YER129W | Other | CaMKK | ELM | D1 (183) |
| Tos3 | YGL179C | Other | CaMKK | ELM | D1 (183) |
| Cdc7 | YDL017W | Other | CDC7 | D1 (44, 156) | |
| Npr1 | YNL183C | Other | HAL | B2 (190); C1 (82) | |
| Ptk1 | YKL198C | Other | HAL | U | |
| Hrk1 | YOR267C | Other | HAL | U | |
| Hal5 | YJL165C | Other | HAL | U | |
| Kkq8 | YKL168C | Other | HAL | U | |
| Prr2 | YDL214C | Other | HAL | U | |
| Ptk2 | YJR059W | Other | HAL | U | |
| Sat4 | YCR008W | Other | HAL | U | |
| YDL025C | Other | HAL | U | ||
| Alk1 | YGL021W | Other | Haspin | F (146) | |
| Alk2 | YBL009W | Other | Haspin | F (146) | |
| Iks1 | YJL057C | Other | IKS | U | |
| Ire1 | YHR079C | Other | IRE | A1 (197); C2 (67); D1 (197) D2 (153) | |
| Akl1 | YBR059C | Other | NAK | U | |
| Ark1 | YNL020C | Other | NAK | G (33) | |
| Prk1 | YIL095W | Other | NAK | G (33) | |
| YPL236C | Other | NAK | U | ||
| Kin3 | YAR018C | Other | NEK | U | |
| Isr1 | YPR106W | Other | Unique | U | |
| YGR052W | Other | Unique | U | ||
| Gcn2 | YDR283C | Other | PEK | GCN2 | D1 (42, 61); D2 (170); E1 (171) |
| Cdc5 | YMR001C | Other | PLK | A2 (141); G (6, 201) | |
| Sks1 | YPL026C | Other | RAN | U | |
| Ksp1 | YHR082C | Other | RAN | G (58) | |
| Vhs1 | YDR247W | Other | RAN | U | |
| Scy1 | YGL083W | Other | SCY1 | U | |
| Mps1 | YDL028C | Other | TTK | B1 (83); D1 (193); F (83) | |
| Atg1 | YGL180W | Other | ULK | B1 (227); B2 (90, 227); D1 (90) | |
| Vps15 | YBR097W | Other | VPS15 | D1 (199, 202) | |
| Swe1 | YJL187C | Other | WEE | B1 (72); B2 (6, 186); D2 (198); F (6, 88, 131, 186) | |
| Bck1 | YJL095W | STE | STE11 | A2 (36, 102) | |
| Ssk2 | YNR031C | STE | STE11 | A1 (164); D1 (164); G (244) | |
| Ssk22 | YCR073C | STE | STE11 | A1 (164); D1 (164) | |
| Ste11 | YLR362W | STE | STE11 | A2 (45, 165); D1 (1, 71, 167, 236); F (48); G (218, 235) | |
| Cdc15 | YAR019C | STE | STE20 | MST | C1 (84, 240); D1 (5, 178); D2 (10); G (27, 223) |
| Cla4 | YNL298W | STE | STE20 | PAK | D1 (14, 20, 217); D2 (126); E1 (233); G (186) |
| Skm1 | YOL113W | STE | STE20 | PAK | D2 (126) |
| Ste20 | YHL007C | STE | STE20 | PAK | B1 (237); D1 (8, 99, 105, 161, 234); D2 (59, 99); G (4, 8, 237) |
| Kic1 | YHR102W | STE | STE20 | YSK | D1 (205) |
| Sps1 | YDR523C | STE | STE20 | YSK | F (160) |
| Mkk1 | YOR231W | STE | STE7 | A2 (81) | |
| Mkk2 | YPL140C | STE | STE7 | A2 (81) | |
| Pbs2 | YJL128C | STE | STE7 | A2 (118); D1 (71, 118, 165, 215); G (172, 177) | |
| Ste7 | YDL159W | STE | STE7 | A2 (145); D1 (194); F (226) | |
| Sln1 | YIL147C | Atypical | HisK | B1 (166); E1 (119, 214) | |
| Ypd1 | YDL235C | Atypical | HCPT | B1 (166); G (115) | |
| YGL059W | Atypical | PDHK | U | ||
| Pkp1 | YIL042C | Atypical | PDHK | U | |
| Tel1 | YBL088C | Atypical | PIKK | ATM | G (111, 213) |
| Mec1 | YBR136W | Atypical | PIKK | ATR | D1 (143, 182); G (213) |
| Tor1 | YJR066W | Atypical | PIKK | FRAP | D1 (176); G (107, 231) |
| Tor2 | YKL203C | Atypical | PIKK | FRAP | D1 (238); G (231) |
| Rio1 | YOR119C | Atypical | RIO | RIO1 | U |
| Rio2 | YNL207W | Atypical | RIO | RIO2 | U |
aAKT, kinase from the AKT8 retrovirus; AMPK, AMP-activated protein kinase; ATM, ataxia-telangiectasia mutated; ATR, ATM-Rad3 related; AUR, aurora; BUB, budding uninhibited by benomyl; CaMKK, CaMK kinase; CaMKL, CaMK like; CK1, casein kinase 1; CK1-D, casein kinase 1 delta; CK1-G, casein kinase 1 gamma; CK2, casein kinase 2; CLK, CDK-like kinase; ELM, elongated morphology; ERK, extracellular signal-regulated kinase; FRAP, FKBP-rapamycin associated protein; HAL, halotolerance; Haspin, haploid germ cell-specific nuclear protein kinase; HCPT, histidine-containing phosphotransmitter; HisK, histidine kinase; IRE, inositol required; MARK, microtubule affinity-regulating kinase; MST, mammalian STE20-like protein kinase; NAK, NF-κ B-activating kinase; NDR, nuclear Dbf2 related; NEK, NIMA-related kinase; NMR, NIM related; PEK, pancreatic eIF-2a kinase; PIKK, phosphoinositide-3-kinase-related protein kinase; PLK, polo-like kinase; RAN, RAN (nuclear import/export) related; RCK, radiation sensitivity-complementing kinase; RSK, ribosomal S6 kinase; SRPK, serine/arginine-rich protein-specific kinase; TTK, dual-specificity protein kinase; ULK, UNC-51-like kinase; WEE, Schizosaccharomyces pombe wee1 homolog; YAK, yet another kinase.
See Table 2 for mechanism abbreviations.
TABLE 2.
Kinase regulatory themes
| Regulatory mechanisma | Theme(s) for indicated kinase group
|
||||||
|---|---|---|---|---|---|---|---|
| AGC | CaMK | CMGC | STE | CK1b | Other | Atypical | |
| A. Activation loop phosphorylation | |||||||
| 1. Autophosphorylation | Rad53 | Mck1, Ime2, Rim11, Yak1 | Ssk2, Ssk22 | Bud32, Ipl1, Ire1 | |||
| 2. Upstream kinase | Dbf2, Pkc1, Sch9, Ypk1, Ypk2 | Dun1, Gin4, Hsl1, Kcc4, Snf1 | Bur1, Cdc28, Ctk1, Fus3, Hog1, Ime2, Kin28, Kss1, Slt2, Smk1 | Bck1, Ste11, Mkk1, Mkk2, Pbs2, Ste7 | Cdc5 | ||
| B. Phosphorylation outside loop | |||||||
| 1. Activating | Chk1, Dun1, Gin4, Rad53, Rck2 | Pho85 | Ste20 | Atg1, Mps1, Swe1 | Sln1, Ypd1 | ||
| 2. Inactivating | Rim15 | Hsl1 | Cdc28, Yak1 | Atg1, Npr1, Swe1 | |||
| C. Dephosphorylation | |||||||
| 1. Activating | Cdc28, | Cdc15 | Yck1, Yck2 | Npr1 | |||
| 2. Inactivating | Snf1 | Cdc28, Fus3, Hog1, Slt2 | Ire1 | ||||
| D. Protein binding | |||||||
| 1. Activating | Cbk1, Dbf2, Pkc1 | Cmk1, Cmk2, Dun1, Gin4, Hsl1, Kcc4, Kin1, Kin2, Mek1, Psk1, Psk2, Snf1 | Bur1, Cdc28, Cka1, Cak2, Ctk1, Fus3, Kin28, Pho85, Slt2, Srb10 | Cdc15, Cla4, Kic1, Pbs2, Ssk2, Ssk22, Ste7, Ste11, Ste20 | Hrr25, Yck1 | Atg1, Bub1, Cdc7, Elm1, Gcn2, Ire1, Mps1, Sak1, Tos3, Vps15 | Mec1, Tor1, Tor2 |
| 2. Inactivating | Tpk1, Tpk2, Tpk3 | Hsl1, Kin1, Kin2, Rck2, Snf1 | Cdc28, Ime2, Pho85 | Cdc15, Cla4, Skm1, Ste20 | Gcn2, Ire1, Swe1 | ||
| E. Binding nonprotein ligands | |||||||
| 1. Activating | Pkc1, Pkh1, Pkh2, Tpk1, Tpk2, Tpk3 | Cmk1, Cmk2 | Cla4 | Gcn2 | Sln1 | ||
| 2. Inactivating | |||||||
| F. Accumulation | Hsl1, Rck2 | Cak1, Ime2, Smk1, Yak1 | Ste7, Ste11, Sps1 | Alk1, Alk2, Mps1, Swe1 | |||
| G. Localization | Dbf2, Pkc1, Rim15 | Gin4, Hsl1, Kcc4, Kin4, Snf1 | Cdc28, Fus3, Hog1, Slt2, Yak1 | Cdc15, Cla4, Pbs2, Ssk2, Ste11, Ste20 | Hrr25, Yck1, Yck2, Yck3 | Ark1, Bub1, Cdc5, Elm1, Ipl1, Ksp1, Prk1 | Mec1, Tel1, Tor1, Tor2, Ypd1 |
| H. Constitutive | Cak1, Kns1, Sky1 | ||||||
| U. Unknown | Dbf20, Kin82, Pkh3, YBR028C, YNR047W | Prr1, Rck1, Ygk3, YKL171W, YMR291W, YPL141C, YPL150W | Mrk1, Ygk3, YKL161C | Akl1, Hal5, Hrk1, Kin3, Kkq8, Iks1, Isr1, Prr2, Ptk1, Ptk2, Sat4, Scy1, Sks1, Vhs1, YDL025C, YGR052W, YPL236C | Pkp1, Rio1, Rio2, YGL059W | ||
The letters and numbers designating the regulatory mechanisms are used in Table 1.
