Abstract
Pneumococcal capsular polysaccharide (PPS) vaccines are less immunogenic in immunocompromised than immunocompetent individuals. However, neither the efficacy of PPS vaccines in immunocompromised individuals nor the host cellular subsets required for vaccine efficacy against pneumococcal disease have been directly investigated. In this study, we vaccinated CD4-deficient (CD4−/−), CD8-deficient (CD8−/−), and secretory immunoglobulin M-deficient (sIgM−/−) mice and wild-type C57BL/6 (Wt) mice with a conjugate of PPS of serotype 3 and tetanus toxoid (PPS3-TT) and determined the antibody response and efficacy of vaccination against systemic and pulmonary challenge with serotype 3 pneumococcus in immunized and control mice. Our results showed that the isotype and predominant IgG subclass of the PPS3 response differed between immunodeficient mouse strains and between immunodeficient and Wt mice, with CD8−/− mice having the most robust response. Vaccination protected Wt, CD4−/−, and sIgM−/− mice from death resulting from both systemic and pulmonary challenge, whereas CD8−/− mice were protected only from systemic and not from pulmonary challenge. Passive vaccination with PPS3-TT-induced sera from Wt, CD4−/−, CD8−/−, and sIgM−/− mice protected naïve Wt mice from death due to pulmonary challenge; however, CD8−/− mice were not protected by sera from Wt or CD8−/− mice. Our findings suggest that PPS-based vaccines can be effective in the setting of CD4 T-cell deficiency but that CD8 T cells could be required for vaccine-mediated protection against pulmonary challenge with serotype 3 pneumococcus.
Pneumococcus is the most common identifiable cause of pneumonia in the United States and many other countries (4, 7, 27, 47). The incidence of pneumococcal disease remains high, despite the fact that pneumococcal vaccines have been in use in adults in the United States for over 20 years (25). Alarmingly, there are more deaths due to pneumococcal disease in the United States than to any other vaccine-preventable illness (37). Initial studies of pneumococcal capsular polysaccharide (PPS)-based vaccine efficacy demonstrated the ability of such vaccines to prevent bacteremic pneumonia in individuals who were at risk for disease by virtue of their employment as miners (31, 78). However, subsequent efforts to establish PPS-based vaccine efficacy against pneumonia have proven to be inconclusive. Uncertainty regarding the efficacy of these vaccines against pneumonia has been attributed to multiple factors, include the difficulty in making a definitive diagnosis of pneumococcal pneumonia in the absence of positive blood cultures; the relatively low prevalence of disease, which rendered prospective trials unfeasible; and the varied study designs used to address this question (22, 23, 34, 85). In addition, there are no universally accepted surrogates for vaccine efficacy, although the use of a standardized opsophagocytic assay to assess serum antibody biological activity has been advocated in recent years (9, 32, 70, 71). The use of this or related assays was invaluable in establishing the efficacy of a PPS-based protein conjugate vaccine in infants and young children (21, 79, 83). At present, in the United States, this 7-valent PPS-based conjugate vaccine is recommended for infants and young children, while 23-valent unconjugated PPS-based vaccines are recommended for adults (16, 17).
The burden of pneumococcal pneumonia in the United States and the developing world, particularly in human immunodeficiency virus (HIV)-infected children, cannot be overstated (43). Therefore, children are a major target population for PPS-based vaccines in the United States and globally, and there is evidence that the use of these vaccines in infants and children protects adults through herd immunity (18). Despite the success of conjugate vaccines in children, concerns about serotype replacement remain. A serotype of potential concern is serotype 3, as it is not included in the conjugate vaccine used in the United States and is an important cause of adult pneumococcal disease. It is possible that future PPS-based conjugate vaccines will include the PPS of serotype 3 (PPS3), which is included in vaccines under investigation outside the United States (64). Conjugate vaccination may also be useful in certain adult populations, particularly those with immune deficiency (19, 51, 55). In addition to questions regarding PPS-based vaccine efficacy against pneumonia, it has been recognized for more than 2 decades that PPS-based vaccines are less effective in immunocompromised adults (13, 24, 62), but vaccine efficacy in immunocompromised individuals has not been directly evaluated. This study was undertaken to investigate this question. We determined the cellular subsets required for efficacy of an experimental PPS3-protein conjugate vaccine against pulmonary and systemic infection with serotype 3 pneumococcus in mice.
MATERIALS AND METHODS
Streptococcus pneumoniae and PPS.
S. pneumoniae serotype 3 strain WU2 (kindly provided by Susan Hollingshead, University of Alabama at Birmingham, Birmingham, AL) has been used extensively in studies of antibody immunity against pneumococcus (67, 82). Pneumococci were grown in tryptic soy broth (Difco Laboratories, Detroit, MI) to mid-log phase at 37°C in 5% CO2 as described previously (74). Aliquots were frozen in tryptic soy broth-10% glycerol at −80°C for use as needed. Purified PPS3 (6303) was obtained from the ATCC. PPS3 conjugated to tetanus toxoid (PPS3-TT), the conjugate used for mouse immunization (obtained from the University of Massachusetts Biologic Laboratories, Worcester, MA), was described previously (20).
Mouse strains and vaccination protocols.
Breeding pairs of secretory immunoglobulin M-deficient (sIgM−/−) mice were bred at the Albert Einstein College of Medicine Animal Facility. CD4+ T-cell knockout (CD4−/−) mice and CD8+ T-cell knockout (CD8−/−) mice were also bred at the Albert Einstein College of Medicine Animal Facility. These mice were all on a C57BL/6 background. C57BL/6 mice were obtained from the National Cancer Institute (Charles River Labs, Wilmington, MA). The sIgM−/− mouse does not secrete IgM but expresses membrane-bound IgM and IgD and undergoes class switching to express other Ig isotypes (8). The CD4−/− mouse lacks CD4+ T cells but can perform certain T-cell functions, including generating T killer cells and T helper function with non-CD4+ cells (65). The CD8−/− mouse is deficient in functional cytotoxic T cells with normal T helper cell development and function (29). Overall, 5 or 10 mice per group (depending on mouse strain availability) were vaccinated with a total dose of 2.5 μg of PPS3-TT with alhydrogel (Brenntag Biosector, Frederikssund, Denmark) as described previously (20). The vaccinations were administered subcutaneously at the base of the tail. The mice were boosted twice on days 14 and 28 after primary immunization. Phosphate-buffered saline (PBS) and TT were used as control immunogens.
Serological studies of immunized mice.
