Abstract
Interactions between macrophages and lymphocytes through costimulatory molecules and cytokines are essential for mounting an efficient immune response and controlling its pathogenic potential. Here we demonstrate the immunomodulatory capacity of Trypanosoma cruzi, the causative agent of Chagas' disease, through its ability to induce differential expression of costimulatory molecules and cytokines by monocytes and T cells. Costimulatory molecule and cytokine modulation was evaluated using cells from noninfected individuals and from patients with the asymptomatic indeterminate form and those with the severe cardiac clinical form of Chagas' disease. Our results show that while exposure of monocytes to live T. cruzi leads to an increase in the frequency of CD80+ monocytes in all groups, it decreases both the frequency and intensity of CD86 expression by monocytes from patients with the cardiac form but not from those with the indeterminate form. Conversely, exposure of lymphocytes to monocytes infected with T. cruzi increased the surface expression of cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) by T cells from indeterminate but not from cardiac patients, compared to that from control patients. These data suggest that T. cruzi induces a potentially down-regulatory environment in indeterminate subjects, which is associated with higher CD80 and CTLA-4 expression. To test the functional importance of this modulation, we evaluated the expression of cytokines after in vitro infection. Although exposure of lymphocytes to parasite-infected monocytes induced high expression of inflammatory and anti-inflammatory cytokines by T cells in all groups, indeterminate patients displayed a higher ratio of monocytes expressing interleukin 10 than tumor necrosis factor alpha following infection than did controls. These data show the ability of T. cruzi to actively change the expression of costimulatory molecules and cytokines, suggesting molecular mechanisms for the differential clinical evolution of human Chagas' disease.
Following infection in humans by pathogens, immune responses are mounted that lead to the control of the infectious agent. In many instances, the control is not associated with a sterile elimination of the pathogen but rather effective control of its replication in vivo. A well-balanced, adaptive immune response plays a critical role in maintaining control of the pathogen in these cases. This is especially true for parasitic infections. Interestingly, regulation of the adaptive immune response is essential not only for controlling parasite replication but also for minimizing immune-mediated pathology (2, 7, 15). It has been suggested that parasites can induce production of cytokines that decrease the expression of molecules critical for T-cell stimulation, such as major histocompatibility complex (MHC) class II and costimulatory molecules, possibly as a strategy for survival in the host (38). On the other hand, exacerbated responses, while efficient in eliminating the pathogen, may lead to tissue pathology (14) because they are highly detrimental to the host. Understanding the mechanisms involved in the control of cellular responses to infection by parasites provides important information toward possible strategies related to the control/exacerbation of cellular responses.
T-cell activation involves the engagement of the T-cell receptor (TCR)-MHC-peptide complex as well as appropriate costimulation. One of the most important costimulatory pathways consists of the interaction between CD28 expressed from T cells and their counterparts, CD80 and CD86, expressed by antigen-presenting cells (APC) (47). This engagement leads to lymphocyte proliferation and cytokine production (25). Cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) is expressed by T cells, and while structurally similar to CD28, it exerts opposite functions (35, 39). This molecule is able to interact with CD80 and CD86, inhibiting T-cell proliferation and the lytic ability of CD8+ cytotoxic T cells in a nonspecific and an antigen-specific manner (40). As a consequence of activation, T cells are capable of producing cytokines that will orchestrate immune responses by controlling cell differentiation, adhesion and costimulatory molecule expression, and migration and recruitment, among many other activities. Proinflammatory cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), are secreted mainly by T cells and stimulate APC to efficiently eliminate pathogens (33, 41). In turn, by properly activating T cells, monocytes elicit a cellular immune reactivity that may cooperate in parasite elimination (18). These functions are regulated by anti-inflammatory cytokines, which can decrease the expression of effector as well as T-cell-activating molecules (43). Thus, proper interactions between APC and T cells, and the consequent immune responses generated by such interactions, are critical in determining the fate of an infection.
Chagas' disease is a morbid parasitic infection caused by Trypanosoma cruzi, which affects millions of people on the American continents. Infection leads to an acute phase that may last between 2 and 4 months, characterized by high numbers of parasites in the bloodstream as well as in tissues. The control of parasite replication leads to chronic, often life-long disease. Most individuals in the chronic phase remain in a silent, asymptomatic clinical form of Chagas' disease and are classified as indeterminate patients. However, approximately 30% of the chronically infected individuals develop a very severe clinical form in which digestive and/or cardiac alterations often lead to death (1). This differential clinical evolution has motivated many researchers to study the factors involved in the morbidity of Chagas' disease. While parasite-related factors may influence the development of different clinical forms (45), it is clear that the host's immune response, especially T cells, is critical in the development of pathology in experimental models (44) as well as in human disease (12). T cells act through the production of cytokines (6, 10, 17) and as mediators of tissue destruction in Chagas' disease (22, 34). Previous studies have evaluated the role of costimulatory molecules in murine infection with T. cruzi. It has been shown that mice that are deficient in CD28 are highly susceptible to T. cruzi infection (28), whereas CTLA-4 blocking increases resistance to infection (29). In humans, it has been demonstrated that chagasic patients have a higher frequency of CD28− T cells in their bloodstream and that these cells display a heterogeneous repertoire (9, 30).
Since T-cell responses are critical in Chagas' disease and are directly influenced by costimulation and cytokines, we evaluated the ability of T. cruzi infection to modulate the expression of costimulatory molecules and cytokines by monocytes and lymphocytes from patients with two distinct and polar clinical forms of Chagas' disease, the indeterminate form and the severe cardiac form. Our data demonstrate that T. cruzi infection is capable of modulating the expression of costimulatory molecules and cytokines by immune cells. We show, for the first time, that T. cruzi infection induces a differential modulation of such molecules in cells from indeterminate and cardiac chagasic patients, consistent with their distinct clinical evolution.