CK1, casein kinase 1.
MECHANISMS OF KINASE REGULATION
We have organized what is known about the regulation of protein kinases in Saccharomyces cerevisiae in two different fashions. In Table 1, the kinases are sorted by phylogenetic group, family, and subfamily. The regulatory mechanism(s) for each kinase is shown along with the appropriate citation(s). Table 2 “plots” the regulatory mechanism by phylogenetic group in a manner that allows visualization of the distribution of mechanisms controlling catalytic activity among the evolutionary groups. Every kinase can be found in both tables. The mechanisms listed in Table 1 are shown as abbreviations based on the schema in Table 2. For instance, activation loop autophosphorylation is shown in Table 1 as A1. The information included in Tables 1 and 2 reflects only that which has been specifically published regarding each yeast kinase. For the data in Tables 1 and 2, we have made no inferences from what is known about the regulation of related kinases in yeast or in other species. We have attempted to create a snapshot of the state of knowledge in the field as it stands. Our knowledge of protein kinase regulation is itself an evolving entity. Clearly, what is currently documented about any given kinase is not all that will ever be known about that particular kinase. Similarly, the mechanisms regulating uncharacterized or poorly characterized protein kinases will one day be uncovered.
The mechanisms regulating the activities of protein kinases in Saccharomyces cerevisiae can be divided into seven major categories: phosphorylation within the activation loop, phosphorylation outside the activation loop, dephosphorylation, protein binding, binding of nonprotein ligands, protein accumulation, and subcellular localization. Of these, several can be further divided into subcategories. A few kinases appear to be unregulated with constitutive activity (for example, Cak1), while many others (55 kinases) are regulated in complex manners, involving more than one regulatory modality. For example, Cdc28 is subject to at least seven distinct mechanisms for its regulation.
Activation loop phosphorylation.
One of the most well-characterized mechanisms by which protein kinases are activated is phosphorylation of the activation loop (also called the T loop), the flexible polypeptide segment that connects the N and C lobes of the kinase domain (149). Activation loops are typically 20 to 35 residues long and bounded by the conserved residues DFG at the segment's N terminus and APE at the C terminus. The sequence of the loop itself is less well conserved but often contains one or two conserved phosphorylatable residues. The conformation of the activation loop relative to the kinase domain changes with its phosphorylation status. The conformational shift controls the activity state of the kinase by either relieving the steric hindrance of the substrates to the active site, aligning the catalytic residues, or both (2, 149).
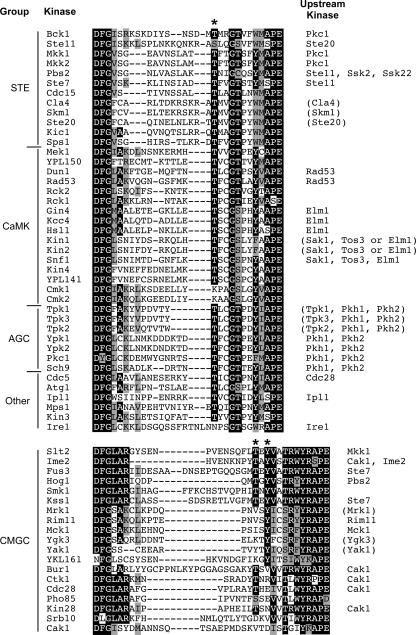
Two alignments of the activation loops from 58 yeast kinases are shown in Fig. 2. The kinases are arranged by group. The top alignment contains representatives from four of the six groups of ePKs present in yeast. The position of the conserved phosphorylated threonine residue is indicated. The lower alignment shows selected members of the CMGC group (a kinase family that includes cyclin-dependent kinases [CDKs], mitogen-activated protein kinases [MAPKs], glycogen synthase kinases [GSKs], and CDK-like kinases), including the MAPKs and CDKs that are phosphorylated on threonine, tyrosine, or both residues and contain a slightly different spacing of the phosphorylated site(s). Only 37 of 117 yeast protein kinases in the ePK family are currently known to be activated by phosphorylation of one or more critical residues within the activation loop (Table 2). However, sequence conservation suggests that many more kinases may ultimately be added to this list. Examination of the alignment of these activation loop sequences allows several predictions to be made. First, many kinases lack the conserved phosphorylatable residues in their loops and are probably not regulated by activation loop phosphorylation. For instance, the yeast calcium/calmodulin-activated kinases (CaMKs) (Cmk1 and Cmk2) lack the conserved target sites in their loops (Fig. 2) even though orthologues from other species contain the conserved sites and are known to be activated by upstream kinases (196). Of the six MAPKs in yeast, one, YKL161C, is uncharacterized and is the only MAPK in yeast to lack the conserved threonine in its activation loop. Most MAPKs are phosphorylated at two nearby sites in the activation loop sequence TXY. YKL161C has the sequence KGY at this position, suggesting that its regulation is likely to be different from those of the other MAPKs. Likewise, Srb10 is a CDK that lacks the conserved threonine that is present in the activation loops of the other CDKs, suggesting that Srb10 may not be regulated by activation loop phosphorylation. Experiments with Srb10 and a temperature-sensitive Cak1 support this idea (50). However, sequence analysis alone is not always sufficient for accurate prediction. Pho85, another CDK, contains potential phosphorylation sites; yet, they are not required for activation (147). Conversely, Bud32 lacks the conserved phosphorylation sites in its unusually small activation loop and yet it is activated by activation loop autophosphorylation (53).
FIG. 2.
Multiple sequence alignments of kinase activation loops. Activation loops were selected to highlight the presence or absence of the conserved phosphorylation sites (indicated by asterisks). The upstream kinase(s) that phosphorylates the activation loop is shown on the right. Upstream kinases predicted from results for other species or from paralogues are shown within parentheses.
While tyrosine phosphorylation is relatively rare in S. cerevisiae, one site where it does occur more frequently is in the activation loops of kinases in the CMGC group (Fig. 2). The MAPKs are typically phosphorylated on both threonine and tyrosine residues in the TXY motif. This reaction is carried out by their respective MAPK kinases, which are capable of phosphorylating both residues (23, 47, 127). Kinases in the dual-specificity tyrosine phosphorylation-regulated protein kinase (DYRK) and GSK families are autophosphorylated on a single tyrosine residue in their activation loops. Mck1 and Rim11, two of the four yeast GSKs, have been shown to autophosphorylate on activation loop tyrosine residues (109, 247). Recent work with metazoan DYRKs and GSKs has illuminated the mechanism by which serine/threonine kinases can autophosphorylate tyrosine residues. These protein kinases are maintained during translation in an intermediate, metastable conformation by protein chaperones. In this state, they autophosphorylate their activation loop tyrosine residues in cis. Following the completion of translation and tyrosine autophosphorylation, these kinases adopt their mature and more stable conformation such that their substrate specificities become restricted to serine and threonine residues (113, 114). These studies predict that the two remaining yeast GSKs, Mrk1 and Ygk3, as well as the yeast DYRK, Yak1, will be similarly regulated.
Phosphorylation outside the activation loop.
Many kinases are regulated by phosphorylation at sites outside their activation loops. In contrast to activation loop phosphorylation, which is always activating, phosphorylation outside the activation loop can either stimulate or inhibit a kinase. The morphogenetic checkpoint illustrates two salient examples of this type of regulation. The Swe1 kinase phosphorylates Tyr19 of Cdc28, inactivating the kinase activity of Cdc28 needed for cell cycle progression. Swe1-mediated phosphorylation declines when Swe1 protein at the bud neck is itself phosphorylated by Cdc5 and Cla4 (186) as well as by Cdc28 (6, 72, 131), leading to its ubiquitination and subsequent proteolysis. This one regulatory pathway shows two examples of inactivating phosphorylation events, one causing reduced kinase catalytic activity and the other leading to degradation. Examples of activating phosphorylation events outside the loop include those that promote binding of other proteins (12) or counter autoinhibition (17).
Dephosphorylation.
For those studying the regulation of protein kinases, it is not difficult to develop (even unwittingly) a kinase-centric worldview, whereby the phosphorylation events regulating pathways and enzymes are analyzed in terms of phosphate addition alone. In reality, the phosphorylation statuses of most substrates are a reflection of the equilibrium of phosphate addition by a kinase and phosphate removal by a phosphatase. At the moment, kinases are better studied than are phosphatases. Indeed, in analysis of phosphorylation sites, the responsible kinase has been identified in many more cases than has the phosphatase. Nonetheless, the dephosphorylation of protein kinases has regulatory consequences and can be either activating or inactivating. The CDK Cdc28 is an excellent example of both forms of regulation. Cdc28 activation requires both the phosphorylation of its activation loop by Cak1 (89) and the dephosphorylation of the Tyr19 site in its N terminus by the dual-specificity phosphatase Mih1 (184).
Protein binding.
Protein-protein interaction is another major regulatory motif controlling the activities of protein kinases. Members of each major group of protein kinases are regulated by interaction of the kinase domain with other protein domains in either cis or trans. Association with protein binding partners can modulate the activation states of kinases in multiple ways. Elegant structural studies have elucidated the manner by which protein binding dictates the activities of several kinases.
Crystallographic studies have illuminated the mechanism by which the mammalian p21-activated kinases (PAKs) are activated by physical association with GTP-bound p21 proteins (106, 158). Binding of p21 shifts a mammalian PAK from a homodimeric autoinhibited conformation to an active form capable of trans-autophosphorylation. In Saccharomyces cerevisiae, three sterile (STE) group constituents are PAK family members: Cla4, Skm1, and Ste20. Two of the three, Cla4 and Ste20, have been shown to be activated by association with p21 family member Cdc42. Skm1 contains a highly homologous N-terminal autoinhibitory domain and is predicted to be similarly activated. Based on the regulation of their metazoan orthologues, we predict that binding of Cdc42 to the yeast PAKs promotes trans-autophosphorylation of the conserved threonine residues in their activation loops (Fig. 2). Another example of a kinase being activated by the binding of a small GTP-binding protein is Pkc1. Binding of the GTP-bound form of Rho1 to Pkc1 enables Pkc1 to respond to activating cofactors (91). This regulatory mechanism makes the yeast Pkc1 similar to the mammalian PRK2 kinase, which is activated by Rho binding (222).