The mice were bled from the retro-orbital sinus at 7 days after the last immunization; the sera were separated by centrifugation and stored at −20°C until analyzed. Titers of PPS3-specific antibodies were determined by an antigen capture enzyme-linked immunosorbent assay (ELISA) as described elsewhere (20, 74). Briefly, 96-well polystyrene ELISA plates (Corning Glass Works, Corning, NY) were coated with 10 μg/ml PPS3 in PBS for 3 h at room temperature, washed with PBS-0.05% Tween using a SkanWasher 400 (Molecular Devices, Sunnyvale, CA), and blocked with 1% bovine serum albumin-PBS. After washing, the titers of the serum samples, which were preadsorbed at 37°C for 30 min with 50 μg/ml purified pneumococcal cell wall polysaccharide (Statens Seruminstitut, Copenhagen, Denmark), were determined starting at a 1:10 dilution, and the samples were incubated at 37°C for 1 h. After further washing, the plates were incubated at 37°C for 1 h with alkaline phosphatase-conjugated goat antibodies to mouse IgG, IgM, IgG1, IgG2c, IgG2b, and IgG3 (Southern Biotechnology, Birmingham, AL). IgG2c was examined because in C57BL/6 mice, the IgG2a gene is deleted and an IgG2c isotype is expressed (54). Antibody binding was detected with p-nitrophenyl phosphate substrate (Sigma) in diethanolamine buffer (pH 9.8). Optical densities (ODs) were measured at 405 nm with a Sunrise absorbance reader (Tecan US, Durham, NC). Antibody levels were defined as the average OD of duplicate wells of the samples minus the background. The background for each plate was defined as the OD of the detection antibody alone. The titers of TT-specific antibodies were determined by ELISA as described above, except that the plates were coated with 10 μg/ml TT in PBS.
Mouse pneumococcus challenge experiments.
Two experimental challenge models were used: (i) an active challenge model to evaluate the efficacy of PPS3-TT against intraperitoneal (i.p.) and intranasal (i.n.) challenge in immunodeficient mice and (ii) a passive immunization model to evaluate the efficacy of PPS3-TT-elicited sera against i.n. challenge in naïve mice. In the first model, PPS3-TT-, TT-, or PBS-treated mice were injected with 100 CFU i.p. or 2 × 107 CFU i.n. at 7 days after the last immunization. In the second model, sera from 10 to 15 mice in each group of mice were pooled 7 days after the last immunization, diluted 1:10 in PBS, and heat inactivated for 30 min at 56°C. One hundred microliters of the diluted sera was given to groups of eight 6- to 8-week-old female C57BL/6 mice i.p. at 1 h before i.n. infection with 108 pneumococci. The same model was used to determine the efficacy of immune sera (IS) from C57BL/6 or CD8−/− mice in naïve CD8−/− mice. Preimmune serum (PS) from the relevant mouse strain was used as the control for each infection group in the passive challenge experiments. The number of live bacteria used was confirmed by counting the CFU on blood agar plates immediately before and after the mouse inoculations. The mice were monitored twice daily, and survival was recorded. Here we report the results of three independent experiments in the passive immunization model with naïve wild-type (Wt) mice, two independent experiments in the passive immunization model with CD8−/− mice, and one experiment in the active immunization model. The number of mice used in each experiment is indicated in the figure legends.
Opsonophagocytic killing assay.
The capacity for the sera from PPS3-TT-immunized and control mice to enhance phagocytosis and the killing activity of mouse polymorphoneutrophils (PMNs) was determined using an opsonophagocytic killing assay as described previously (56). Briefly, healthy C57BL/6 mice were bled, and PMNs were isolated from whole blood by using a Ficoll-Paque gradient as described previously (89). Sera from PPS3-TT-immunized immunodeficient mice were heat inactivated for 30 min at 56°C. Then, 2 ×103 CFU of serotype 3 pneumococcus were combined with 10 μl of the sera, and the volume was adjusted to 50 μl with Hanks balanced salt solution (Cambrex, Walkersville, MD). After incubation for a total of 30 min at room temperature, 40 μl of PMNs at a concentration of 2.5 × 107 cells/ml and 10 μl of mouse serum (Sigma-Aldrich, St. Louis, MO) used as a complement source were added, and the mixture was allowed to incubate for 1 h at 37°C with shaking. The ratio of PMNs to pneumococci for all experiments was 500:1. This effector-to-target ratio has previously been reported as effective for mediating opsonophagocytosis of serotype 3 pneumococcus (28). After incubation, Hanks balanced salt solution was added to the samples to bring the volume up to 1 ml, and immediately thereafter 50 μl of the solution was plated onto blood agar plates (Becton Dickinson, Franklin Lakes, NJ) in triplicate. The plates were incubated overnight at 37°C with 5% CO2, and then the colonies were counted.
Statistics.
Mouse survival was statistically evaluated by the Kaplan-Meier log rank test. Differences in antibody titers were determined using a one-way analysis of variance (ANOVA). If ANOVA indicated a group difference, then Bonferroni's multiple-comparison test was used to test for a significant difference among groups. Comparisons between the effects of the IS and PS in the opsonophagocytosis were performed with an unpaired t test. Statistical comparisons were not made among IS from different immunodeficient mice strains because these sera were not standardized. All statistical analyses were performed using Prism (v.4.02 for Windows; GraphPad Software, San Diego, CA). A P value of <0.05 was considered statistically significant.
RESULTS
Serological studies.
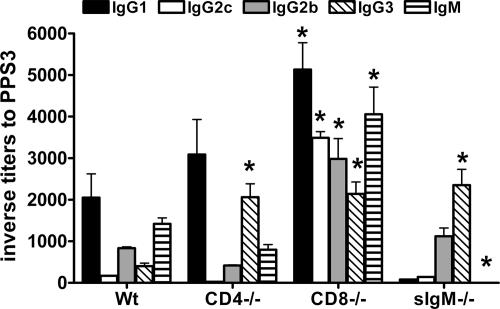
The titers of PPS3-specific IgM and IgG in the sera of PPS3-TT immunized mice were determined by ELISA (Fig. 1). PPS3-TT induced an antibody response in all mouse strains, but the isotype and IgG subclass distribution differed as a function of the mouse strain. In C57BL/6 (Wt) mice, the Ig subclasses were, in decreasing order, IgG1 > IgM > IgG2b > IgG3 > IgG2c. In CD4−/− mice, they were IgG1 > IgG3 > IgM > IgG2b > IgG2c; in CD8−/− mice, they were IgG1 > IgM > IgG2c > IgG2b > IgG3; and in sIgM−/− mice, they were IgG3 > IgG2b ≫ IgG1 ≈ IgG2c. IgG1 titers were the highest in C57BL/6, CD4−/−, and CD8−/− mice and the lowest in sIgM−/− mice. Overall, the highest titers were found among CD8−/− mice. The titer of IgG2c was highest in CD8−/− mice, being more than 100-fold higher than that among Wt, CD4−/−, and sIgM−/− mice. Titers of IgA were determined but were below the level of detection in most mice. All mice produced antibody to TT. The predominant IgG subclass of these antibodies was IgG1 in all the mouse strains. The titers of TT-specific IgG1 in Wt, CD4−/−, CD8−/−, and sIgM−/− mice were 1,450, 1,080, 420, and 220, respectively.