MATERIALS AND METHODS
Patients.
Chagasic patients included in our studies were from areas of endemicity within the state of Minas Gerais, Brazil, and were under the medical responsibility of Manoel Otávio C. Rocha. Serologic tests indicative of Chagas' disease (indirect immunofluorescence, enzyme-linked immunosorbent assay, or indirect hemagglutination) were positive for all chagasic patients studied. A detailed evaluation, including physical examinations, electrocardiograms, chest X rays, and echo Doppler cardiographic evaluations were performed with each patient in order to define indeterminate or cardiac clinical forms, according to the criteria described by Rocha et al. (36). Esophagograms and barium enemas were also performed to exclude digestive disease. Patients classified as indeterminate (n, 11) had positive serology results for T. cruzi infection, normal electrocardiograms, and normal cardiac and digestive radiological evaluations. Patients classified as cardiac (n, 13) had positive serology results for T. cruzi infection and displayed several alterations in the electrocardiogram, such as right- or left-bundle branch blocks and dilated left ventricles, as shown by echocardiography and chest X rays. The average age of the chagasic patients was 43 ± 13 (standard deviation) years for the cardiac group and 43 ± 10 years for the indeterminate group. The control group was composed of noninfected individuals (n, 8), as indicated by negative serology tests specific for Chagas' disease. The noninfected individual group was also from the state of Minas Gerais and had an average age of 38 ± 16 years. We excluded from our study individuals with a history of other parasitic diseases or any other chronic inflammatory diseases, diabetes, or heart/circulatory illnesses or bacterial infections. This study protocol was approved by the Ethical Committee of the Universidade Federal de Minas Gerais. All individuals included in this work were volunteers, and treatment and clinical care were offered to all patients, as needed, independent of their enrollment in this research project.
T. cruzi trypomastigotes and parasite antigen.
Trypanosoma cruzi trypomastigotes (Y strain) were grown in a VERO or a L929 cell line, as we described in a previous study (42). Briefly, cells were infected with a ratio of 10 trypomastigotes/cell, and after free trypomastigotes were removed by washing with culture medium, they were maintained in RPMI medium (Sigma, St. Louis, MO) enriched with 5% fetal calf serum and antibiotic (penicillin, 500 U/ml, and streptomycin, 0.5 mg/ml [Sigma, St. Louis, MO]) for approximately 5 days. After this period, a large number of trypomastigotes ruptured the cells and were collected from the supernatant. Contamination with amastigote forms was always below 3%. Parasites obtained in such a manner were used for the infection of adherent cells from patients and from noninfected individuals, as well as for obtaining antigen. Antigen preparation was performed as previously described (31) by homogenizing the parasites in a sonicator in the presence of a 1% protease inhibitor solution (1 mM EDTA, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 50 μg/ml TLCK [Nα-p-tosyl-l-lysine chloromethyl ketone], 100 μg/ml phenylmethylsulfonyl fluoride [PMSF; Sigma, St. Louis, MO]). After three cycles of rupture, the mixture was centrifuged at 12,000 × g for 10 min at 4°C, and the supernatant was submitted to protein quantification by the Bradford method. T. cruzi trypomastigote antigens were stored at −70°C, and a final concentration of 20 μg/ml of the antigen was used for all experiments.
Preparation of peripheral blood mononuclear cells for in vitro infection or exposure to parasite antigens.
Purification of peripheral blood mononuclear cells (PBMC) was performed as we described previously (42). Briefly, heparinized blood was diluted to a proportion of 1:1 with phosphate-buffered saline (PBS; Sigma, St. Louis, MO) and applied over a Ficoll (Amersham Biosciences, Pittsburgh, PA) gradient. The mixture was centrifuged, and PBMC were collected at the interface between the plasma and the Ficoll. Cells were washed three times by centrifugation with PBS and resuspended in RPMI medium supplemented with antibiotic-antimycotic (0.25 μg/ml amphotericin B, 200 U/ml penicillin, and 0.1 mg/ml streptomycin [Sigma, St. Louis, MO]) and l-glutamine (1 mM [Sigma, St. Louis, MO]) to a concentration of 107 cells/ml.
In vitro infection of adherent cells from patients and from noninfected individuals by T. cruzi trypomastigotes.
This procedure was performed as we described in a previous study (42). Briefly, 1 × 106 PBMC/well were plated in a 24-well plate and incubated at 37°C in 5% CO2 for 1 h with gentle agitation after each 20-min period. This procedure allowed obtaining adherent cells, which were used for in vitro T. cruzi infection. Infection was performed using a ratio of 10 trypomastigotes/adherent cell, based on ∼50,000 monocytes per well. Cells and parasites were incubated at 37°C in 5% CO2 for a period of 3 h. After this time, cells were washed with warm RPMI medium to remove free trypomastigotes. This procedure led to an infection of 80% for monocytes and only 1% for T cells and 5% for B cells from all groups of individuals analyzed, as we described in a previous study (42). Nonadherent cells were obtained by washing the wells where the adherence was performed using RPMI, right after the adherence time and prior to infection. After the infection of adherent cells, as described above, nonadherent cells were added to adherent cells infected in vitro, and the mixture was cultured for approximately 18 h at 37°C in 5% CO2.
In vitro culture of PBMC from patients and noninfected individuals with T. cruzi antigens.