Another well-characterized example of regulation by physical association with protein binding partners is the activation of CDKs by their cognate cyclins. Again, X-ray crystal structures with a mammalian CDK and cyclin pair serve as a paradigm for understanding the mechanism by which physical association of cyclin with CDK results in kinase activation (85). Cyclin binding induces the proper alignment of active site residues such that they are catalysis competent. Additionally, the activation loop, once crisscrossing and occluding the active site, shifts position upon association with cyclin, relieving steric hindrance to ATP entry as well as becoming available for phosphorylation by a CDK-activating kinase. Yeast encodes multiple CDKs (Cdc28, Pho85, Srb10, Kin28, and Bur1) that are activated by interaction with one or multiple cyclin partners. Additionally, Ctk1 activation requires interaction with the cyclin-related protein Ctk2 (73, 203).
In addition to activating interactions, some protein-protein interactions lead to inhibition of kinase activity. Some kinases, Rck2 and Snf1 for example, contain autoinhibitory domains within the kinase polypeptide but outside the kinase domain. Though the molecular mechanism of the inhibition is not yet known, the inhibitory effects of these domains can be overcome by phosphorylation in the case of Rck2 (17) or additional protein-protein interactions in the case of Snf1 (86). In the case of the cyclic AMP-dependent protein kinase, the association of the regulatory subunit places a pseudosubstrate peptide in the active site of the catalytic subunit, thereby blocking substrate access (40, 204). Whether other inhibitory interactions also involve a pseudosubstrate mechanism remains to be determined.
Binding nonprotein ligands.
While many kinases are controlled by their interactions with other proteins or protein domains, a few are regulated by binding nonprotein ligands. In all documented cases in yeast, these interactions stimulate the activities of the respective kinases. Though the biochemical mechanism is not yet clear, the sphingoid long-chain base phytosphingosine activates kinase Pkh1 of the AGC group (a kinase family that includes protein kinase A [PKA], PKG, and PKC) (112). Cyclic AMP binding of the PKA regulatory subunit Bcy1 results in its dissociation from and subsequent activation of redundant catalytic subunits Tpk1, Tpk2, and Tpk3 (87). The related proteins Cmk1 and Cmk2 are both activated by interaction with calmodulin protein complexed with four calcium ions (152, 159). In addition to interaction with Cdc42 (discussed above), Cla4 requires association with the plasma membrane lipid phosphatidylinositol 4-phosphate (PI4P) via its pleckstrin homology domain for its role in regulating cellular morphogenesis and the mitotic exit network (233). The protein kinase Gcn2 senses and is activated by amino acid starvation by virtue of binding uncharged tRNA molecules (171). In addition, the PAS domain kinases (PASKs), Psk1 and Psk2, as well as Snf1 may someday be added to the list of kinases regulated by ligands since these kinases (or associated subunits) have domains that are known to bind ligands in other systems (34, 195).
Accumulation.
Kinases are also regulated by management of protein accumulation. Hsp90 and its cochaperone Cdc37 play an important role in the folding and accumulating of many if not most yeast protein kinases (121). The interaction of kinases with chaperones may also regulate kinase activity by controlling the transition between active and inactive conformations (1, 42, 63). The abundance of specific kinases may be modulated by changes in expression at the level of mRNA, protein, or both. We have made no attempt to review or assess the volumes of microarray data. Here, we have limited our review to kinases whose abundance has been studied individually. For instance, Smk1 is required for spore morphogenesis, and its mRNA expression is induced during sporulation (163). Ste7 (226) and Swe1 (25), by contrast, are regulated at the level of protein stability via the ubiquitin degradation pathway.
Localization.
The final paradigm of kinase regulation is subcellular localization. Like protein accumulation, this mechanism does not necessarily involve a change in intrinsic catalytic activity but serves to position the enzyme at the right place and time to perform its respective function. The protein kinase Elm1 is localized to the bud neck in its role in regulating the morphogenetic checkpoint; mutations that misdirect the subcellular localization of Elm1 prevent Elm1 from phosphorylating critical substrates, resulting in aberrantly elongated morphology (21). In another example of regulated subcellular localization, PKA-dependent phosphorylation of Rim15 (outside its activation loop) tethers Rim15 in the cytoplasm by promoting association with 14-3-3 proteins Bmh1 and Bmh2. Upon dephosphorylation, Rim15 dissociates from the 14-3-3s and translocates to the nucleus, whence it initiates the G0 program (229). Many more examples of kinases that are regulated by their localization will no doubt be uncovered in the future.
CONCLUSIONS
Organizing the protein kinases of Saccharomyces cerevisiae by regulatory mechanisms provides a useful genome-wide perspective on how these enzymes are controlled. While some regulatory motifs are represented more heavily in particular groups and families, there is an otherwise broad distribution of mechanisms across the phylogenetic spectrum. This review highlights the wealth of research that has been conducted to understand how protein kinases are regulated. It also makes clear that there is still much work to be done. Currently, no regulatory mechanism has been reported for 36 yeast kinases. However, for several of these uncharacterized enzymes, we have used the regulation of related kinases to predict plausible modes for their regulation. We hope that this review will help investigators as they design experiments to test these and other predictions based on the regulation of related kinases.
Acknowledgments
We are indebted to Gerard Manning for current kinase classification and Pekka Lappalainen for explaining the misannotation of twinfilin. We thank Jeremy Thorner, Ed Winter, and our anonymous reviewers for helpful comments.
This work was supported by grant GM46443 from the National Institutes of Health (to M.S.) and American Heart Association predoctoral fellowship 0615379U (to E.R.).
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Abbas-Terki, T., O. Donze, and D. Picard. 2000. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 467:111-116. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. A. 2003. Activation loop phosphorylation and catalysis in protein kinases: is there functional evidence for the autoinhibitor model? Biochemistry 42:601-607. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal, R., Z. Tang, H. Yu, and O. Cohen-Fix. 2003. Two distinct pathways for inhibiting pds1 ubiquitination in response to DNA damage. J. Biol. Chem. 278:45027-45033. [DOI] [PubMed] [Google Scholar]
- 4.Ahn, S. H., W. L. Cheung, J. Y. Hsu, R. L. Diaz, M. M. Smith, and C. D. Allis. 2005. Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell 120: 25-36. [DOI] [PubMed] [Google Scholar]
- 5.Asakawa, K., S. Yoshida, F. Otake, and A. Toh-e. 2001. A novel functional domain of Cdc15 kinase is required for its interaction with Tem1 GTPase in Saccharomyces cerevisiae. Genetics 157:1437-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asano, S., J. E. Park, K. Sakchaisri, L. R. Yu, S. Song, P. Supavilai, T. D. Veenstra, and K. S. Lee. 2005. Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast. EMBO J. 24:2194-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano, S., J. E. Park, L. R. Yu, M. Zhou, K. Sakchaisri, C. J. Park, Y. H. Kang, J. Thorner, T. D. Veenstra, and K. S. Lee. 2006. Direct phosphorylation and activation of a Nim1-related kinase Gin4 by Elm1 in budding yeast. J. Biol. Chem. 281:27090-27098. [DOI] [PubMed] [Google Scholar]
- 8.Ash, J., C. Wu, R. Larocque, M. Jamal, W. Stevens, M. Osborne, D. Y. Thomas, and M. Whiteway. 2003. Genetic analysis of the interface between Cdc42p and the CRIB domain of Ste20p in Saccharomyces cerevisiae. Genetics 163:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babu, P., R. J. Deschenes, and L. C. Robinson. 2004. Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J. Biol. Chem. 279:27138-27147. [DOI] [PubMed] [Google Scholar]
- 10.Bardin, A. J., M. G. Boselli, and A. Amon. 2003. Mitotic exit regulation through distinct domains within the protein kinase Cdc15. Mol. Cell. Biol. 23:5018-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barral, Y., M. Parra, S. Bidlingmaier, and M. Snyder. 1999. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 13:176-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashkirov, V. I., E. V. Bashkirova, E. Haghnazari, and W. D. Heyer. 2003. Direct kinase-to-kinase signaling mediated by the FHA phosphoprotein recognition domain of the Dun1 DNA damage checkpoint kinase. Mol. Cell. Biol. 23:1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beeler, J. F., W. J. LaRochelle, M. Chedid, S. R. Tronick, and S. A. Aaronson. 1994. Prokaryotic expression cloning of a novel human tyrosine kinase. Mol. Cell. Biol. 14:982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benton, B. K., A. Tinkelenberg, I. Gonzalez, and F. R. Cross. 1997. Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis. Mol. Cell. Biol. 17:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkey, C. D., and M. Carlson. 2006. A specific catalytic subunit isoform of protein kinase CK2 is required for phosphorylation of the repressor Nrg1 in Saccharomyces cerevisiae. Curr. Genet. 50:1-10. [DOI] [PubMed] [Google Scholar]
