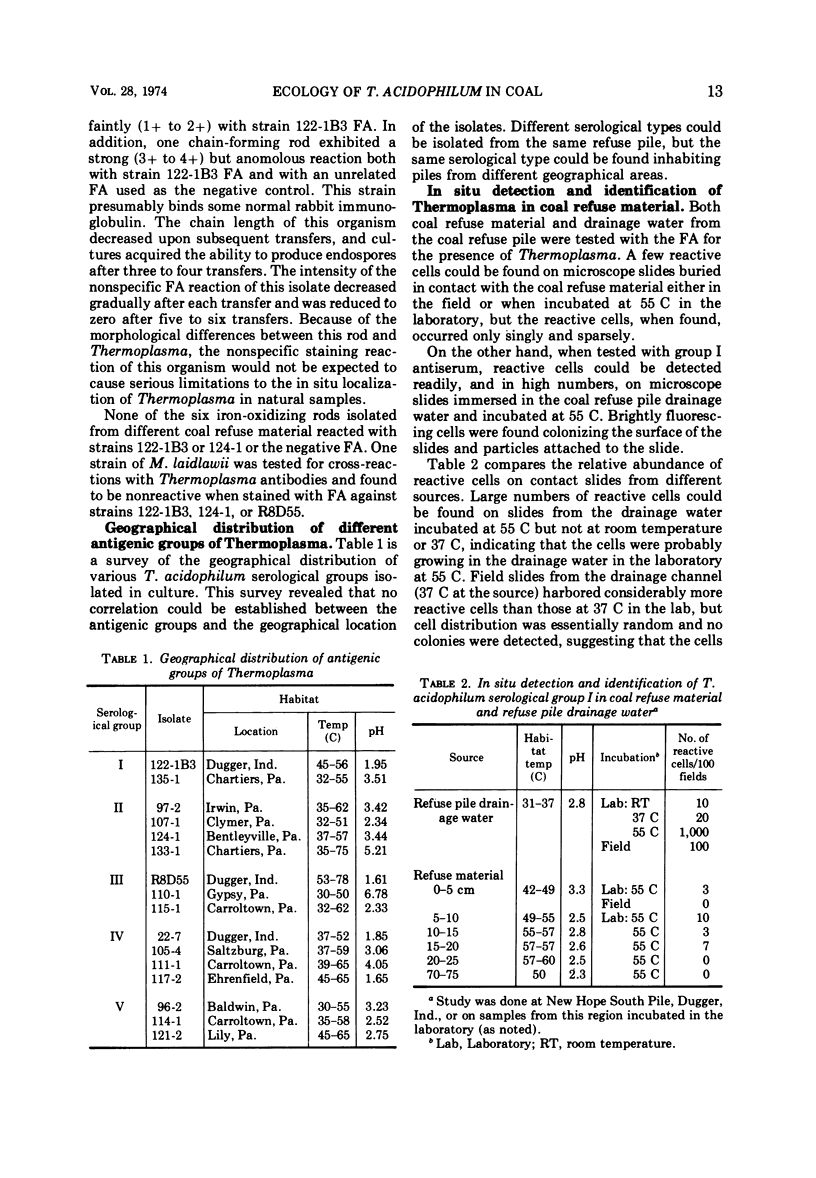
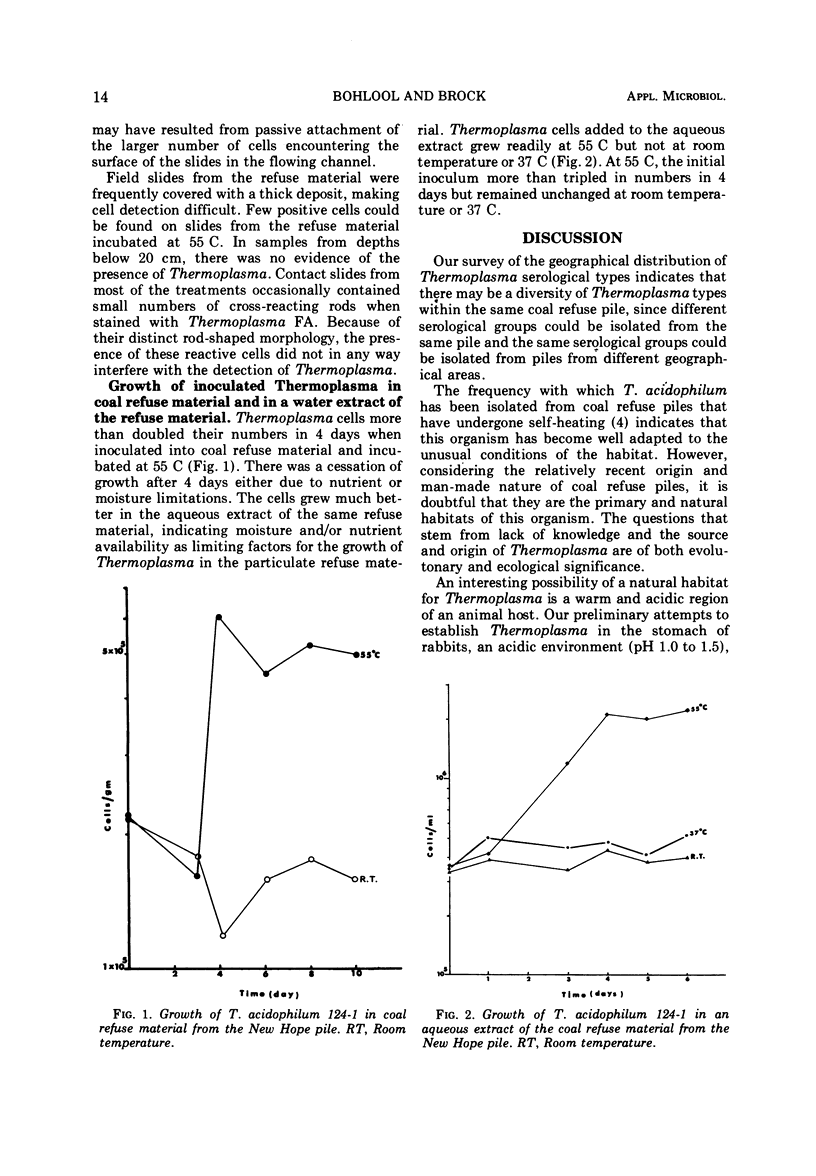
Abstract
Specific immunofluorescence staining was applied to the study of the localization, distribution, and growth of Thermoplasma acidophilum in its natural habitat, the coal refuse pile. Different antigenic groups of T. acidophilum could be isolated from the same refuse pile, and the same antigenic groups were isolated from piles from different geographical areas. No correlation could be established between the antigenic groups and the pH or temperature of the habitats. Brightly fluorescing cells of T. acidophilum were detected on microscope slides buried in contact with the coal refuse material or immersed in the water in the stream draining a refuse pile. T. acidophilum grew when inoculated into either coal refuse material and/or an aqueous extract of coal refuse when incubated at its optimal temperature of 55 C, but not when incubated at room temperature or 37 C. The coal refuse pile appears to be a primary habitat for T. acidophilum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- Belly R. T., Brock T. D. Cellular stability of a thermophilic, acidophilic mycoplasma. J Gen Microbiol. 1972 Dec;73(3):465–469. doi: 10.1099/00221287-73-3-465. [DOI] [PubMed] [Google Scholar]
- Belly R. T., Brock T. D. Ecology of iron-oxidizing bacteria in pyritic materials associated with coal. J Bacteriol. 1974 Feb;117(2):726–732. doi: 10.1128/jb.117.2.726-732.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Darland G., Brock T. D., Samsonoff W., Conti S. F. A thermophilic, acidophilic mycoplasma isolated from a coal refuse pile. Science. 1970 Dec 25;170(3965):1416–1418. doi: 10.1126/science.170.3965.1416. [DOI] [PubMed] [Google Scholar]