Adherent cells were incubated with RPMI medium or parasite antigen for 3 h, and after that, nonadherent cells were added to wells, maintaining the original proportion. Cultures were carried out in the presence or absence of T. cruzi antigens (20 μg/ml, final concentration) and incubated for approximately 18 h under the same conditions as the infected cultures.
Analysis of expression of costimulatory molecules in CD14+ monocytes and CD4+ or CD8+ T lymphocytes from chagasic patients and noninfected individuals.
We evaluated the surface expression of CD80 and CD86 in monocytes and CD28 and CTLA-4 in T lymphocytes from individuals belonging to the different groups. Analyses were performed in cells cultured in the absence of any stimulus to access the ex vivo expression of these molecules in each group. Moreover, we performed analysis after in vitro infection with T. cruzi or exposure to T. cruzi antigens to determine the effect of such treatments on the expression of these molecules.
PBMC after each culture condition were submitted to incubation with phycoerythrin (PE)-labeled anti-CD80 or anti-CD86 monoclonal antibodies in conjunction with fluorescein isothiocyanate (FITC)-labeled anti-CD14 monoclonal antibodies, following protocols we describe in a previous study (4, 42). For lymphocyte analysis, PE-labeled anti-CTLA-4 or anti-CD28 monoclonal antibodies together with FITC-labeled anti-CD4 or anti-CD8 were used. Briefly, cells and antibody solutions were incubated for 15 min at 4°C, washed with PBS, fixed with 2% formaldehyde, and read in a flow cytometer. A minimum of 20,000 events was counted, and the parameters were analyzed in the monocyte or lymphocyte populations by gating in the region classically occupied by these cells, in a size-versus-granularity plot (30, 42). CTLA-4 intracellular expression was also evaluated, as described below. Antibodies were purchased from Caltag (Carlsbad, CA).
Analysis of cytokine expression by lymphocytes from chagasic patients and noninfected individuals.
Expression of interleukin 10 (IL-10), IL-4, IFN-γ, and TNF-α by CD4+ or CD8+ T lymphocytes or that of IL-10 and TNF-α by monocytes was determined using intracellular cytokine staining, as we describe previously (13). We also evaluated the CTLA-4 staining in the intracellular compartment of T lymphocytes. Samples destined for intracellular molecule analysis were cultured in the presence or absence of any stimulus for a period of approximately 18 h, as described above. During the last 4 h of culture, 1 μg/ml brefeldin A (Sigma, St. Louis, MO) was added to the cultures to prevent molecule secretion. Cells were then harvested, labeled for CD4, CD8, or CD14, as described above, and fixed in 2% formaldehyde solution for 20 min. After the fixing solution was removed by centrifugation, cells were permeabilized by incubation for 10 min with a 0.5% saponin solution and submitted to the intracellular molecule labeling. Samples were incubated with PE-labeled anticytokine or anti-CTLA-4 monoclonal antibodies for 20 min at room temperature, washed twice with the 0.5% saponin solution, resuspended in PBS, and read in a flow cytometer. Events (30,000) were counted, and parameters were analyzed for the CD4+ or CD8+ lymphocyte population and CD14+ monocytes by gating in the regions classically occupied by lymphocytes or monocytes in a size-versus-granularity plot (30, 42). Unlabeled cells and PE- and FITC-labeled isotype controls were added to the experiments. Antibodies used in all of our experiments (surface and intracellular) were purchased from Caltag (Carlsbad, CA).
Statistical analysis.
We compared our results among different groups using Tukey-Kramer all-pair comparison analysis of variance contained in JMP software from SAS. As for the comparisons between different treatments within the same group, we used the Wilcoxon paired statistical analysis. Results were considered statistically significant when P was <0.05.
RESULTS
Indeterminate chagasic patients displayed higher surface expression of CTLA-4 in freshly isolated CD8+ T cells than cardiac chagasic patients and noninfected individuals.
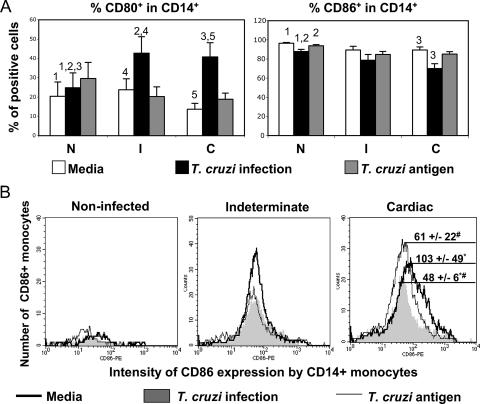
The ex vivo expression of CD28 and CTLA-4 as well as the expression of their ligands CD80 and CD86 allowed determination of the steady-state immunological environment in chagasic patients. We accessed the expression of CD80 and CD86 by CD14+ cells from indeterminate and cardiac patients or noninfected individuals by using double labeling with anti-CD14-FITC and anti-CD80-PE or anti-CD86-PE. The frequencies of expression of CD80 and CD86 by nonstimulated CD14+ cells were similar among the different groups (Fig. 1A, clear bars). Similarly, the intensities of expression of these molecules in nonstimulated CD14+ monocytes did not differ among groups (Fig. 1B, dark line curves).
FIG. 1.