- 16.Biggins, S., F. F. Severin, N. Bhalla, I. Sassoon, A. A. Hyman, and A. W. Murray. 1999. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 13:532-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilsland-Marchesan, E., J. Arino, H. Saito, P. Sunnerhagen, and F. Posas. 2000. Rck2 kinase is a substrate for the osmotic stress-activated mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 20:3887-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell, E., I. M. Halatek, H. J. Kim, A. T. Ellicott, A. A. Obukhov, and D. E. Stone. 2003. Effect of the pheromone-responsive Gα and phosphatase proteins of Saccharomyces cerevisiae on the subcellular localization of the Fus3 mitogen-activated protein kinase. Mol. Cell. Biol. 23:1135-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booher, R. N., R. J. Deshaies, and M. W. Kirschner. 1993. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 12:3417-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bose, I., J. E. Irazoqui, J. J. Moskow, E. S. Bardes, T. R. Zyla, and D. J. Lew. 2001. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276:7176-7186. [DOI] [PubMed] [Google Scholar]
- 21.Bouquin, N., Y. Barral, R. Courbeyrette, M. Blondel, M. Snyder, and C. Mann. 2000. Regulation of cytokinesis by the Elm1 protein kinase in Saccharomyces cerevisiae. J. Cell Sci. 113:1435-1445. [DOI] [PubMed] [Google Scholar]
- 22.Bowdish, K. S., and A. P. Mitchell. 1993. Bipartite structure of an early meiotic upstream activation sequence from Saccharomyces cerevisiae. Mol. Cell. Biol. 13:2172-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brewster, J. L., T. de Valoir, N. D. Dwyer, E. Winter, and M. C. Gustin. 1993. An osmosensing signal transduction pathway in yeast. Science 259:1760-1763. [DOI] [PubMed] [Google Scholar]
- 24.Burgess, S. M., M. Ajimura, and N. Kleckner. 1999. GCN5-dependent histone H3 acetylation and RPD3-dependent histone H4 deacetylation have distinct, opposing effects on IME2 transcription, during meiosis and during vegetative growth, in budding yeast. Proc. Natl. Acad. Sci. USA 96:6835-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton, J. L., and M. J. Solomon. 2000. Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex. Mol. Cell. Biol. 20:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casamayor, A., P. D. Torrance, T. Kobayashi, J. Thorner, and D. R. Alessi. 1999. Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr. Biol. 9:186-197. [DOI] [PubMed] [Google Scholar]
- 27.Cenamor, R., J. Jimenez, V. J. Cid, C. Nombela, and M. Sanchez. 1999. The budding yeast Cdc15 localizes to the spindle pole body in a cell-cycle-dependent manner. Mol. Cell Biol. Res. Commun. 2:178-184. [DOI] [PubMed] [Google Scholar]
- 28.Chen, S. H., M. B. Smolka, and H. Zhou. 2007. Mechanism of Dun1 activation by Rad53 phosphorylation in Saccharomyces cerevisiae. J. Biol. Chem. 282:986-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng, A., K. E. Ross, P. Kaldis, and M. J. Solomon. 1999. Dephosphorylation of cyclin-dependent kinases by type 2C protein phosphatases. Genes Dev. 13:2946-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi, K. Y., J. E. Kranz, S. K. Mahanty, K. S. Park, and E. A. Elion. 1999. Characterization of Fus3 localization: active Fus3 localizes in complexes of varying size and specific activity. Mol. Biol. Cell 10:1553-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clotet, J., X. Escote, M. A. Adrover, G. Yaakov, E. Gari, M. Aldea, E. de Nadal, and F. Posas. 2006. Phosphorylation of Hsl1 by Hog1 leads to a G2 arrest essential for cell survival at high osmolarity. EMBO J. 25:2338-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collister, M., M. P. Didmon, F. MacIsaac, M. J. Stark, N. Q. MacDonald, and S. M. Keyse. 2002. YIL113w encodes a functional dual-specificity protein phosphatase which specifically interacts with and inactivates the Slt2/Mpk1p MAP kinase in S. cerevisiae. FEBS Lett. 527:186-192. [DOI] [PubMed] [Google Scholar]
- 33.Cope, M. J., S. Yang, C. Shang, and D. G. Drubin. 1999. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J. Cell Biol. 144:1203-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crews, S. T., and C. M. Fan. 1999. Remembrance of things PAS: regulation of development by bHLH-PAS proteins. Curr. Opin. Genet. Dev. 9:580-587. [DOI] [PubMed] [Google Scholar]
- 35.D'Aquino, K. E., F. Monje-Casas, J. Paulson, V. Reiser, G. M. Charles, L. Lai, K. M. Shokat, and A. Amon. 2005. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol. Cell 19:223-234. [DOI] [PubMed] [Google Scholar]
- 36.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 37.deHart, A. K., J. D. Schnell, D. A. Allen, and L. Hicke. 2002. The conserved Pkh-Ypk kinase cascade is required for endocytosis in yeast. J. Cell Biol. 156:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denis, V., and M. S. Cyert. 2005. Molecular analysis reveals localization of Saccharomyces cerevisiae protein kinase C to sites of polarized growth and Pkc1p targeting to the nucleus and mitotic spindle. Eukaryot. Cell 4:36-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deshaies, R. J., V. Chau, and M. Kirschner. 1995. Ubiquitination of the G1 cyclin Cln2p by a Cdc34p-dependent pathway. EMBO J. 14:303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diller, T. C., Madhusudan, N. H. Xuong, and S. S. Taylor. 2001. Molecular basis for regulatory subunit diversity in cAMP-dependent protein kinase: crystal structure of the type II beta regulatory subunit. Structure 9:73-82. [DOI] [PubMed] [Google Scholar]
- 41.Dobbelaere, J., M. S. Gentry, R. L. Hallberg, and Y. Barral. 2003. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev. Cell 4:345-357. [DOI] [PubMed] [Google Scholar]
- 42.Donzé, O., and D. Picard. 1999. Hsp90 binds and regulates Gcn2, the ligand-inducible kinase of the alpha subunit of eukaryotic translation initiation factor 2. Mol. Cell. Biol. 19:8422-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donzeau, M., and W. Bandlow. 1999. The yeast trimeric guanine nucleotide-binding protein alpha subunit, Gpa2p, controls the meiosis-specific kinase Ime2p activity in response to nutrients. Mol. Cell. Biol. 19:6110-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowell, S. J., P. Romanowski, and J. F. Diffley. 1994. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science 265:1243-1246. [DOI] [PubMed] [Google Scholar]
- 45.Drogen, F., S. M. O'Rourke, V. M. Stucke, M. Jaquenoud, A. M. Neiman, and M. Peter. 2000. Phosphorylation of the MEKK Ste11p by the PAK-like kinase Ste20p is required for MAP kinase signaling in vivo. Curr. Biol. 10:630-639. [DOI] [PubMed] [Google Scholar]
- 46.Elbert, M., G. Rossi, and P. Brennwald. 2005. The yeast par-1 homologs kin1 and kin2 show genetic and physical interactions with components of the exocytic machinery. Mol. Biol. Cell 16:532-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Errede, B., A. Gartner, Z. Zhou, K. Nasmyth, and G. Ammerer. 1993. MAP kinase-related FUS3 from S. cerevisiae is activated by STE7 in vitro. Nature 362:261-264. [DOI] [PubMed] [Google Scholar]
- 48.Esch, R. K., and B. Errede. 2002. Pheromone induction promotes Ste11 degradation through a MAPK feedback and ubiquitin-dependent mechanism. Proc. Natl. Acad. Sci. USA 99:9160-9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Espinoza, F. H., A. Farrell, H. Erdjument-Bromage, P. Tempst, and D. O. Morgan. 1996. A cyclin-dependent kinase-activating kinase (CAK) in budding yeast unrelated to vertebrate CAK. Science 273:1714-1717. [DOI] [PubMed] [Google Scholar]
- 50.Espinoza, F. H., A. Farrell, J. L. Nourse, H. M. Chamberlin, O. Gileadi, and D. O. Morgan. 1998. Cak1 is required for Kin28 phosphorylation and activation in vivo. Mol. Cell. Biol. 18:6365-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Espinoza, F. H., J. Ogas, I. Herskowitz, and D. O. Morgan. 1994. Cell cycle control by a complex of the cyclin HCS26 (PCL1) and the kinase PHO85. Science 266:1388-1391. [DOI] [PubMed] [Google Scholar]
- 52.Eyers, P. A., E. Erikson, L. G. Chen, and J. L. Maller. 2003. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13:691-697. [DOI] [PubMed] [Google Scholar]
- 53.Facchin, S., R. Lopreiato, S. Stocchetto, G. Arrigoni, L. Cesaro, O. Marin, G. Carignani, and L. A. Pinna. 2002. Structure-function analysis of yeast piD261/Bud32, an atypical protein kinase essential for normal cell life. Biochem. J. 364:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farrell, A., and D. O. Morgan. 2000. Cdc37 promotes the stability of protein kinases Cdc28 and Cak1. Mol. Cell. Biol. 20:749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrigno, P., F. Posas, D. Koepp, H. Saito, and P. A. Silver. 1998. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flandez, M., I. C. Cosano, C. Nombela, H. Martin, and M. Molina. 2004. Reciprocal regulation between Slt2 MAPK and isoforms of Msg5 dual-specificity protein phosphatase modulates the yeast cell integrity pathway. J. Biol. Chem. 279:11027-11034. [DOI] [PubMed] [Google Scholar]
- 57.Flatauer, L. J., S. F. Zadeh, and L. Bardwell. 2005. Mitogen-activated protein kinases with distinct requirements for Ste5 scaffolding influence signaling specificity in Saccharomyces cerevisiae. Mol. Cell. Biol. 25:1793-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleischmann, M., I. Stagljar, and M. Aebi. 1996. Allele-specific suppression of a Saccharomyces cerevisiae prp20 mutation by overexpression of a nuclear serine/threonine protein kinase. Mol. Gen. Genet. 250:614-625. [DOI] [PubMed] [Google Scholar]
- 59.Fujita, A., A. Tonouchi, T. Hiroko, F. Inose, T. Nagashima, R. Satoh, and S. Tanaka. 1999. Hsl7p, a negative regulator of Ste20p protein kinase in the Saccharomyces cerevisiae filamentous growth-signaling pathway. Proc. Natl. Acad. Sci. USA 96:8522-8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gadura, N., L. C. Robinson, and C. A. Michels. 2006. Glc7-Reg1 phosphatase signals to Yck1,2 casein kinase 1 to regulate transport activity and glucose-induced inactivation of Saccharomyces maltose permease. Genetics 172:1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Barrio, M., J. Dong, S. Ufano, and A. G. Hinnebusch. 2000. Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation. EMBO J. 19:1887-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gartner, A., K. Nasmyth, and G. Ammerer. 1992. Signal transduction in Saccharomyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes Dev. 6:1280-1292. [DOI] [PubMed] [Google Scholar]