FIG. 1.
Serum isotype and IgG subclass profiles of PPS3-TT-immunized mice. Inverse titers to PPS3, determined by ELISA, are plotted on the y axis for the mouse strains indicated on the x axis. Mean values and standard deviations are shown for the indicated groups of five mice. Asterisks indicate P values of <0.05 by one-way ANOVA followed by Bonferroni's multiple-comparison test, comparing IS obtained 35 days after primary immunization from immunodeficient (CD4−/−, CD8−/−, or sIgM−/−) mice to IS from Wt (C57BL/6) mice. The data shown are from a representative experiment that was repeated three times, with similar results.
Active protection experiments. (i) Systemic infection.
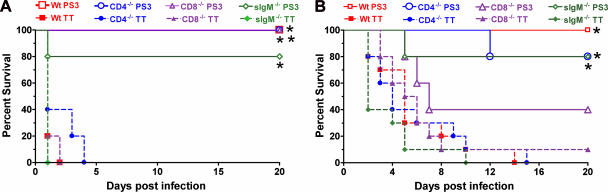
All PPS3-TT-immunized Wt, CD4−/−, and CD8−/− mice and 80% of PPS3-TT-immunized sIgM−/− mice survived i.p. challenge, whereas no TT- or PBS-immunized control mice survived after 4 days (Fig. 2A). The survival of each strain of PPS3-TT-immunized mice was significantly longer than that of PBS- or TT-treated mice (P < 0.05) (Fig. 2A).
FIG. 2.
(A) Survival of PPS3-TT-immunized mice after i.p. challenge with serotype 3 pneumococcus. PS3, mice immunized with PPS3-TT; TT. mice treated with TT. Survival of all PS3 mouse groups (C57BL/6 [Wt], CD4−/−, CD8−/−, and sIgM−/−) was significantly prolonged compared to that of TT- and PBS-treated mice (P < 0.015 [asterisk]; Kaplan-Meier log rank survival test [n = 5 mice per group]). (B) Survival of PPS3-TT-immunized mice after i.n. challenge with serotype 3 pneumococcus. Survival of PS3-immunized C57BL/6, CD4−/−, and sIgM−/− mice was significantly prolonged compared to that of the corresponding TT- and PBS-treated mice (P < 0.006 [asterisk]; Kaplan-Meier log rank survival test). There was no significant difference in survival for PS3- and control-treated CD8−/− mice (P = 0.08 compared to PBS; P = 0.17 compared to TT) (n = 5 mice per group for PPS3-TT-immunized mice; n = 10 mice per group for TT-treated mice). Survival of the PPS-treated mice is not shown, as there was no statistical difference between the survival of mice that received TT alone or PBS.
(ii) Pulmonary infection.
The survival of PPS3-TT-immunized Wt, CD4−/−, and sIgM−/− mice was significantly longer than that of both control groups, but the survival of PPS3-TT-immunized CD8−/− mice was not prolonged compared to that of either control group (Fig. 2B).
Passive immunization/challenge experiments.
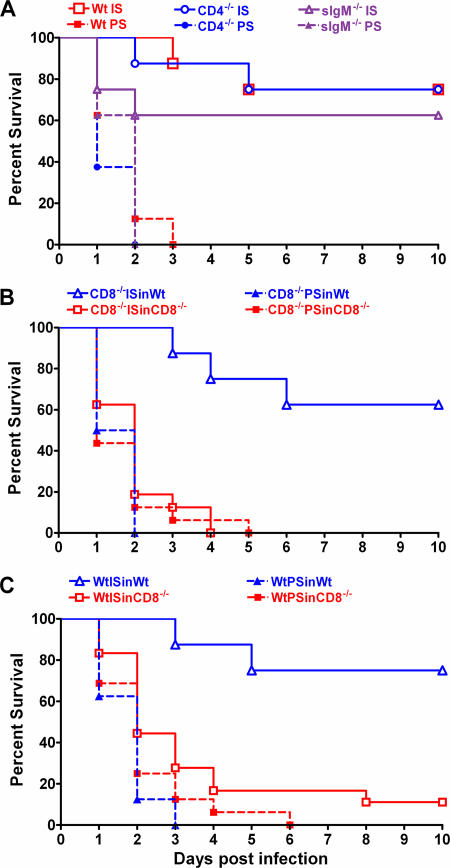
IS from Wt, CD4−/−, sIgM−/−, and CD8−/− mice significantly prolonged survival of naïve Wt mice compared to PS from each respective mouse strain (Fig. 3). PPS3-TT-elicited sera from each mouse strain protected naïve Wt mice compared to PS from the respective mouse strain (Fig. 3). Sera from CD4−/− mice protected 75% of naïve Wt mice (P < 0.001 compared to PS) (Fig. 3A), and sera from CD8−/− mice (P < 0.001 compared to PS) (Fig. 3B) and sIgM−/− mice (P < 0.05 compared to PS) (Fig. 3A) protected 63% of naïve Wt mice against i.n. challenge. Since active challenge studies showed that PPS3-TT immunization did not significantly prolong survival of CD8−/− mice against i.n. challenge (Fig. 2B) but sera from CD8−/− mice protected naïve Wt mice, we investigated whether sera from PPS3-TT-immunized CD8−/− and Wt mice could protect CD8−/− mice using the same passive protection protocol. IS from CD8−/− and Wt mice protected naïve Wt mice compared to PS (Fig. 3B and C), but neither IS prolonged survival of naïve CD8−/− mice after a lethal i.n. challenge (Fig. 3B and C).
FIG. 3.