Cardiac chagasic patients display a lower percentage of CD14+ CD86+ cells and a lower intensity of expression of CD86 by monocytes following T. cruzi infection than from exposure to noninfected cultures. Analysis of in vitro infection (10 parasites/monocytes) or exposure to parasite antigens (20 mg/ml) is shown by costimulatory molecule expression of CD14+ monocytes from indeterminate (I; n, 11) and cardiac (C; n, 13) chagasic patients and from noninfected individuals (N; n, 8). Cells were double stained for CD14 and CD80 or CD86 and analyzed by flow cytometry, as described in Materials and Methods. (A) Analysis of the frequency of CD14+ CD80+ and CD14+ CD86+ cells from N, I, and C subjects submitted to different treatments. Clear, dark, and gray bars represent the average values ± standard errors obtained by analysis of unstimulated cells, cells infected in vitro with T. cruzi, and cells exposed to parasite antigen, respectively. Identical numbers above the bars indicate statistical significance using Tukey-Kramer or Wilcoxon tests, as described in Materials and Methods. (B) Representative individual histograms of mean intensity of CD86 expression by CD14+ monocytes from N, I, and C subjects after in vitro treatments. The rank line, gray area, and thin line represent the intensity of CD86 expression by CD14+ unstimulated monocytes, cells infected in vitro with T. cruzi, and monocytes exposed to parasite antigen, respectively. The numbers reflect the averages ± standard errors for each group. #, statistically significant differences between cultures infected with T. cruzi and those exposed to parasite antigen; *, statistically significant differences between cultures infected with T. cruzi and cultures performed with medium only.
Analysis of costimulatory molecules in unstimulated lymphocyte populations revealed that noninfected individuals showed a higher frequency of CD28+ CD8+ cells than chagasic patients, as we have shown previously (Table 1). The frequencies of CD4+ CD28+ T cells were not different among the groups. Analysis of CTLA-4 expression by unstimulated CD4+ T cells revealed that the surface expression of this molecule was similar among the groups (Table 1). However, the intracytoplasmic expression of CTLA-4 was higher in CD4+ T cells from chagasic patients than in those from noninfected individuals (Table 1). Within the CD8+ T-cell subpopulation, surface and intracellular expressions of CTLA-4 were significantly increased in cells from indeterminate patients compared to those of noninfected and cardiac patients (Table 1).
TABLE 1.
Ex vivo analysis of costimulatory molecules CD28 and CTLA-4 expression by CD4+ and CD8+ lymphocytes from indeterminate and cardiac chagasic patients and noninfected individualsa
| Study group | CD4+ T cells
|
CD8+ T cellsc
|
||||
|---|---|---|---|---|---|---|
| % CD28+ (S) | % of CTLA-4+ (S) | % of CTLA-4+ (IC) | % of CD28+ (S) | % of CTLA-4+ (S) | % of CTLA-4+ (IC) | |
| Noninfected (n, 8) | 44 ± 11 | 0.15 ± 0.11 | 0.62 ± 0.38b | 11.1 ± 3.6b | 0.21 ± 0.09 | 0.21 ± 0.16 |
| Indeterminate (n, 11) | 37 ± 9 | 0.34 ± 0.26 | 2.29 ± 1.57 | 4.4 ± 1.8 | 0.68 ± 0.47c | 0.9 ± 0.39c |
| Cardiac (n, 13) | 36 ± 12 | 0.2 ± 0.18 | 5.05 ± 3.54 | 4.8 ± 3.4 | 0.14 ± 0.15 | 0.18 ± 0.16 |
Cells were double stained for costimulatory molecules CD28 and CTLA-4 and CD4 or CD8 expression and analyzed by flow cytometry, as described in Materials and Methods. Results are expressed as percentages of CD4+ and CD8+ lymphocytes expressing CD28 or CTLA-4 and are indicated as means ± standard deviations for each analysis. Expression of the molecules was evaluated at the surface (S) or intracellularly (IC).
Bold numbers are statistically different from those for indeterminate and cardiac subjects, as determined using the Tukey-Kramer test as described in Materials and Methods.
Bold numbers are statistically different from those for noninfected and cardiac subjects, as determined using the Tukey-Kramer test as described in Materials and Methods.
T. cruzi infection in vitro induces a down-regulatory profile in indeterminate but not in cardiac patients, as determined by the expression of CD86 and CTLA-4.
Ex vivo analysis of costimulatory molecule expression patterns can give important information concerning the dynamics of immune regulation and the role that the host-parasite interaction may have on this regulation in vivo. After determining the ex vivo profiles, we sought to investigate whether the parasite was able to modulate the expression of costimulatory molecules and their ligands by exposing host cells to live trypomastigotes in vitro. We evaluated the expression of CD80 and CD86 by monocytes infected with T. cruzi or the expression of CD28 and CTLA-4 by lymphocytes exposed to monocytes infected in vitro with live trypomastigotes. As we determined earlier, the T. cruzi infectivity rates of monocytes from the different groups were similar (42). Two different approaches were taken to analyze the data from in vitro-infected PBMC, as follows: (i) we compared cells infected with T. cruzi, cells exposed to T. cruzi antigens, and cells with no treatment within the same patient group by using the Wilcoxon test; and (ii) we compared each treatment among indeterminate, cardiac, and noninfected individuals, using the nonparametric Tukey-Kramer test. Exposure to T. cruzi antigens was used to determine whether infection or simply exposure to parasite antigens would be responsible for any observed changes.
Our results showed that the frequency of CD80 expression by CD14+ monocytes was significantly increased after T. cruzi infection in cells from all groups, compared to that of the respective unstimulated cells (Fig. 1A). Comparisons among the groups revealed that infection with T. cruzi led to a higher frequency of CD80 expression by CD14+ monocytes from the indeterminate and cardiac groups than by those from the noninfected group samples submitted to the same treatment (Fig. 1A). Analysis of the intensity of expression of CD80 per cell did not show statistically significant differences among groups (data not shown).