- 63.Gerber, M. R., A. Farrell, R. J. Deshaies, I. Herskowitz, and D. O. Morgan. 1995. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc. Natl. Acad. Sci. USA 92:4651-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillett, E. S., C. W. Espelin, and P. K. Sorger. 2004. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J. Cell Biol. 164:535-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grandin, N., and S. I. Reed. 1993. Differential function and expression of Saccharomyces cerevisiae B-type cyclins in mitosis and meiosis. Mol. Cell. Biol. 13:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grava, S., F. Schaerer, M. Faty, P. Philippsen, and Y. Barral. 2006. Asymmetric recruitment of dynein to spindle poles and microtubules promotes proper spindle orientation in yeast. Dev. Cell 10:425-439. [DOI] [PubMed] [Google Scholar]
- 67.Guo, J., and M. Polymenis. 2006. Dcr2 targets Ire1 and downregulates the unfolded protein response in Saccharomyces cerevisiae. EMBO Rep. 7:1124-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guttmann-Raviv, N., S. Martin, and Y. Kassir. 2002. Ime2, a meiosis-specific kinase in yeast, is required for destabilization of its transcriptional activator, Ime1. Mol. Cell. Biol. 22:2047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hahn, J. S., and D. J. Thiele. 2002. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 277:21278-21284. [DOI] [PubMed] [Google Scholar]
- 70.Hanrahan, J., and M. Snyder. 2003. Cytoskeletal activation of a checkpoint kinase. Mol. Cell 12:663-673. [DOI] [PubMed] [Google Scholar]
- 71.Harris, K., R. E. Lamson, B. Nelson, T. R. Hughes, M. J. Marton, C. J. Roberts, C. Boone, and P. M. Pryciak. 2001. Role of scaffolds in MAP kinase pathway specificity revealed by custom design of pathway-dedicated signaling proteins. Curr. Biol. 11:1815-1824. [PubMed] [Google Scholar]
- 72.Harvey, S. L., A. Charlet, W. Haas, S. P. Gygi, and D. R. Kellogg. 2005. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 122:407-420. [DOI] [PubMed] [Google Scholar]
- 73.Hautbergue, G., and V. Goguel. 2001. Activation of the cyclin-dependent kinase CTDK-I requires the heterodimerization of two unstable subunits. J. Biol. Chem. 276:8005-8013. [DOI] [PubMed] [Google Scholar]
- 74.Hedbacker, K., S. P. Hong, and M. Carlson. 2004. Pak1 protein kinase regulates activation and nuclear localization of Snf1-Gal83 protein kinase. Mol. Cell. Biol. 24:8255-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hedbacker, K., R. Townley, and M. Carlson. 2004. Cyclic AMP-dependent protein kinase regulates the subcellular localization of Snf1-Sip1 protein kinase. Mol. Cell. Biol. 24:1836-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong, S. P., F. C. Leiper, A. Woods, D. Carling, and M. Carlson. 2003. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl. Acad. Sci. USA 100:8839-8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang, D., J. Moffat, W. A. Wilson, L. Moore, C. Cheng, P. J. Roach, and B. Andrews. 1998. Cyclin partners determine Pho85 protein kinase substrate specificity in vitro and in vivo: control of glycogen biosynthesis by Pcl8 and Pcl10. Mol. Cell. Biol. 18:3289-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang, S., D. A. Jeffery, M. D. Anthony, and E. K. O'Shea. 2001. Functional analysis of the cyclin-dependent kinase inhibitor Pho81 identifies a novel inhibitory domain. Mol. Cell. Biol. 21:6695-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hunter, T., and G. D. Plowman. 1997. The protein kinases of budding yeast: six score and more. Trends Biochem. Sci. 22:18-22. [DOI] [PubMed] [Google Scholar]
- 80.Inagaki, M., T. Schmelzle, K. Yamaguchi, K. Irie, M. N. Hall, and K. Matsumoto. 1999. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol. 19:8344-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irie, K., M. Takase, K. S. Lee, D. E. Levin, H. Araki, K. Matsumoto, and Y. Oshima. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13:3076-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacinto, E., B. Guo, K. T. Arndt, T. Schmelzle, and M. N. Hall. 2001. TIP41 interacts with TAP42 and negatively regulates the TOR signaling pathway. Mol. Cell 8:1017-1026. [DOI] [PubMed] [Google Scholar]
- 83.Jaspersen, S. L., B. J. Huneycutt, T. H. Giddings, Jr., K. A. Resing, N. G. Ahn, and M. Winey. 2004. Cdc28/Cdk1 regulates spindle pole body duplication through phosphorylation of Spc42 and Mps1. Dev. Cell 7:263-274. [DOI] [PubMed] [Google Scholar]
- 84.Jaspersen, S. L., and D. O. Morgan. 2000. Cdc14 activates cdc15 to promote mitotic exit in budding yeast. Curr. Biol. 10:615-618. [DOI] [PubMed] [Google Scholar]
- 85.Jeffrey, P. D., A. A. Russo, K. Polyak, E. Gibbs, J. Hurwitz, J. Massague, and N. P. Pavletich. 1995. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature 376:313-320. [DOI] [PubMed] [Google Scholar]
- 86.Jiang, R., and M. Carlson. 1996. Glucose regulates protein interactions within the yeast SNF1 protein kinase complex. Genes Dev. 10:3105-3115. [DOI] [PubMed] [Google Scholar]
- 87.Johnson, K. E., S. Cameron, T. Toda, M. Wigler, and M. J. Zoller. 1987. Expression in Escherichia coli of BCY1, the regulatory subunit of cyclic AMP-dependent protein kinase from Saccharomyces cerevisiae. Purification and characterization. J. Biol. Chem. 262:8636-8642. [PubMed] [Google Scholar]
- 88.Kaiser, P., R. A. Sia, E. G. Bardes, D. J. Lew, and S. I. Reed. 1998. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 12:2587-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaldis, P., A. Sutton, and M. J. Solomon. 1996. The Cdk-activating kinase (CAK) from budding yeast. Cell 86:553-564. [DOI] [PubMed] [Google Scholar]
- 90.Kamada, Y., T. Funakoshi, T. Shintani, K. Nagano, M. Ohsumi, and Y. Ohsumi. 2000. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol. 150:1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamada, Y., H. Qadota, C. P. Python, Y. Anraku, Y. Ohya, and D. E. Levin. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193-9196. [DOI] [PubMed] [Google Scholar]
- 92.Kassis, S., T. Melhuish, R. S. Annan, S. L. Chen, J. C. Lee, G. P. Livi, and C. L. Creasy. 2000. Saccharomyces cerevisiae Yak1p protein kinase autophosphorylates on tyrosine residues and phosphorylates myelin basic protein on a C-terminal serine residue. Biochem. J. 348 (Pt. 2):263-272. [PMC free article] [PubMed] [Google Scholar]
- 93.Keogh, M. C., E. J. Cho, V. Podolny, and S. Buratowski. 2002. Kin28 is found within TFIIH and a Kin28-Ccl1-Tfb3 trimer complex with differential sensitivities to T-loop phosphorylation. Mol. Cell. Biol. 22:1288-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kimmelman, J., P. Kaldis, C. J. Hengartner, G. M. Laff, S. S. Koh, R. A. Young, and M. J. Solomon. 1999. Activating phosphorylation of the Kin28p subunit of yeast TFIIH by Cak1p. Mol. Cell. Biol. 19:4774-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kranz, J. E., B. Satterberg, and E. A. Elion. 1994. The MAP kinase Fus3 associates with and phosphorylates the upstream signaling component Ste5. Genes Dev. 8:313-327. [DOI] [PubMed] [Google Scholar]
- 96.Krause-Buchholz, U., U. Gey, J. Wunschmann, S. Becker, and G. Rodel. 2006. YIL042c and YOR090c encode the kinase and phosphatase of the Saccharomyces cerevisiae pyruvate dehydrogenase complex. FEBS Lett. 580:2553-2560. [DOI] [PubMed] [Google Scholar]
- 97.Kubinski, K., K. Domanska, E. Sajnaga, E. Mazur, R. Zielinski, and R. Szyszka. 2007. Yeast holoenzyme of protein kinase CK2 requires both beta and beta′ regulatory subunits for its activity. Mol. Cell. Biochem. 295:229-236. [DOI] [PubMed] [Google Scholar]
- 98.Kuchin, S., P. Yeghiayan, and M. Carlson. 1995. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc. Natl. Acad. Sci. USA 92:4006-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamson, R. E., M. J. Winters, and P. M. Pryciak. 2002. Cdc42 regulation of kinase activity and signaling by the yeast p21-activated kinase Ste20. Mol. Cell. Biol. 22:2939-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaRonde-LeBlanc, N., and A. Wlodawer. 2005. A family portrait of the RIO kinases. J. Biol. Chem. 280:37297-37300. [DOI] [PubMed] [Google Scholar]
- 101.Lee, K., C. Du, M. Horn, and L. Rabinow. 1996. Activity and autophosphorylation of LAMMER protein kinases. J. Biol. Chem. 271:27299-27303. [DOI] [PubMed] [Google Scholar]
- 102.Lee, K. S., and D. E. Levin. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee, S. J., M. F. Schwartz, J. K. Duong, and D. F. Stern. 2003. Rad53 phosphorylation site clusters are important for Rad53 regulation and signaling. Mol. Cell. Biol. 23:6300-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leech, A., N. Nath, R. R. McCartney, and M. C. Schmidt. 2003. Isolation of mutations in the catalytic domain of the snf1 kinase that render its activity independent of the snf4 subunit. Eukaryot. Cell 2:265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leeuw, T., C. Wu, J. D. Schrag, M. Whiteway, D. Y. Thomas, and E. Leberer. 1998. Interaction of a G-protein beta-subunit with a conserved sequence in Ste20/PAK family protein kinases. Nature 391:191-195. [DOI] [PubMed] [Google Scholar]
- 106.Lei, M., W. Lu, W. Meng, M. C. Parrini, M. J. Eck, B. J. Mayer, and S. C. Harrison. 2000. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102:387-397. [DOI] [PubMed] [Google Scholar]
- 107.Li, H., C. K. Tsang, M. Watkins, P. G. Bertram, and X. F. Zheng. 2006. Nutrient regulates Tor1 nuclear localization and association with rDNA promoter. Nature 442:1058-1061. [DOI] [PubMed] [Google Scholar]
- 108.Liao, S. M., J. Zhang, D. A. Jeffery, A. J. Koleske, C. M. Thompson, D. M. Chao, M. Viljoen, H. J. van Vuuren, and R. A. Young. 1995. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature 374:193-196. [DOI] [PubMed] [Google Scholar]
- 109.Lim, M. Y., D. Dailey, G. S. Martin, and J. Thorner. 1993. Yeast MCK1 protein kinase autophosphorylates at tyrosine and serine but phosphorylates exogenous substrates at serine and threonine. J. Biol. Chem. 268:21155-21164. [PubMed] [Google Scholar]