Efficacy of passive transfer of IS from PPS3-TT-immunized mice against i.n. challenge with pneumococcus in naïve mice. IS, pooled sera obtained on day 35 after primary immunization of the indicated mice. All survival comparisons were done with the Kaplan-Meier log rank test (n = 8 mice per group for naïve Wt [C57BL/6] mice; n = 16 mice per group for naïve CD8−/− mice). (A). Efficacy of IS from PPS3-TT-immunized Wt (C57BL/6), CD4−/−, or sIgM−/− mice against i.n. challenge with serotype 3 pneumococcus in naïve Wt mice. For the PPS3-TT-immunized CD8−/− mice data, see panel B. Wt IS denotes IS from PPS3-TT-immunized Wt mice, etc. Survival of mice receiving sera from each PPS3-TT immunized group was significantly prolonged compared to that for PS-treated mice (P < 0.05). The data shown are from a representative experiment that was repeated three times with similar results. (B). Efficacy of IS from PPS3-TT-immunized CD8−/− mice against i.n. challenge with serotype 3 pneumococcus in naïve Wt and CD8−/− mice. ISinWt denotes passive transfer of the IS into naïve Wt mice, etc. (P = 0.6, comparing survival in CD8−/− mice treated with IS to those treated with PS; P < 0.001, comparing survival in Wt mice treated with IS to those treated with PS and CD8−/− mice treated with IS). (C). Efficacy of IS from PPS3-TT-immunized Wt mice against i.n. challenge with serotype 3 pneumococcus in naïve Wt and CD8−/− mice. ISinCD8−/− denotes passive transfer of the IS into naïve CD8−/− mice, etc. (P = 0.1, comparing survival in CD8−/− mice treated with IS to those treated with PS; P < 0.002, comparing CD8−/− to Wt mice treated with IS).
Opsonophagocytic killing assay.
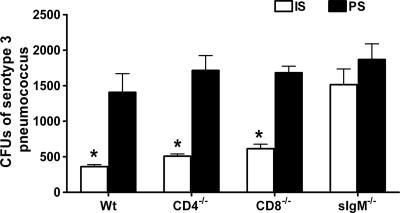
The ability of sera from PPS3-TT-immunized mice to promote killing of type 3 pneumococcus by mouse PMNs was evaluated. There was a statistically significant reduction in the number of CFU with sera from Wt, CD4−/−, and CD8−/− mice (Fig. 4). Sera from sIgM−/− mice did not promote killing. The data shown are from a representative experiment that was repeated three times with similar results.
FIG. 4.
IS-mediated opsonophagocytic killing of type 3 pneumococcus. The numbers of CFU after incubation of serotype 3 pneumococcus with C57BL/6 mouse neutrophils and IS from PPS3-TT-immunized C57BL/6, CD4−/−, CD8−/−, or sIgM−/− mice were compared in an opsonophagocytic killing assay. Mean values and standard deviations are shown for the indicated groups of 10 mice. Asterisks indicate P values of <0.05, comparing IS to PS, (unpaired t test); P < 0.02, comparing IS to PS for Wt mice; P < 0.01, comparing IS to PS for CD4−/− and CD8−/− mice; and P = 0.40, comparing IS to PS for sIgM−/− mice. The data shown are from a representative experiment that was repeated three times with similar results.
DISCUSSION
PPS-based vaccines have been shown to be less immunogenic in the setting of immune impairment (33, 39, 52, 59, 62), but their efficacy against pneumococcal disease in immunocompromised patients has not been established. Our results in this study show that although the isotype and IgG subclass response to immunization with a PPS3-TT conjugate differed between immunodeficient and normal mice, immunization protected mice with CD4 and CD8 T-cell deficiency and secretory IgM deficiency against a lethal systemic challenge with serotype 3 pneumococcus. PPS3-TT-immunized Wt, CD4−/−, and sIgM−/− mice were also protected from a lethal pulmonary challenge, but CD8−/− mice were not. The lack of protection in CD8−/− mice was a function of CD8 deficiency, rather than the nature of the antibody response per se, because passive immunization with PPS3-TT-elicited sera from CD8−/− mice protected Wt but not CD8−/− mice from death due to i.n. challenge.
PPS3-TT was immunogenic in each mouse strain. Other studies have shown that the predominant IgG subclass elicited by PPS-based vaccines in normal mice is IgG3 (60, 73). IgG3 is considered representative of a T-cell-independent (TI) immune response. In contrast, the response to PPS when conjugated to a protein carrier is dominated by IgG1 in mice and humans (45, 50, 63, 80), and this is considered representative of a T-cell-dependent (TD) response. Although the T-cell response to PPS-based conjugate vaccines has been shown to have TD features, the response can also have characteristics of a TI or an “incomplete” TD response (3, 30, 38, 49, 76). The antibody response of Wt mice to PPS3 in our study was consistent with a TD response, with IgG1 as the predominant IgG subclass. The most robust response was observed in CD8−/− mice. Compared to Wt mice, CD8−/− mice produced significantly higher titers of IgG1, IgG2c, IgG2b, and IgG3. The response of the CD8−/− mice is consistent with and extends to a new serotype previous studies showing that the magnitude of the PPS antibody response in CD8-deficient mice is higher than that in normal mice, a finding which has been attributed to CD8+ T-lymphocyte suppression of the PPS response (6, 36).
CD4−/− mice produced an IgG1 response similar in magnitude to that of Wt mice. This is not unexpected, since CD4−/− mice are not completely deficient in helper cell function (5). Although the CD4−/− mouse strain we used does not express CD4, these mice have normal cytotoxic T-lymphocyte (CTL) responses and a population of major histocompatibility complex (MHC) class II-restricted CD4− CD8− TcRαβ+ T cells that have the potential for helper function (66). Previous studies of the response to other antigens have shown that the response of CD4−/− mice to TD antigens is more robust than that of MHC class II-deficient mice (44). Studies of MHC class II-deficient mice were beyond the scope of this study. Nevertheless, although their response could be less than that of CD4−/− mice, CD4−/− mice are a plausible model of HIV-associated T-cell deficiency, since CD4+ T lymphocytes are depleted in HIV infection. We found that vaccination induced high titers of TT-specific IgG1, even in CD4−/− mice. Although it is possible that CD4−/− mice produced IgG1 via non-CD4+ helper cells (see above), it is also possible that these mice generated IgG1 by mechanisms that do not depend entirely on CD4+ T helper cell function. For example, CD8+ and CD4+ T cells have redundant abilities to produce cytokines, particularly gamma interferon (IFN-γ) (86). The predominant IgG subclass of sIgM−/− mice was IgG3, the major subclass of TI type 2 responses. These mice produced low levels of IgG1 and IgG2c and no detectable IgM. These findings are consistent with their inability to produce a robust response to a TD antigen and decreased antigen trapping, which have been described previously (8). Since sIgM−/− mice produced only small amounts of IgG1 and IgG2c, our data suggest that the regulatory properties of natural IgM are important for the generation of a TD response to PPS3.