T. cruzi infection led to a statistically significant decrease in the frequency of CD86+ monocytes from noninfected and cardiac individuals but not in those from indeterminate patients, compared to that of nonstimulated cultures (Fig. 1A). Moreover, in vitro infection by the parasite induced a decrease in the intensity of expression of CD86 in CD14+ monocytes only for cardiac patients (Fig. 1B).
We also evaluated the expression of CD28 and CTLA-4 (for both surface expression and intracellular expression of the latter) by CD4+ or CD8+ T lymphocytes from chagasic and noninfected individuals exposed or not to infected monocytes. Exposure of T cells to monocytes infected with live parasites did not significantly change the expression of CD28 by CD4+ or by CD8+ T cells (data not shown).
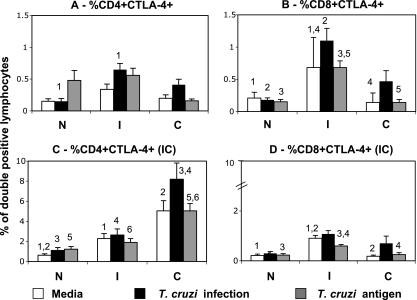
On the other hand, exposure to monocytes infected with T. cruzi differentially modulated the expression of CTLA-4 by T cells from chagasic patients. We observed a higher expression of CTLA-4 on the surface of CD4+ T cells from indeterminate but not cardiac patients exposed to monocytes infected with the parasite than that for cells from noninfected individuals submitted to the same conditions (Fig. 2A). Moreover, the frequency of CD8+ CTLA-4+ was higher in cells from indeterminate individuals than in those from the other groups, regardless of the treatment (Fig. 2B). Evaluation of intracytoplasmic (IC) CTLA-4 expression by CD4+ T cells showed that all treatments led to an increase in CD4+ CTLA-4+ IC expression in cells from cardiac individuals (Fig. 2C). The frequency of CD8+ CTLA-4+ IC expression was higher in cells from indeterminate patients than in those from other groups (Fig. 2D), both for medium alone and after antigen stimulation.
FIG. 2.
Indeterminate chagasic patients display a higher frequency of CTLA-4+ CD8+ T cells than do cardiac or noninfected individuals. Analysis of the influence of exposure to monocytes infected with T. cruzi (10 parasites/monocyte) or incubated with parasite antigen (20 mg/ml) on CTLA-4 expression by CD4+ and CD8+ T cells from indeterminate (I; n, 11) and cardiac (C; n, 13) chagasic patients and noninfected individuals (N; n, 8). Cells were double stained for CD4 or CD8 and CTLA-4 and analyzed by flow cytometry. The CTLA-4 staining was performed for surface and IC localizations of this costimulatory molecule, as described in Materials and Methods. Clear, dark, and gray bars represent the average values obtained by analysis of unstimulated cells, cells exposed to monocytes infected in vitro with T. cruzi, and cells exposed to monocytes pulsed with parasite antigen, respectively. Results are expressed as averages for double-positive cells ± standard errors. Identical numbers above the bars indicate statistical significance values using Tukey-Kramer or Wilcoxon tests, as described in Materials and Methods.
Our data demonstrated that in vitro infection with T. cruzi or exposure of T cells to infected monocytes led to an increase in the frequency of CD80+ and CTLA-4+ cells from indeterminate patients compared to that of cells from noninfected patients submitted to the same treatment (Fig. 1 and 2A and B). In contrast, similar treatment led to a decrease in CD86 expression (Fig. 1B) and no significant increase in CTLA-4 on cell surfaces (Fig. 2A and B) of cells from cardiac individuals. Thus, T. cruzi induces the expression of down-modulatory molecules on the surface of T cells from indeterminate but not from cardiac patients.
In vitro infection with T. cruzi induces a skewed regulatory environment in indeterminate compared to cardiac patients.
In order to determine if the induction of the expression of down-regulatory molecules by the parasite in cells from indeterminate patients would be reflected by the expression of cytokines leading to a regulatory environment, we evaluated the expression of inflammatory and anti-inflammatory cytokines by T cells and monocytes from the different clinical groups. Since the major effects on costimulatory molecule expression were induced by infection with the parasite rather than solely by exposure to its antigen, we evaluated the expression of key immunoregulatory cytokines by lymphocytes (IFN-γ, TNF-α, IL-10, and IL-4) and by monocytes (IL-10 and TNF-α) after in vitro infection with T. cruzi or exposure of T cells to infected monocytes, by using flow cytometry as described in Materials and Methods.
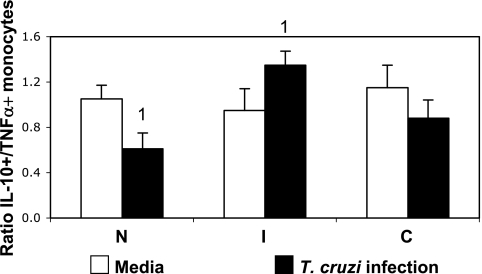
We have previously shown that infection with T. cruzi leads to changes in cytokine expression by monocytes from chagasic individuals with different clinical outcomes (42). Here, we evaluated the ratio of two important immunoregulatory cytokines produced by monocytes, IL-10 and TNF-α, considering that the balance between inflammatory versus anti-inflammatory cytokines is likely to be a determining factor in the type of response established in patients, rather than the production of a single cytokine. We observed that while the ratio was similar between groups without any stimulation, in vitro infection induced a higher IL-10/TNF-α ratio in cells from indeterminate patients, but not in those from cardiac patients, than in cells from noninfected individuals submitted to the same treatment (Fig. 3).
FIG. 3.