- 110.Lin, F. C., and K. T. Arndt. 1995. The role of Saccharomyces cerevisiae type 2A phosphatase in the actin cytoskeleton and in entry into mitosis. EMBO J. 14:2745-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lisby, M., J. H. Barlow, R. C. Burgess, and R. Rothstein. 2004. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118:699-713. [DOI] [PubMed] [Google Scholar]
- 112.Liu, K., X. Zhang, R. L. Lester, and R. C. Dickson. 2005. The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. J. Biol. Chem. 280:22679-22687. [DOI] [PubMed] [Google Scholar]
- 113.Lochhead, P. A., R. Kinstrie, G. Sibbet, T. Rawjee, N. Morrice, and V. Cleghon. 2006. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol. Cell 24:627-633. [DOI] [PubMed] [Google Scholar]
- 114.Lochhead, P. A., G. Sibbet, N. Morrice, and V. Cleghon. 2005. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell 121:925-936. [DOI] [PubMed] [Google Scholar]
- 115.Lu, J. M., R. J. Deschenes, and J. S. Fassler. 2003. Saccharomyces cerevisiae histidine phosphotransferase Ypd1p shuttles between the nucleus and cytoplasm for SLN1-dependent phosphorylation of Ssk1p and Skn7p. Eukaryot. Cell 2:1304-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma, D., J. G. Cook, and J. Thorner. 1995. Phosphorylation and localization of Kss1, a MAP kinase of the Saccharomyces cerevisiae pheromone response pathway. Mol. Biol. Cell 6:889-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma, J. L., S. J. Lee, J. K. Duong, and D. F. Stern. 2006. Activation of the checkpoint kinase Rad53 by the phosphatidyl inositol kinase-like kinase Mec1. J. Biol. Chem. 281:3954-3963. [DOI] [PubMed] [Google Scholar]
- 118.Maeda, T., M. Takekawa, and H. Saito. 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269:554-558. [DOI] [PubMed] [Google Scholar]
- 119.Maeta, K., S. Izawa, and Y. Inoue. 2005. Methylglyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 280:253-260. [DOI] [PubMed] [Google Scholar]
- 120.Mah, A. S., J. Jang, and R. J. Deshaies. 2001. Protein kinase Cdc15 activates the Dbf2-Mob1 kinase complex. Proc. Natl. Acad. Sci. USA 98: 7325-7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mandal, A. K., P. Lee, J. A. Chen, N. Nillegoda, A. Heller, S. Distasio, H. Oen, J. Victor, D. M. Nair, J. L. Brodsky, and A. J. Caplan. 2007. Cdc37 has distinct roles in protein kinase quality control that protect nascent chains from degradation and promote posttranslational maturation. J. Cell Biol. 176:319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manning, G., G. D. Plowman, T. Hunter, and S. Sudarsanam. 2002. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 27:514-520. [DOI] [PubMed] [Google Scholar]
- 123.Mapes, J., and I. M. Ota. 2004. Nbp2 targets the Ptc1-type 2C Ser/Thr phosphatase to the HOG MAPK pathway. EMBO J. 23:302-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marcus, S., A. Polverino, M. Barr, and M. Wigler. 1994. Complexes between STE5 and components of the pheromone-responsive mitogen-activated protein kinase module. Proc. Natl. Acad. Sci. USA 91:7762-7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin, D. E., A. Soulard, and M. N. Hall. 2004. TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell 119:969-979. [DOI] [PubMed] [Google Scholar]
- 126.Martin, H., A. Mendoza, J. M. Rodriguez-Pachon, M. Molina, and C. Nombela. 1997. Characterization of SKM1, a Saccharomyces cerevisiae gene encoding a novel Ste20/PAK-like protein kinase. Mol. Microbiol. 23:431-444. [DOI] [PubMed] [Google Scholar]
- 127.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 128.Mattison, C. P., and I. M. Ota. 2000. Two protein tyrosine phosphatases, Ptp2 and Ptp3, modulate the subcellular localization of the Hog1 MAP kinase in yeast. Genes Dev. 14:1229-1235. [PMC free article] [PubMed] [Google Scholar]
- 129.Mattison, C. P., S. S. Spencer, K. A. Kresge, J. Lee, and I. M. Ota. 1999. Differential regulation of the cell wall integrity mitogen-activated protein kinase pathway in budding yeast by the protein tyrosine phosphatases Ptp2 and Ptp3. Mol. Cell. Biol. 19:7651-7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McCartney, R. R., and M. C. Schmidt. 2001. Regulation of Snf1 kinase. Activation requires phosphorylation of threonine 210 by an upstream kinase as well as a distinct step mediated by the Snf4 subunit. J. Biol. Chem. 276:36460-36466. [DOI] [PubMed] [Google Scholar]
- 131.McMillan, J. N., C. L. Theesfeld, J. C. Harrison, E. S. Bardes, and D. J. Lew. 2002. Determinants of Swe1p degradation in Saccharomyces cerevisiae. Mol. Biol. Cell 13:3560-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Measday, V., L. Moore, J. Ogas, M. Tyers, and B. Andrews. 1994. The PCL2 (ORFD)-PHO85 cyclin-dependent kinase complex: a cell cycle regulator in yeast. Science 266:1391-1395. [DOI] [PubMed] [Google Scholar]
- 133.Measday, V., L. Moore, R. Retnakaran, J. Lee, M. Donoviel, A. M. Neiman, and B. Andrews. 1997. A family of cyclin-like proteins that interact with the Pho85 cyclin-dependent kinase. Mol. Cell. Biol. 17:1212-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Melcher, M. L., and J. Thorner. 1996. Identification and characterization of the CLK1 gene product, a novel CaM kinase-like protein kinase from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 271:29958-29968. [DOI] [PubMed] [Google Scholar]
- 135.Mendenhall, M. D. 1993. An inhibitor of p34CDC28 protein kinase activity from Saccharomyces cerevisiae. Science 259:216-219. [DOI] [PubMed] [Google Scholar]
- 136.Miller, M. E., and F. R. Cross. 2000. Distinct subcellular localization patterns contribute to functional specificity of the Cln2 and Cln3 cyclins of Saccharomyces cerevisiae. Mol. Cell. Biol. 20:542-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Millson, S. H., A. W. Truman, V. King, C. Prodromou, L. H. Pearl, and P. W. Piper. 2005. A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in the activity of a stress-activated mitogen-activated protein kinase, Slt2p (Mpk1p). Eukaryot. Cell 4:849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mizunuma, M., D. Hirata, R. Miyaoka, and T. Miyakawa. 2001. GSK-3 kinase Mck1 and calcineurin coordinately mediate Hsl1 down-regulation by Ca2+ in budding yeast. EMBO J. 20:1074-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moriya, H., and M. Johnston. 2004. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. USA 101:1572-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moriya, H., Y. Shimizu-Yoshida, A. Omori, S. Iwashita, M. Katoh, and A. Sakai. 2001. Yak1p, a DYRK family kinase, translocates to the nucleus and phosphorylates yeast Pop2p in response to a glucose signal. Genes Dev. 15:1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mortensen, E. M., W. Haas, M. Gygi, S. P. Gygi, and D. R. Kellogg. 2005. Cdc28-dependent regulation of the Cdc5/Polo kinase. Curr. Biol. 15:2033-2037. [DOI] [PubMed] [Google Scholar]
- 142.Mortensen, E. M., H. McDonald, J. Yates III, and D. R. Kellogg. 2002. Cell cycle-dependent assembly of a Gin4-septin complex. Mol. Biol. Cell 13:2091-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakada, D., Y. Hirano, Y. Tanaka, and K. Sugimoto. 2005. Role of the C terminus of Mec1 checkpoint kinase in its localization to sites of DNA damage. Mol. Biol. Cell 16:5227-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nath, N., R. R. McCartney, and M. C. Schmidt. 2003. Yeast Pak1 kinase associates with and activates Snf1. Mol. Cell. Biol. 23:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Neiman, A. M., and I. Herskowitz. 1994. Reconstitution of a yeast protein kinase cascade in vitro: activation of the yeast MEK homologue STE7 by STE11. Proc. Natl. Acad. Sci. USA 91:3398-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nespoli, A., R. Vercillo, L. di Nola, L. Diani, M. Giannattasio, P. Plevani, and M. Muzi-Falconi. 2006. Alk1 and Alk2 are two new cell cycle-regulated haspin-like proteins in budding yeast. Cell Cycle 5:1464-1471. [DOI] [PubMed] [Google Scholar]
- 147.Nishizawa, M., K. Suzuki, M. Fujino, T. Oguchi, and A. Toh-e. 1999. The Pho85 kinase, a member of the yeast cyclin-dependent kinase (Cdk) family, has a regulation mechanism different from Cdks functioning throughout the cell cycle. Genes Cells 4:627-642. [DOI] [PubMed] [Google Scholar]
- 148.Niu, H., L. Wan, B. Baumgartner, D. Schaefer, J. Loidl, and N. M. Hollingsworth. 2005. Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol. Biol. Cell 16:5804-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nolen, B., S. Taylor, and G. Ghosh. 2004. Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell 15:661-675. [DOI] [PubMed] [Google Scholar]
- 150.Nolen, B., C. Y. Yun, C. F. Wong, J. A. McCammon, X. D. Fu, and G. Ghosh. 2001. The structure of Sky1p reveals a novel mechanism for constitutive activity. Nat. Struct. Biol. 8:176-183. [DOI] [PubMed] [Google Scholar]
- 151.Nugroho, T. T., and M. D. Mendenhall. 1994. An inhibitor of yeast cyclin-dependent protein kinase plays an important role in ensuring the genomic integrity of daughter cells. Mol. Cell. Biol. 14:3320-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ohya, Y., H. Kawasaki, K. Suzuki, J. Londesborough, and Y. Anraku. 1991. Two yeast genes encoding calmodulin-dependent protein kinases. Isolation, sequencing and bacterial expressions of CMK1 and CMK2. J. Biol. Chem. 266:12784-12794. [PubMed] [Google Scholar]
- 153.Okamura, K., Y. Kimata, H. Higashio, A. Tsuru, and K. Kohno. 2000. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem. Biophys. Res. Commun. 279:445-450. [DOI] [PubMed] [Google Scholar]
- 154.Okuzaki, D., and H. Nojima. 2001. Kcc4 associates with septin proteins of Saccharomyces cerevisiae. FEBS Lett. 489:197-201. [DOI] [PubMed] [Google Scholar]
- 155.Okuzaki, D., S. Tanaka, H. Kanazawa, and H. Nojima. 1997. Gin4 of S. cerevisiae is a bud neck protein that interacts with the Cdc28 complex. Genes Cells 2:753-770. [DOI] [PubMed] [Google Scholar]