The efficacy of PPS-based vaccines against bacteremic pneumococcal disease was established in the first half of the 20th century (31) but has not been determined in immunodeficient individuals. Currently available PPS-based vaccines are known to be less immunogenic in patients with immunodeficiency (26, 35, 46). Our data show that CD4+ and CD8+ T cells and natural IgM were each dispensable for PPS3-TT-elicited protection from death due to i.p. (systemic) challenge. Based on previous studies with a similar model, the cause of death in our model is almost certainly bacteremia (11). Since the antibody responses of the mouse strains were different, our findings suggest that there is redundancy in the antibody isotypes and T-cell subsets that protect against death due to systemic disease in our model. The ability of IS from CD4−/−, CD8−/−, and sIgM−/− mice to protect naïve Wt mice from a lethal systemic challenge suggests that CD4+ and CD8+ T cells and natural IgM are dispensable for the induction of a protective PPS3-elicited antibody response. However, further studies are required to determine which cellular subset(s) is required to mediate the response when one of the foregoing is absent.
Since IgG3 was produced by each mouse strain, whereas the other IgG subclasses either were not present or were present in very small amounts, our findings appear to implicate IgG3 in protection against systemic disease. PPS-IgG3 was shown to mediate protection against systemic challenge with another serotype (11), and phosphorylcholine-reactive IgG3 was protective against systemic challenge with serotype 3 pneumococcus (10). Determination of the identity of the actual antibody mediator(s) of protection in our model requires further study. Nonetheless, our findings hold promise for the ability of PPS-based vaccines to prevent systemic pneumococcal disease in the setting of certain immunodeficiencies.
There is a large body of information that opsonic IgG mediates protection against encapsulated microbes, including pneumococcus (41, 48, 58, 68, 89). In fact, the opsonophagocytic activity of vaccine-elicited antibody in a pneumococcal killing assay has been proposed as a suitable surrogate for vaccine efficacy (9, 70). Our data show that IS from Wt, CD4−/−, and CD8−/− mice promoted PMN-mediated killing of serotype 3 in vitro. In contrast, IS from sIgM−/− mice did not. This suggests that IgM or IgG1 or IgG2c, the IgG subclasses that sIgM−/− mice did not produce, is required for antibody-dependent PMN-mediated killing in vitro, with the caveat that the overall antibody levels of sIgM−/− mice could have been less than those of the other mice. However, the role of defined antibody isotypes/subsets is difficult to dissect with polyclonal serum (15). The nature of the protective antibodies that were produced by sIgM−/− mice requires further investigation with monoclonal or highly purified agents, which were not produced as part of this study. Nonetheless, the protection we observed against systemic and pulmonary challenge in IgM−/− mice demonstrates that the induction of antibodies that are opsonic in vitro may not be required for vaccine efficacy against bacteremia or pneumonia in our model, with the caveat that sera that are not opsonic in vitro could contain antibodies that promote effector cell killing in vivo by PMNs or other cell types. The relevance of our findings to human disease requires further study.
Our data demonstrate that CD4+ T cells and secreted IgM are dispensable for vaccine efficacy against a lethal pulmonary challenge with pneumococcus in our model. Notably, although CD4+ T-cell counts predict the magnitude of PPS-based conjugate vaccine responses in HIV-infected children, particularly those on highly active antiretroviral therapy (2), the response to PPS-based vaccines in HIV-infected adults is not a function of the CD4+ T-cell count (69). Our group has shown that reduced PPS-based vaccine responses in the setting of HIV infection are most likely due to an HIV-associated B-cell repertoire defect (1, 81). This defect does not exist in the mice. Hence, our findings with CD4−/− mice raise the hope that appropriately immunogenic vaccines could be effective in patients with CD4+ T-cell deficiency. The requirement for CD4+ T lymphocytes in antibody-mediated immunity against other microbes has been shown to be a function of CD4+ T-lymphocyte-derived cytokines (14, 87). However, since our mice were likely to have non-CD4+ T lymphocyte-mediated helper function, further studies are needed to ascertain whether T helper functions mediated by non-CD4+ T lymphocytes are required for antibody immunity against either pneumonia or systemic infection in our model.
Protection in our i.n. model was almost certainly antibody mediated, since passive transfer of heat-inactivated IS from each mouse strain was protective in naïve Wt mice. Although immunized CD8−/− mice were protected against systemic challenge, CD8−/− mice were not protected against active pulmonary challenge after immunization with PPS3-TT or passive administration of IS from Wt or CD8−/− mice. Nonetheless, our data show that CD8−/− mice had the highest titers of PPS3-reactive antibodies and that IS from these mice was opsonic in vitro and protective in naïve Wt mice. Hence, our findings suggest that although CD8+ T lymphocytes are essential for antibody-mediated protection against pulmonary challenge, they are dispensable for protection against systemic challenge. An important caveat to the foregoing is that our data do not rule out the possibility that antibodies of a defined specificity, affinity, or isotype or in a certain amount could mediate protection in the setting of CD8+ T-cell deficiency. Studies to identify such antibodies require the use of defined antibody agents, which are currently under development by our group. Nonetheless, it is interesting to note that vaccine-mediated protection against Ebola virus infection in mice required both antibody and CD8+ T lymphocytes (84). In addition, CD8+ T lymphocytes were required for clearance of West Nile virus from mouse tissues, but antibody was sufficient to abrogate viremia (77). Interestingly, the latter observation parallels our finding that CD8+ T lymphocytes were dispensable for protection against systemic disease, which involves a reduction in bacteremia (12, 88), but not against tissue-specific disease (e.g., pneumonia). Our data implicate CD8+ T lymphocytes in protection against pneumonia. Although it is possible that PPS3-induced antibodies are detrimental in CD8−/− mice, our findings that IS from CD8−/− mice protected Wt mice and that Wt IS was not protective in CD8−/− mice make this possibility unlikely.
To our knowledge, our finding that CD8+ T lymphocytes are required for antibody immunity to pulmonary challenge with pneumococcus is novel. At present, we do not know the mechanism by which CD8+ T lymphocytes could contribute to or enhance antibody immunity. This question is currently a subject of investigation in our laboratory. One possibility is that CD8+ T cell-derived IFN-γ is required for antibody efficacy. Interestingly, and similar to our findings for CD8+ T lymphocytes, IFN-γ was required for protection against pulmonary but not systemic challenge with another bacterium, Klebsiella pneumoniae (53). Along the same lines, CD8+ T-lymphocyte-derived IFN-γ was essential for CD4+ T-lymphocyte-independent control of experimental Cryptococcus neoformans pneumonia (42). Since IFN-γ was previously shown to be important for clearance of pulmonary infection with pneumococcus (72) and IFN-γ-mediated activation of phagocytes is a common mechanism of host defense, our data suggest the possibility that activated CD8+ T lymphocytes produce IFN-γ that contributes to protection against pneumococcal pneumonia. However, CD8−/− mice are also deficient in CTL function (5). Hence, our data also raise the possibility that CTLs could also be required for protection in the lung. There is ample precedent for this for other pulmonary pathogens (40, 57, 61, 75). Studies to evaluate CD8+ T-lymphocyte production of IFN-γ and CTL function in our model were beyond the scope of this study but are now under way in our laboratory.