- Garcia M. M., Neil D. H., McKay K. A. Application of immunofluorescence to studies on the ecology of Sphaerophorus necrophorus. Appl Microbiol. 1971 May;21(5):809–814. doi: 10.1128/am.21.5.809-814.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. A., Johnson R. C., Bohlool B. B., Schmidt E. L. Detection of Leptospira in soil and water by immunofluorescence staining. Appl Microbiol. 1971 May;21(5):953–956. doi: 10.1128/am.21.5.953-956.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill I. R., Gray T. R. Application of the fluorescent-antibody technique to an ecological study of bacteria in soil. J Bacteriol. 1967 Jun;93(6):1888–1896. doi: 10.1128/jb.93.6.1888-1896.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langworthy T. A., Smith P. F., Mayberry W. R. Lipids of Thermoplasma acidophilum. J Bacteriol. 1972 Dec;112(3):1193–1200. doi: 10.1128/jb.112.3.1193-1200.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Langworth T. A., Mayberry W. R., Houghland A. E. Characterization of the membranes of Thermoplasma acidophilum. J Bacteriol. 1973 Nov;116(2):1019–1028. doi: 10.1128/jb.116.2.1019-1028.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey M. R., Brock T. D. Dactylaria gallopava, a cause of avian encephalitis, in hot spring effluents, thermal soils and self-heated coal waste piles. Nature. 1973 Mar 16;242(5394):202–203. doi: 10.1038/242202a0. [DOI] [PubMed] [Google Scholar]