- 156.Oshiro, G., J. C. Owens, Y. Shellman, R. A. Sclafani, and J. J. Li. 1999. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol. Cell. Biol. 19:4888-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ostapenko, D., and M. J. Solomon. 2005. Phosphorylation by Cak1 regulates the C-terminal domain kinase Ctk1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 25:3906-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parrini, M. C., M. Lei, S. C. Harrison, and B. J. Mayer. 2002. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell 9:73-83. [DOI] [PubMed] [Google Scholar]
- 159.Pausch, M. H., D. Kaim, R. Kunisawa, A. Admon, and J. Thorner. 1991. Multiple Ca2+/calmodulin-dependent protein kinase genes in a unicellular eukaryote. EMBO J. 10:1511-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Percival-Smith, A., and J. Segall. 1986. Characterization and mutational analysis of a cluster of three genes expressed preferentially during sporulation of Saccharomyces cerevisiae. Mol. Cell. Biol. 6:2443-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Peter, M., A. M. Neiman, H. O. Park, M. van Lohuizen, and I. Herskowitz. 1996. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 15:7046-7059. [PMC free article] [PubMed] [Google Scholar]
- 162.Petronczki, M., J. Matos, S. Mori, J. Gregan, A. Bogdanova, M. Schwickart, K. Mechtler, K. Shirahige, W. Zachariae, and K. Nasmyth. 2006. Monopolar attachment of sister kinetochores at meiosis I requires casein kinase 1. Cell 126:1049-1064. [DOI] [PubMed] [Google Scholar]
- 163.Pierce, M., M. Wagner, J. Xie, V. Gailus-Durner, J. Six, A. K. Vershon, and E. Winter. 1998. Transcriptional regulation of the SMK1 mitogen-activated protein kinase gene during meiotic development in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:5970-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Posas, F., and H. Saito. 1998. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 17:1385-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Posas, F., and H. Saito. 1997. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science 276:1702-1705. [DOI] [PubMed] [Google Scholar]
- 166.Posas, F., S. M. Wurgler-Murphy, T. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 167.Printen, J. A., and G. F. Sprague, Jr. 1994. Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics 138:609-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Purnapatre, K., M. Gray, S. Piccirillo, and S. M. Honigberg. 2005. Glucose inhibits meiotic DNA replication through SCFGrr1p-dependent destruction of Ime2p kinase. Mol. Cell. Biol. 25:440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Qi, M., and E. A. Elion. 2005. Formin-induced actin cables are required for polarized recruitment of the Ste5 scaffold and high level activation of MAPK Fus3. J. Cell Sci. 118:2837-2848. [DOI] [PubMed] [Google Scholar]
- 170.Qiu, H., M. T. Garcia-Barrio, and A. G. Hinnebusch. 1998. Dimerization by translation initiation factor 2 kinase GCN2 is mediated by interactions in the C-terminal ribosome-binding region and the protein kinase domain. Mol. Cell. Biol. 18:2697-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qiu, H., C. Hu, J. Dong, and A. G. Hinnebusch. 2002. Mutations that bypass tRNA binding activate the intrinsically defective kinase domain in GCN2. Genes Dev. 16:1271-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Raitt, D. C., F. Posas, and H. Saito. 2000. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 19:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ramne, A., E. Bilsland-Marchesan, S. Erickson, and P. Sunnerhagen. 2000. The protein kinases Rck1 and Rck2 inhibit meiosis in budding yeast. Mol. Gen. Genet. 263:253-261. [DOI] [PubMed] [Google Scholar]
- 174.Rayner, T. F., J. V. Gray, and J. W. Thorner. 2002. Direct and novel regulation of cAMP-dependent protein kinase by Mck1p, a yeast glycogen synthase kinase-3. J. Biol. Chem. 277:16814-16822. [DOI] [PubMed] [Google Scholar]
- 175.Reinders, A., N. Burckert, T. Boller, A. Wiemken, and C. De Virgilio. 1998. Saccharomyces cerevisiae cAMP-dependent protein kinase controls entry into stationary phase through the Rim15p protein kinase. Genes Dev. 12:2943-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reinke, A., J. C. Chen, S. Aronova, and T. Powers. 2006. Caffeine targets TOR complex I and provides evidence for a regulatory link between the FRB and kinase domains of Tor1p. J. Biol. Chem. 281:31616-31626. [DOI] [PubMed] [Google Scholar]
- 177.Reiser, V., S. M. Salah, and G. Ammerer. 2000. Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat. Cell Biol. 2:620-627. [DOI] [PubMed] [Google Scholar]
- 178.Ro, H. S., S. Song, and K. S. Lee. 2002. Bfa1 can regulate Tem1 function independently of Bub2 in the mitotic exit network of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 99:5436-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roberts, B. T., K. A. Farr, and M. A. Hoyt. 1994. The Saccharomyces cerevisiae checkpoint gene BUB1 encodes a novel protein kinase. Mol. Cell. Biol. 14:8282-8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roelants, F. M., P. D. Torrance, and J. Thorner. 2004. Differential roles of PDK1- and PDK2-phosphorylation sites in the yeast AGC kinases Ypk1, Pkc1 and Sch9. Microbiology 150:3289-3304. [DOI] [PubMed] [Google Scholar]
- 181.Ross, K. E., P. Kaldis, and M. J. Solomon. 2000. Activating phosphorylation of the Saccharomyces cerevisiae cyclin-dependent kinase, cdc28p, precedes cyclin binding. Mol. Biol. Cell 11:1597-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rouse, J., and S. P. Jackson. 2002. Lcd1p recruits Mec1p to DNA lesions in vitro and in vivo. Mol. Cell 9:857-869. [DOI] [PubMed] [Google Scholar]
- 183.Rubenstein, E. M., R. R. McCartney, and M. C. Schmidt. 2006. Regulatory domains of Snf1-activating kinases determine pathway specificity. Eukaryot. Cell 5:620-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Russell, P., S. Moreno, and S. I. Reed. 1989. Conservation of mitotic controls in fission and budding yeasts. Cell 57:295-303. [DOI] [PubMed] [Google Scholar]
- 185.Rutter, J., B. L. Probst, and S. L. McKnight. 2002. Coordinate regulation of sugar flux and translation by PAS kinase. Cell 111:17-28. [DOI] [PubMed] [Google Scholar]
- 186.Sakchaisri, K., S. Asano, L. R. Yu, M. J. Shulewitz, C. J. Park, J. E. Park, Y. W. Cho, T. D. Veenstra, J. Thorner, and K. S. Lee. 2004. Coupling morphogenesis to mitotic entry. Proc. Natl. Acad. Sci. USA 101:4124-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schaber, M., A. Lindgren, K. Schindler, D. Bungard, P. Kaldis, and E. Winter. 2002. CAK1 promotes meiosis and spore formation in Saccharomyces cerevisiae in a CDC28-independent fashion. Mol. Cell. Biol. 22:57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schindler, K., K. R. Benjamin, A. Martin, A. Boglioli, I. Herskowitz, and E. Winter. 2003. The Cdk-activating kinase Cak1p promotes meiotic S phase through Ime2p. Mol. Cell. Biol. 23:8718-8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schindler, K., and E. Winter. 2006. Phosphorylation of Ime2 regulates meiotic progression in Saccharomyces cerevisiae. J. Biol. Chem. 281:18307-18316. [DOI] [PubMed] [Google Scholar]
- 190.Schmidt, A., T. Beck, A. Koller, J. Kunz, and M. N. Hall. 1998. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17:6924-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schmidt, M. C., and R. R. McCartney. 2000. β-Subunits of Snf1 kinase are required for kinase function and substrate definition. EMBO J. 19:4936-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schneider, K. R., R. L. Smith, and E. K. O'Shea. 1994. Phosphate-regulated inactivation of the kinase PHO80-PHO85 by the CDK inhibitor PHO81. Science 266:122-126. [DOI] [PubMed] [Google Scholar]
- 193.Schutz, A. R., T. H. Giddings, Jr., E. Steiner, and M. Winey. 1997. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J. Cell Biol. 136:969-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schwartz, M. A., and H. D. Madhani. 2006. Control of MAPK signaling specificity by a conserved residue in the MEK-binding domain of the yeast scaffold protein Ste5. Curr. Genet. 49:351-363. [DOI] [PubMed] [Google Scholar]
- 195.Scott, J. W., S. A. Hawley, K. A. Green, M. Anis, G. Stewart, G. A. Scullion, D. G. Norman, and D. G. Hardie. 2004. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Investig. 113:274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Selbert, M. A., K. A. Anderson, Q. H. Huang, E. G. Goldstein, A. R. Means, and A. M. Edelman. 1995. Phosphorylation and activation of Ca(2+)-calmodulin-dependent protein kinase IV by Ca(2+)-calmodulin-dependent protein kinase Ia kinase. Phosphorylation of threonine 196 is essential for activation. J. Biol. Chem. 270:17616-17621. [DOI] [PubMed] [Google Scholar]
- 197.Shamu, C. E., and P. Walter. 1996. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15:3028-3039. [PMC free article] [PubMed] [Google Scholar]
- 198.Shulewitz, M. J., C. J. Inouye, and J. Thorner. 1999. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:7123-7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Slessareva, J. E., S. M. Routt, B. Temple, V. A. Bankaitis, and H. G. Dohlman. 2006. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell 126:191-203. [DOI] [PubMed] [Google Scholar]
- 200.Smith, A., M. P. Ward, and S. Garrett. 1998. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Song, S., T. Z. Grenfell, S. Garfield, R. L. Erikson, and K. S. Lee. 2000. Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol. Cell. Biol. 20:286-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stack, J. H., P. K. Herman, P. V. Schu, and S. D. Emr. 1993. A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 12:2195-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sterner, D. E., J. M. Lee, S. E. Hardin, and A. L. Greenleaf. 1995. The yeast carboxyl-terminal repeat domain kinase CTDK-I is a divergent cyclin-cyclin-dependent kinase complex. Mol. Cell. Biol. 15:5716-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Su, Y., W. R. Dostmann, F. W. Herberg, K. Durick, N. H. Xuong, L. Ten Eyck, S. S. Taylor, and K. I. Varughese. 1995. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science 269:807-813. [DOI] [PubMed] [Google Scholar]