Acknowledgments
This work was supported by grants R01AI045459 and R01044374 from NIH to L.-A.P.
We thank Kausik Datta for assistance with statistical analysis.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Abadi, J., J. Friedman, R. Jefferis, R. A. Mageed, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J. Infect. Dis. 178:707-716. [DOI] [PubMed] [Google Scholar]
- 2.Abzug, M. J., S. I. Pelton, L. Y. Song, T. Fenton, M. J. Levin, S. A. Nachman, W. Borkowsky, H. M. Rosenblatt, J. F. Marcinak, A. Dieudonne, E. J. Abrams, and I. Pathak. 2006. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr. Infect. Dis. J. 25:920-929. [DOI] [PubMed] [Google Scholar]
- 3.Anttila, M., M. Voutilainen, V. Jantti, J. Eskola, and H. Kayhty. 1999. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 118:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artz, A. S., W. B. Ershler, and D. L. Longo. 2003. Pneumococcal vaccination and revaccination of older adults. Clin. Microbiol. Rev. 16:308-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., A. Oxenius, T. W. Mak, and R. M. Zinkernagel. 1995. T cell development in CD8−/− mice. Thymic positive selection is biased toward the helper phenotype. J. Immunol. 155:3727-3733. [PubMed] [Google Scholar]
- 6.Baker, P. J. 1992. T cell regulation of the antibody response to bacterial polysaccharide antigens: an examination of some general characteristics and their implications. J. Infect. Dis. 165(Suppl. 1):S44-S48. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett, J. G., S. F. Dowell, L. A. Mandell, J. T. M. File, D. M. Musher, and M. J. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boes, M., C. Esau, M. B. Fischer, T. Schmidt, M. Carroll, and J. Chen. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776-4787. [PubMed] [Google Scholar]
- 9.Bogaert, D., M. Sluijter, R. de Groot, and P. W. Hermans. 2004. Multiplex opsonophagocytosis assay (MOPA): a useful tool for the monitoring of the 7-valent pneumococcal conjugate vaccine. Vaccine 22:4014-4020. [DOI] [PubMed] [Google Scholar]
- 10.Briles, D. E., C. Forman, and M. Crain. 1992. Mouse antibody to phosphorylcholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect. Immun. 60:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchwald, U. K., A. Lees, M. Steinitz, and L. Pirofski. 2005. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infect. Immun. 73:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchwald, U. K., and L. Pirofski. 2003. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 9:945-968. [DOI] [PubMed] [Google Scholar]
- 13.Butler, J. C., R. F. Breiman, J. F. Campbell, H. B. Lipman, C. V. Broome, and R. R. Facklam. 1993. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA 270:1826-1831. [PubMed] [Google Scholar]
- 14.Casadevall, A., and L. A. Pirofski. 2004. New concepts in antibody-mediated immunity. Infect. Immun. 72:6191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadevall, A., and M. D. Scharff. 1994. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob. Agents Chemotherap. 38:1695-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 46:1-24. [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 49:1-35. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2005. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998-2003. Morb. Mortal. Wkly. Rep. 54:893-897. [PubMed] [Google Scholar]
- 19.Chan, C. Y., D. C. Molrine, S. George, N. J. Tarbell, P. Mauch, L. Diller, R. C. Shamberger, N. R. Phillips, A. Goorin, and D. M. Ambrosino. 1996. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin's disease. J. Infect. Dis. 173:256-258. [DOI] [PubMed] [Google Scholar]
- 20.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Concepcion, N., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin. Diagn. Lab. Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornu, C., D. Yzebe, P. Leophonte, J. Gaillat, J. P. Boissel, and M. Cucherat. 2001. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 19:4780-4790. [DOI] [PubMed] [Google Scholar]
- 23.Dear, K., J. Holden, R. Andrews, and D. Tatham. 2003. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. CD000422. [DOI] [PubMed]
- 24.Dorner, T., H. P. Brezinschek, S. J. Foster, R. I. Brezinschek, N. L. Farner, and P. E. Lipsky. 1998. Delineation of selective influences shaping the mutated and expressed human Ig heavy chain repertoire. J. Immunol. 160:2831-2841. [PubMed] [Google Scholar]
- 25.Edwards, K. M., and M. R. Griffin. 2003. Great expectations for a new vaccine. N. Engl. J. Med. 349:1312-1314. [DOI] [PubMed] [Google Scholar]
- 26.Fedson, D. S., and C. Liss. 2004. Precise answers to the wrong question: prospective clinical trials and the meta-analyses of pneumococcal vaccine in elderly and high-risk adults. Vaccine 22:927-946. [DOI] [PubMed] [Google Scholar]
- 27.File, T. M. 2003. Community-acquired pneumonia. Lancet 362:1991-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine, D. P., J. L. Kirk, G. Schiffman, J. E. Schwinle, and J. C. Guckinan. 1988. Analysis of humoral and phagocytic defenses against Streptococcus pneumoniae serotypes 1 and 3. J. Lab. Clin. Med. 112:487-497. [PubMed] [Google Scholar]
- 29.Fung-Leung, W. P., M. W. Schilham, A. Rahemtulla, T. M. Kundig, M. Vollenweider, J. Potter, E. W. van, and T. W. Mak. 1991. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 65:443-449. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Ojeda, P. A., M. E. Monser, L. J. Rubenstein, H. J. Jennings, and K. E. Stein. 2000. Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent conjugate vaccine. Infect. Immun. 68:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffron, R., B. White, and R. Austin. 1979. The biology of pneumococcus. Harvard University Press, Cambridge, MA.