T. cruzi infection induces a higher IL-10/TNF-α ratio in monocytes from indeterminate but not from cardiac chagasic patients. Analysis of in vitro infection (10 parasites/monocyte) influences on the cytokine ratio in monocytes from indeterminate (I; n, 11) and cardiac (C; n, 13) chagasic patients and noninfected individuals (N; n, 8). Cells were double stained for CD14 and IL-10 and TNF-α and analyzed by flow cytometry. Clear and black bars represent the averages ± standard errors obtained by the individual ratios of IL-10/TNF-α in nonstimulated and infected cultures, respectively. Identical numbers above the bars indicate statistical significance values using the Tukey-Kramer test, as described in Materials and Methods.
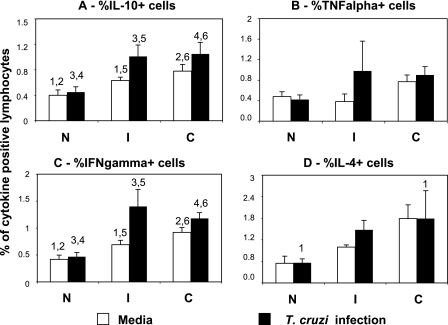
Exposure of T cells to monocytes infected with T. cruzi led to an increase in the expression of IFN-γ and IL-10 by lymphocytes from indeterminate and cardiac patients, compared to that in cells from noninfected individuals submitted to the same treatment (Fig. 4). Expression of these cytokines was parasite specific, as seen by the fact that the averages of cytokine-expressing lymphocytes were significantly higher after exposure to infected monocytes than exposure to medium only in both groups of chagasic patients, but not in noninfected individuals (Fig. 4). Furthermore, expression of IFN-γ and IL-10 was higher in cells from chagasic patients than in cells from noninfected individuals in cultures with medium only (Fig. 4). Expression of IL-4 by lymphocytes from cardiac patients was increased after exposure to infected monocytes, compared to that by lymphocytes from noninfected individuals submitted to the same treatment (Fig. 4). TNF-α expression by lymphocytes was not significantly altered after exposure to in vitro infected monocytes (Fig. 4).
FIG. 4.
Exposure to T. cruzi-infected monocytes induces a higher expression of inflammatory and anti-inflammatory cytokines by lymphocytes from chagasic patients. Percentages of cells positive for IL-10 (A), TNF-α (B), IFN-γ (C), and IL-4 (D) were determined within the lymphocyte gate, using PE-labeled monoclonal antibodies to each of the cytokines. Clear and black bars represent the average values obtained in nonstimulated cells or in cells exposed to parasite-infected monocytes, respectively. Results are expressed as averages ± standard errors. Identical numbers above the bars indicate statistical significance values using the Wilcoxon or Tukey-Kramer test, as described in Materials and Methods.
DISCUSSION
In this work, we demonstrate that infection by T. cruzi, the causative agent of Chagas' disease, alters the expression of costimulatory molecules and cytokines in immune cells from noninfected as well as from chagasic individuals and that the observed changes are consistent with the clinical characteristics observed in indeterminate and cardiac chagasic patients.
The interaction between T. cruzi and macrophages is certainly an important event in regulating cellular reactivity in human Chagas' disease, as previously suggested (31). Macrophages process and present antigens, produce cytokines, and express costimulatory molecules that initiate and influence the outcome of the immune response, as well as trigger initial events in the innate immune response against T. cruzi infection (reviewed in reference 37). T cells play a crucial role in the establishment and development of human Chagas' disease, displaying both immunoregulatory and effector functions, which determine disease dynamics (11, 17, 20, 34). We have previously shown that monocytes from cardiac or indeterminate chagasic patients and noninfected individuals are similarly infected by T. cruzi in vitro (42), but the functional potential of these cells has not yet been determined.
Similarities in the ex vivo expression of CD80 and CD86 by monocytes from individuals of the different groups analyzed (Fig. 1A, clear bars) suggest that monocytes obtained from chagasic patients with different clinical forms and from noninfected individuals have similar costimulatory potential. However, the parasite does indeed differentially modulate the expression of these molecules. A striking observation was the decreased percentage of CD14+ CD86+ cells as well as a decrease in the intensity of CD86 expression by monocytes from cardiac but not from indeterminate patients following in vitro infection (Fig. 1A and B). Moreover, cells from indeterminate patients infected with the parasite showed a higher percentage of monocytes expressing CD80 than cells from the control group submitted to the same treatment. While CD86 has more abundant expression, CD80 is not expressed in resting antigen-presenting cells (19, 23). It has been shown that CD86 has 20- to 100-fold higher affinity than CD28 for CTLA-4 (27). Thus, a decreased expression of CD86, associated with no changes in CD80 expression by monocytes from cardiac patients upon parasite infection, would result in less down-modulated cellular responses due to a lower association of CD86 with available CTLA-4. As a result, these individuals, when exposed to the parasite, could mount a more intense cellular response, which is consistent with the inflammation observed in the hearts of these cardiac patients and in the activated inflammatory profile seen for both monocytes and T cells from these individuals (data shown here and in references 8, 17, and 42). These findings are also in accordance with studies of experimental models where it was shown that T. cruzi infection leads to a decrease in CD40 and CD86 expression by macrophages and dendritic cells of susceptible BALB/c mice but not in those of resistant C57BL/6 mice (32).