- 205.Sullivan, D. S., S. Biggins, and M. D. Rose. 1998. The yeast centrin, cdc31p, and the interacting protein kinase, Kic1p, are required for cell integrity. J. Cell Biol. 143:751-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sun, B., L. Chen, W. Cao, A. F. Roth, and N. G. Davis. 2004. The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXPhi adaptin sorting signal. Mol. Biol. Cell 15:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Surana, U., H. Robitsch, C. Price, T. Schuster, I. Fitch, A. B. Futcher, and K. Nasmyth. 1991. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell 65:145-161. [DOI] [PubMed] [Google Scholar]
- 208.Sutherland, C. M., S. A. Hawley, R. R. McCartney, A. Leech, M. J. Stark, M. C. Schmidt, and D. G. Hardie. 2003. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr. Biol. 13:1299-1305. [DOI] [PubMed] [Google Scholar]
- 209.Sutton, A., and R. Freiman. 1997. The Cak1p protein kinase is required at G1/S and G2/M in the budding yeast cell cycle. Genetics 147:57-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Svejstrup, J. Q., W. J. Feaver, and R. D. Kornberg. 1996. Subunits of yeast RNA polymerase II transcription factor TFIIH encoded by the CCL1 gene. J. Biol. Chem. 271:643-645. [DOI] [PubMed] [Google Scholar]
- 211.Swaminathan, S., and P. Sunnerhagen. 2005. Degradation of Saccharomyces cervisiae Rck2 upon exposure of cells to high levels of zinc is dependent on Pep4. Mol. Genet. Genomics 273:433-439. [DOI] [PubMed] [Google Scholar]
- 212.Swinnen, E., V. Wanke, J. Roosen, B. Smets, F. Dubouloz, I. Pedruzzi, E. Cameroni, C. De Virgilio, and J. Winderickx. 2006. Rim15 and the crossroads of nutrient signalling pathways in Saccharomyces cerevisiae. Cell Div. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Takata, H., Y. Kanoh, N. Gunge, K. Shirahige, and A. Matsuura. 2004. Reciprocal association of the budding yeast ATM-related proteins Tel1 and Mec1 with telomeres in vivo. Mol. Cell 14:515-522. [DOI] [PubMed] [Google Scholar]
- 214.Tao, W., R. J. Deschenes, and J. S. Fassler. 1999. Intracellular glycerol levels modulate the activity of Sln1p, a Saccharomyces cerevisiae two-component regulator. J. Biol. Chem. 274:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tatebayashi, K., M. Takekawa, and H. Saito. 2003. A docking site determining specificity of Pbs2 MAPKK for Ssk2/Ssk22 MAPKKKs in the yeast HOG pathway. EMBO J. 22:3624-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Teige, M., E. Scheikl, V. Reiser, H. Ruis, and G. Ammerer. 2001. Rck2, a member of the calmodulin-protein kinase family, links protein synthesis to high osmolarity MAP kinase signaling in budding yeast. Proc. Natl. Acad. Sci. USA 98:5625-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tjandra, H., J. Compton, and D. Kellogg. 1998. Control of mitotic events by the Cdc42 GTPase, the Clb2 cyclin and a member of the PAK kinase family. Curr. Biol. 8:991-1000. [DOI] [PubMed] [Google Scholar]
- 218.Truckses, D. M., J. E. Bloomekatz, and J. Thorner. 2006. The RA domain of Ste50 adaptor protein is required for delivery of Ste11 to the plasma membrane in the filamentous growth signaling pathway of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 26:912-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vancura, A., A. Sessler, B. Leichus, and J. Kuret. 1994. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J. Biol. Chem. 269:19271-19278. [PubMed] [Google Scholar]
- 220.van Drogen, F., and M. Peter. 2002. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr. Biol. 12:1698-1703. [DOI] [PubMed] [Google Scholar]
- 221.Vincent, O., R. Townley, S. Kuchin, and M. Carlson. 2001. Subcellular localization of the Snf1 kinase is regulated by specific beta subunits and a novel glucose signaling mechanism. Genes Dev. 15:1104-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vincent, S., and J. Settleman. 1997. The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Mol. Cell. Biol. 17:2247-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Visintin, R., and A. Amon. 2001. Regulation of the mitotic exit protein kinases Cdc15 and Dbf2. Mol. Biol. Cell 12:2961-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wagner, M., M. Pierce, and E. Winter. 1997. The CDK-activating kinase CAK1 can dosage suppress sporulation defects of smk1 MAP kinase mutants and is required for spore wall morphogenesis in Saccharomyces cerevisiae. EMBO J. 16:1305-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wan, L., T. de los Santos, C. Zhang, K. Shokat, and N. M. Hollingsworth. 2004. Mek1 kinase activity functions downstream of RED1 in the regulation of meiotic double strand break repair in budding yeast. Mol. Biol. Cell 15:11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang, Y., Q. Ge, D. Houston, J. Thorner, B. Errede, and H. G. Dohlman. 2003. Regulation of Ste7 ubiquitination by Ste11 phosphorylation and the Skp1-Cullin-F-box complex. J. Biol. Chem. 278:22284-22289. [DOI] [PubMed] [Google Scholar]
- 227.Wang, Z., W. A. Wilson, M. A. Fujino, and P. J. Roach. 2001. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol. Cell. Biol. 21:5742-5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang, Z., W. A. Wilson, M. A. Fujino, and P. J. Roach. 2001. The yeast cyclins Pc16p and Pc17p are involved in the control of glycogen storage by the cyclin-dependent protein kinase Pho85p. FEBS Lett. 506:277-280. [DOI] [PubMed] [Google Scholar]
- 229.Wanke, V., I. Pedruzzi, E. Cameroni, F. Dubouloz, and C. De Virgilio. 2005. Regulation of G0 entry by the Pho80-Pho85 cyclin-CDK complex. EMBO J. 24:4271-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Warmka, J., J. Hanneman, J. Lee, D. Amin, and I. Ota. 2001. Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 21:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wedaman, K. P., A. Reinke, S. Anderson, J. Yates III, J. M. McCaffery, and T. Powers. 2003. Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell 14:1204-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Weiss, E. L., C. Kurischko, C. Zhang, K. Shokat, D. G. Drubin, and F. C. Luca. 2002. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J. Cell Biol. 158:885-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wild, A. C., J. W. Yu, M. A. Lemmon, and K. J. Blumer. 2004. The p21-activated protein kinase-related kinase Cla4 is a coincidence detector of signaling by Cdc42 and phosphatidylinositol 4-phosphate. J. Biol. Chem. 279:17101-17110. [DOI] [PubMed] [Google Scholar]
- 234.Winters, M. J., and P. M. Pryciak. 2005. Interaction with the SH3 domain protein Bem1 regulates signaling by the Saccharomyces cerevisiae p21-activated kinase Ste20. Mol. Cell. Biol. 25:2177-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wu, C., G. Jansen, J. Zhang, D. Y. Thomas, and M. Whiteway. 2006. Adaptor protein Ste50p links the Ste11p MEKK to the HOG pathway through plasma membrane association. Genes Dev. 20:734-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wu, C., E. Leberer, D. Y. Thomas, and M. Whiteway. 1999. Functional characterization of the interaction of Ste50p with Ste11p MAPKKK in Saccharomyces cerevisiae. Mol. Biol. Cell 10:2425-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wu, C., T. Leeuw, E. Leberer, D. Y. Thomas, and M. Whiteway. 1998. Cell cycle- and Cln2p-Cdc28p-dependent phosphorylation of the yeast Ste20p protein kinase. J. Biol. Chem. 273:28107-28115. [DOI] [PubMed] [Google Scholar]
- 238.Wullschleger, S., R. Loewith, W. Oppliger, and M. N. Hall. 2005. Molecular organization of target of rapamycin complex 2. J. Biol. Chem. 280:30697-30704. [DOI] [PubMed] [Google Scholar]
- 239.Wurgler-Murphy, S. M., T. Maeda, E. A. Witten, and H. Saito. 1997. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol. Cell. Biol. 17:1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Xu, S., H. K. Huang, P. Kaiser, M. Latterich, and T. Hunter. 2000. Phosphorylation and spindle pole body localization of the Cdc15p mitotic regulatory protein kinase in budding yeast. Curr. Biol. 10:329-332. [DOI] [PubMed] [Google Scholar]
- 241.Yao, S., A. Neiman, and G. Prelich. 2000. BUR1 and BUR2 encode a divergent cyclin-dependent kinase-cyclin complex important for transcription in vivo. Mol. Cell. Biol. 20:7080-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yao, S., and G. Prelich. 2002. Activation of the Bur1-Bur2 cyclin-dependent kinase complex by Cak1. Mol. Cell. Biol. 22:6750-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Young, C., J. Mapes, J. Hanneman, S. Al-Zarban, and I. Ota. 2002. Role of Ptc2 type 2C Ser/Thr phosphatase in yeast high-osmolarity glycerol pathway inactivation. Eukaryot. Cell 1:1032-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yuzyuk, T., and D. C. Amberg. 2003. Actin recovery and bud emergence in osmotically stressed cells requires the conserved actin interacting mitogen-activated protein kinase kinase kinase Ssk2p/MTK1 and the scaffold protein Spa2p. Mol. Biol. Cell 14:3013-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zappacosta, F., M. J. Huddleston, R. L. Karcher, V. I. Gelfand, S. A. Carr, and R. S. Annan. 2002. Improved sensitivity for phosphopeptide mapping using capillary column HPLC and microionspray mass spectrometry: comparative phosphorylation site mapping from gel-derived proteins. Anal. Chem. 74:3221-3231. [DOI] [PubMed] [Google Scholar]
- 246.Zhan, X. L., R. J. Deschenes, and K. L. Guan. 1997. Differential regulation of FUS3 MAP kinase by tyrosine-specific phosphatases PTP2/PTP3 and dual-specificity phosphatase MSG5 in Saccharomyces cerevisiae. Genes Dev. 11:1690-1702. [DOI] [PubMed] [Google Scholar]
- 247.Zhan, X. L., Y. Hong, T. Zhu, A. P. Mitchell, R. J. Deschenes, and K. L. Guan. 2000. Essential functions of protein tyrosine phosphatases PTP2 and PTP3 and RIM11 tyrosine phosphorylation in Saccharomyces cerevisiae meiosis and sporulation. Mol. Biol. Cell 11:663-676. [DOI] [PMC free article] [PubMed] [Google Scholar]