- 32.Hu, B. T., X. Yu, T. R. Jones, C. Kirch, S. Harris, S. W. Hildreth, D. V. Madore, and S. A. Quataert. 2005. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 12:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huo, Z., J. Miles, T. Harris, and P. Riches. 2002. Effect of Pneumovax II vaccination in high-risk individuals on specific antibody and opsonic capacity against specific and non-specific antigen. Vaccine 20:3532-3534. [DOI] [PubMed] [Google Scholar]
- 34.Hutchison, B. G., A. D. Oxman, H. S. Shannon, S. Lloyd, C. A. Altmayer, and K. Thomas. 1999. Clinical effectiveness of pneumococcal vaccine. Meta-analysis. Can. Fam. Physician 45:2381-2393. [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson, L. A., K. M. Neuzil, O. yu, P. Benson, W. E. Barlow, A. L. Adams, C. A. Hanson, L. D. Mahoney, D. K. Shay, W. W. Thompson, and the Vaccine Study Datalink. 2003. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 348:1755. [DOI] [PubMed] [Google Scholar]
- 36.Jeurissen, A., M. Wuyts, A. Kasran, S. Ramdien-Murli, L. Boon, J. L. Ceuppens, and X. Bossuyt. 2002. Essential role for CD40 ligand interactions in T lymphocyte-mediated modulation of the murine immune response to pneumococcal capsular polysaccharides. J. Immunol. 168:2773-2781. [DOI] [PubMed] [Google Scholar]
- 37.Kadioglu, A., and P. W. Andrew. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25:143-149. [DOI] [PubMed] [Google Scholar]
- 38.Kamboj, K. K., H. L. Kirchner, R. Kimmel, N. S. Greenspan, and J. R. Schreiber. 2003. Significant variation in serotype-specific immunogenicity of the seven-valent Streptococcus pneumoniae capsular polysaccharide-CRM197 conjugate vaccine occurs despite vigorous T cell help induced by the carrier protein. J. Infect. Dis. 187:1629-1638. [DOI] [PubMed] [Google Scholar]
- 39.Kroon, F. P., J. T. van Dissel, E. Ravensbergen, P. H. Nibbering, and R. van Furth. 1999. Antibodies against pneumococcal polysaccharides after vaccination in HIV-infected individuals: 5-year follow-up of antibody concentrations. Vaccine 18:524-530. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence, C. W., R. M. Ream, and T. J. Braciale. 2005. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J. Immunol. 174:5332-5340. [DOI] [PubMed] [Google Scholar]
- 41.Lefeber, D. J., B. Benaissa-Trouw, J. F. Vliegenthart, J. P. Kamerling, W. T. Jansen, K. Kraaijeveld, and H. Snippe. 2003. Th1-directing adjuvants increase the immunogenicity of oligosaccharide-protein conjugate vaccines related to Streptococcus pneumoniae type 3. Infect. Immun. 71:6915-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindell, D. M., T. A. Moore, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J. Immunol. 174:7920-7928. [DOI] [PubMed] [Google Scholar]
- 43.Madhi, S. A., E. Cumin, and K. P. Klugman. 2002. Defining the potential impact of conjugate bacterial polysaccharide-protein vaccines in reducing the burden of pneumonia in human immunodeficiency virus type 1-infected and -uninfected children. Pediatr. Infect. Dis. J. 21:393-399. [DOI] [PubMed] [Google Scholar]
- 44.Madsen, L., N. Labrecque, J. Engberg, A. Dierich, A. Svejgaard, C. Benoist, D. Mathis, and L. Fugger. 1999. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA 96:10338-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makela, O., P. Mattila, N. Rautonen, I. Seppala, J. Eskola, and H. Kayhty. 1987. Isotype concentrations of human antibodies to Haemophilus influenzae type b polysaccharide (Hib) in young adults immunized with the polysaccharide as such or conjugated to a protein (diphtheria toxoid). J. Immunol. 139:1999-2004. [PubMed] [Google Scholar]
- 46.Mangtani, P., F. Cutts, and A. J. Hall. 2003. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect. Dis. 3:71-78. [DOI] [PubMed] [Google Scholar]
- 47.Marrie, T. J. 1999. Pneumococcal pneumonia: epidemiology and clinical features. Semin. Respir. Infect. 14:227-235. [PubMed] [Google Scholar]
- 48.Martinez, J. E., E. A. Clutterbuck, H. Li, S. Romero-Steiner, and G. M. Carlone. 2006. Evaluation of multiplex flow cytometric opsonophagocytic assays for determination of functional anticapsular antibodies to Streptococcus pneumoniae. Clin. Vaccine Immunol. 13:459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCool, T. L., C. V. Harding, N. S. Greenspan, and J. R. Schreiber. 1999. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect. Immun. 67:4862-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLay, J., E. Leonard, S. Petersen, D. Shapiro, N. S. Greenspan, and J. R. Schreiber. 2002. Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J. Immunol. 168:3437-3443. [DOI] [PubMed] [Google Scholar]
- 51.Miiro, G., H. Kayhty, C. Watera, H. Tolmie, J. A. Whitworth, C. F. Gilks, and N. French. 2005. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J. Infect. Dis. 192:1801-1805. [DOI] [PubMed] [Google Scholar]
- 52.Moore, R. A., P. J. Wiffen, and B. A. Lipsky. 2000. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam. Pract. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore, T. A., M. L. Perry, A. G. Getsoian, M. W. Newstead, and T. J. Standiford. 2002. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect. Immun. 70:6310-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgado, M. G., P. Cam, C. Gris-Liebe, P. A. Cazenave, and E. Jouvin-Marche. 1989. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 8:3245-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musher, D. M., J. E. Groover, D. A. Watson, M. C. Rodriguez-Barradas, and R. E. Baughn. 1998. IgG responses to protein-conjugated pneumococcal capsular polysaccharides in persons who are genetically incapable of responding to unconjugated polysaccharides. Clin. Infect. Dis. 27:1487-1490. [DOI] [PubMed] [Google Scholar]
- 56.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698-703. [DOI] [PubMed] [Google Scholar]
- 57.Neff-LaFord, H. D., B. A. Vorderstrasse, and B. P. Lawrence. 2003. Fewer CTL, not enhanced NK cells, are sufficient for viral clearance from the lungs of immunocompromised mice. Cell. Immunol. 226:54-64. [DOI] [PubMed] [Google Scholar]
- 58.Netski, D., and T. R. Kozel. 2002. Fc-dependent and Fc-independent opsonization of Cryptococcus neoformans by anticapsular monoclonal antibodies: importance of epitope specificity. Infect. Immun. 70:2812-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nielsen, H., B. Kvinesdal, T. L. Benfield, J. D. Lundgren, and H. B. Konradsen. 1998. Rapid loss of specific antibodies after pneumococcal vaccination in patients with human immunodeficiency virus-1 infection. Scand. J. Infect. Dis. 30:597-601. [DOI] [PubMed] [Google Scholar]