The interaction of CD80 and CD86 with their ligands, CD28 and CTLA-4, is critical to determine the fate of cellular responses. While CD28 engagement leads to cellular activation, CTLA-4 engagement leads to a down-modulation of cellular response (5). The activation of T cells may lead to reduced CD28 expression and increased CTLA-4 expression and thus render the T cells less responsive to activation stimuli as a negative feedback mechanism (5). Previous studies have shown that CD28-deficient mice were highly susceptible to T. cruzi infection, presenting with higher parasitemia and tissue parasitism but less inflammation in the heart than those of wild-type mice (28). Moreover, it has been shown that modulation via CTLA-4 during the acute phase of experimental infection leads to a decreased ability to clear the parasite and, thus, susceptibility to T. cruzi infection (29). Our previous findings have shown that chagasic patients, despite their clinical form, showed decreased expression of CD28 by freshly isolated T cells compared to that of noninfected individuals (9, 30). In this work, we confirmed this finding while determining that exposure to monocytes infected with T. cruzi does not significantly alter the frequency of T cells expressing CD28 in cultures from noninfected or cardiac individuals (data not shown). However, we observed an increase in the expression of the down-regulatory molecule CTLA-4 on the surface of cells from indeterminate patients (Fig. 2A and B). The up-regulation of CTLA-4 was even more striking within the CD8 subpopulation in indeterminate patients. Interestingly, high expression of CTLA-4 was not seen on the surface of cells from cardiac patients. The lack of this regulatory mechanism in cardiac patients, especially of CD8+ T cells, which are the main cell type in cardiac lesions, strengthens the hypothesis that these cells may be involved in tissue destruction in cardiac patients (20, 34). Conversely, up-regulation of CTLA-4 is consistent with the establishment of a regulatory response in indeterminate patients, possibly preventing pathology.
While CD28 is constitutively expressed on the cell surface, CTLA-4 is absent from the resting state of T cells, and its transcript is induced upon stimulation. The cell surface protein expression is readily detectable at 1 to 2 days (24, 46). Although CTLA-4 mRNA is induced by TCR engagement, most of the protein is accumulated within the cytoplasm (26). After reaching the cell surface, CTLA-4 is immediately endocytosed and transported to lysosomes, where in the absence of any further stimuli, it is degraded (21). Upon further stimulation, CTLA-4-containing vesicles will move toward the TCR-engaged site where it is phosphorylated and induces negative signals. Weak signals will not be able to induce fusion of the CTLA-4-containing vesicles with the plasma membrane to increase the cell surface CTLA-4 (40). The cell surface expression of CTLA-4 is precisely regulated because an expression that is too high or too low could lead the immune response in extreme directions (3, 40). In this study, we observed a high intracellular expression of CTLA-4 by CD4+ T cells from cardiac patients after exposure to monocytes infected in vitro with the parasite, compared to that in cells from indeterminate and noninfected individuals submitted to the same treatment, while surface expression was low (Fig. 2). It is possible that, although capable of producing this molecule, cells from cardiac patients do not succeed in expressing it due to a defect in the exocytosis machinery or through an active halting of this pathway induced by the parasite. It is unlikely that the stimulation provided by the parasite (or its antigen) was not sufficient to induce the expression of this molecule, since it has been shown that antigenic stimulation of cells from cardiac patients induces high cell proliferation and cytokine expression (10, 16). Nonetheless, the result of the low expression of CTLA-4 could be the lack of down-regulation of the cellular responses in the cardiac patients, consistent with the inflammatory response observed in these individuals. Interestingly, most of the effects we observed concerning the expression of surface molecules were induced by exposure to monocytes infected with T. cruzi but not always by exposure to parasite antigen. These data suggest that live T. cruzi has a much higher impact on regulating costimulatory molecule expression, possibly due to higher stimulatory capacity.
The idea that changes in the expression of these costimulatory molecules by the parasite would be associated with a down-regulated versus an up-regulated response in indeterminate and cardiac patients, respectively, was confirmed by cytokine expression after parasite infection, especially in the monocytic population. Exposure of lymphocytes to monocytes infected by T. cruzi led to the increased expression of IFN-γ and IL-10 by these cells from patients of both clinical forms (Fig. 4). Previous studies have demonstrated that circulating memory T lymphocytes produce IFN-γ and IL-10 in response to stimulation with anti-CD3 antibody (48). Thus, our results may reflect the higher frequency of circulating memory T cells in chagasic patients than in noninfected individuals. This is in accordance with previous studies (8) that showed higher frequencies of CD4+ and CD8+ T cells expressing CD45RO in chagasic individuals than in noninfected individuals. We also have shown that indeterminate and cardiac patients display increased mRNA for IL-10 and IFN-γ, as well as other cytokines (11). Evaluating the ratio of IL-10 and TNF-α expressed by monocytes from the different groups, we observed that infection with T. cruzi led to a higher IL-10/TNF-α ratio in cells from indeterminate patients than in those from noninfected individuals (Fig. 3), which was reflective of an increased IL-10 expression by infected cells from indeterminates, as we previously showed (42). Thus, the parasite induces a regulatory cytokine balance among cells from indeterminate but not from cardiac patients. Extrapolations of cause/effect between the expression of down-regulatory molecules and cytokines are not possible. However, our results suggest that changes in the expression of costimulatory molecules and cytokines may be an important mechanism for the establishment of pathogenic versus protective responses in humans infected with T. cruzi. Moreover, these data suggest mechanisms through which parasites and other infective agents may regulate the immune response.