- 60.Perlmutter, R. M., D. Hansburg, D. E. Briles, R. A. Nicolotti, and J. M. Davie. 1978. Subclass restriction of murine anti-carbohydrate antibodies. J. Immunol. 121:566-572. [PubMed] [Google Scholar]
- 61.Pinchuk, I., B. C. Starcher, B. Livingston, A. Tvninnereim, S. Wu, E. Appella, J. Sidney, A. Sette, and B. Wizel. 2005. A CD8+ T cell heptaepitope minigene vaccine induces protective immunity against Chlamydia pneumoniae. J. Immunol. 174:5729-5739. [DOI] [PubMed] [Google Scholar]
- 62.Pirofski, L., and A. Casadevall. 1998. The use of licensed vaccines for active immunization of the immunocompromised host. Clin. Microbiol. Rev. 11:1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poland, G. A. 1999. The burden of pneumococcal disease: the role of conjugate vaccines. Vaccine 17:1674-1679. [DOI] [PubMed] [Google Scholar]
- 64.Puumalainen, T., N. Ekstrom, R. Zeta-Capeding, J. Ollgren, K. Jousimies, M. Lucero, H. Nohynek, and H. Kayhty. 2003. Functional antibodies elicited by an 11-valent diphtheria-tetanus toxoid-conjugated pneumococcal vaccine. J. Infect. Dis. 187:1704-1708. [DOI] [PubMed] [Google Scholar]
- 65.Rahemtulla, A., W. P. Fung-Leung, M. W. Schilham, T. M. Kundig, S. R. Sambhara, A. Narendran, A. Arabian, A. Wakeham, C. J. Paige, R. M. Zinkernagel, et al. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353:180-184. [DOI] [PubMed] [Google Scholar]
- 66.Rahemtulla, A., A. Shahinian, T. Kundig, R. Zinkernagel, and T. W. Mak. 1993. CD4 negative mice as a model for immunodeficiency. Philos. Trans. R. Soc. London B 342:57-58. [DOI] [PubMed] [Google Scholar]
- 67.Ren, B., A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robbins, J. B., R. Schneerson, and S. C. Szu. 1995. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 171:1387-1398. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Barradas, M. C., I. Alexandraki, T. Nazir, M. Foltzer, D. M. Musher, S. Brown, and J. Thornby. 2003. Response of human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy to vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin. Infect. Dis. 37:438-447. [DOI] [PubMed] [Google Scholar]
- 70.Romero-Steiner, S., C. E. Frasch, G. Carlone, R. A. Fleck, D. Goldblatt, and M. H. Nahm. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romero-Steiner, S., D. Libutti, L. B. Pais, et al. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65:2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubinstein, L. J., P. A. Garcia-Ojeda, F. Michon, H. J. Jennings, and K. E. Stein. 1998. Murine immune responses to Neisseria meningitidis group C capsular polysaccharide and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 66:5450-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell, N., J. R. Corvalan, M. L. Gallo, C. G. Davis, and L. Pirofski. 2000. Production of protective human antipneumococcal antibodies by transgenic mice with human immunoglobulin loci. Infect. Immun. 68:1820-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sable, S. B., S. Kaur, I. Verma, and G. K. Khuller. 2005. Immunodominance of low molecular weight secretory polypeptides of Mycobacterium tuberculosis to induce cytotoxic T-lymphocyte response. Vaccine 23:4947-4954. [DOI] [PubMed] [Google Scholar]
- 76.Shapiro, D. A., D. S. Threadgill, M. J. Copfer, D. A. Corey, T. L. McCool, L. L. McCormick, T. R. Magnuson, N. S. Greenspan, and J. R. Schreiber. 1998. Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides undergo normal isotype switching after immunization with polysaccharide-protein conjugate vaccines. J. Immunol. 161:3393-3399. [PubMed] [Google Scholar]
- 77.Shrestha, B., and M. S. Diamond. 2004. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 78:8312-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smit, P., D. Oberholzer, S. Hayden-Smith, H. J. Koornhof, and M. R. Hilleman. 1977. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA 238:2613-2616. [PubMed] [Google Scholar]
- 79.Soininen, A., M. Karpala, S. L. Wahlman, H. Lehtonen, and H. Kayhty. 2002. Specificities and opsonophagocytic activities of antibodies to pneumococcal capsular polysaccharides in sera of unimmunized young children. Clin. Diagn. Lab. Immunol. 9:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sorensen, R. U., L. E. Leiva, P. A. Giangrosso, B. Butler, F. C. Javier III, D. M. Sacerdote, N. Bradford, and C. Moore. 1998. Response to a heptavalent conjugate Streptococcus pneumoniae vaccine in children with recurrent infections who are unresponsive to the polysaccharide vaccine. Pediatr. Infect. Dis. J. 17:685-691. [DOI] [PubMed] [Google Scholar]
- 81.Subramaniam, K. S., R. Segal, R. H. Lyles, M. C. Rodriguez-Barradas, and L. A. Pirofski. 2003. Qualitative change in antibody responses of human immunodeficiency virus-infected individuals to pneumococcal capsular polysaccharide vaccination associated with highly active antiretroviral therapy. J. Infect. Dis. 187:768. [DOI] [PubMed] [Google Scholar]
- 82.Thornton, J., and L. S. McDaniel. 2005. THP-1 monocytes up-regulate intercellular adhesion molecule 1 in response to pneumolysin from Streptococcus pneumoniae. Infect. Immun. 73:6493-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vakevainen, M., W. Jansen, E. Saeland, I. Jonsdottir, H. Snippe, A. Verheul, and H. Kayhty. 2001. Are the opsonophagocytic activities of antibodies in infant sera measured by different pneumococcal phagocytosis assays comparable? Clin. Diagn. Lab. Immunol. 8:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warfield, K. L., G. Olinger, E. M. Deal, D. L. Swenson, M. Bailey, D. L. Negley, M. K. Hart, and S. Bavari. 2005. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J. Immunol. 175:1184-1191. [DOI] [PubMed] [Google Scholar]
- 85.Watson, L., B. J. Wilson, and N. Waugh. 2002. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 20:2166-2173. [DOI] [PubMed] [Google Scholar]
- 86.Wuthrich, M., T. Filutowicz, T. Warner, G. S. Deepe, and B. S. Klein. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan, R., A. Casadevall, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify the course of Cryptococcus neoformans in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong, Z., and L. Pirofski. 1996. Opsonization of Cryptococcus neoformans by human antiglucuronoxylomannan antibodies. Infect. Immun. 64:3446-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]