Acknowledgments
We thank Marcela Lopes, Ricardo Gazzinelli, Luis Carlos Crocco Afonso, and Denise Carmona Machado (members of E. A. Souza's thesis defense committee) for helpful discussions concerning the manuscript.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease (TDR), a CNPq universal grant, and NIH and FINEP CT-Infra; W.O.D., C.A.S.M., and K.J.G. are CNPq fellows.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Andrade, Z. A. 1999. Immunopathology of Chagas disease. Mem. Inst. Oswaldo Cruz 94:71-80. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli, L. R., W. O. Dutra, R. P. Almeida, O. Bacellar, E. M. Carvalho, and K. J. Gollob. 2005. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol. Lett. 101:226-230. [DOI] [PubMed] [Google Scholar]
- 3.Barrat, F. J., F. Le Deist, M. Benkerrou, P. Bousso, J. Feldmann, A. Fischer, and G. de Saint Basile. 1999. Defective CTLA-4 cycling pathway in Chediak-Higashi syndrome: a possible mechanism for deregulation of T lymphocyte activation. Proc. Natl. Acad. Sci. USA 96:8645-8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottrel, R. L., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Neto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers, C. A., and J. P. Allison. 1997. Co-stimulation in T cell responses. Curr. Opin. Immunol. 9:396-404. [DOI] [PubMed] [Google Scholar]
- 6.Cunha-Neto, E., V. J. Dzau, P. D. Allen, D. Stamatiou, L. Benvenutti, M. L. Higuchi, N. S. Koyama, J. S. Silva, J. Kalil, and C. C. Liew. 2005. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas disease cardiomyopathy. Am. J. Pathol. 167:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza, P. E., R. S. Gomez, G. M. Xavier, J. S. dos Santos, K. J. Gollob, and W. O. Dutra. 2005. Systemic leukocyte activation in patients with central giant cell lesions. J. Oral Pathol. Med. 34:312-317. [DOI] [PubMed] [Google Scholar]
- 8.Dutra, W. O., A. O. Martins-Filho, J. R. Cançado, J. C. Pinto-Dias, Z. Brener, G. L. Freeman, Jr., D. G. Colley, G. Gazzinelli, and J. C. Parra. 1994. Activated T and B lymphocytes in peripheral blood of patients with Chagas disease. Int. Immunol. 6:499-506. [DOI] [PubMed] [Google Scholar]
- 9.Dutra, W. O., A. O. Martins-Filho, J. R. Cançado, J. C. Pinto-Dias, Z. Brener, G. Gazzinelli, J. F. Carvalho, and D. G. Colley. 1996. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand. J. Immunol. 43:88-93. [DOI] [PubMed] [Google Scholar]
- 10.Dutra, W. O., D. G. Colley, J. C. Pinto-Dias, G. Gazzinelli, Z. Brener, M. E. Pereira, R. L. Coffman, R. Correa-Oliveira, and J. F. Carvalho-Parra. 2000. Self and non-self stimulatory molecules induce preferential expansion of CD5+ B cells or activated T cells of chagasic patients, respectively. Scand. J. Immunol. 51:91-97. [DOI] [PubMed] [Google Scholar]
- 11.Dutra, W. O., K. J. Gollob, J. C. Pinto-Dias, G. Gazzinelli, R. Correa-Oliveira, R. L. Coffman, and J. F. Carvalho-Parra. 1997. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand. J. Immunol. 45:74-80. [DOI] [PubMed] [Google Scholar]
- 12.Dutra, W. O., M. O. Rocha, and M. M. Teixeira. 2005. The clinical immunology of human Chagas disease. Trends Parasitol. 21:581-587. [DOI] [PubMed] [Google Scholar]
- 13.Dutra, W. O., R. Correa-Oliveira, D. Dunne, L. Cecchini, L. Fraga, M. Roberts, A. Soares-Silveira, M. Webster, H. Yssel, and K. J. Gollob. 2002. Polarized Th2 like cells, in the absence of Th0 cells, are responsible for lymphocyte produced IL-4 in high IgE-producer schistosomiasis patients. BMC Immunol. 6:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faria, D. R., K. J. Gollob, J. Barbosa, Jr., A. Schriefer, P. R. Machado, H. Lessa, L. P. Carvalho, M. A. Romano-Silva, A. R. de Jesus, E. M. Carvalho, and W. O. Dutra. 2005. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect. Immun. 73:7853-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaze, S. T., W. O. Dutra, M. Lessa, H. Lessa, L. H. Guimarães, A. R. Jesus, L. P. Carvalho, P. Machado, E. M. Carvalho, and K. J. Gollob. 2006. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand. J. Immunol. 63:70-78. [DOI] [PubMed] [Google Scholar]
- 16.Gazzinelli, R. T., I. P. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ-activated macrophages. J. Immunol. 148:1792-1796. [PubMed] [Google Scholar]
- 17.Gomes, J. A. S., L. M. G. Bahia-Oliveira, M. O. C. Rocha, O. A. Martins-Filho, G. Gazzinelli, and R. Correa-Oliveira. 2003. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect. Immun. 71:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto, H., and J. A. Lindoso. 2004. Immunity and immunosuppression in experimental visceral leishmaniasis. Braz. J. Med. Biol. Res. 37:615-623. [DOI] [PubMed] [Google Scholar]
- 19.Hathcock, K. S., S. G. Laszlo, C. Pucillo, P. S. Linsley, and R. J. Hodes. 1994. Comparative analysis of B7-1 and B7-2 co-stimulatory ligands: expression and function. J. Exp. Med. 180:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi, M. L., P. S. Gutierrez, V. D. Aiello, S. Palomino, E. Bocchi, J. Kalil, G. Bellotti, and F. Pileggi. 1993. Immunohistochemical characterization of infiltrating cells in human chronic chagasic myocarditis: comparison with myocardial rejection process. Virchows Arch. A 423:157-160. [DOI] [PubMed] [Google Scholar]
- 21.Iida, T., H. Ohno, C. Nakaseko, M. Sakuma, M. Takeda-Ezaki, H. Arase, E. Kominami, T. Fujisawa, and T. Saito. 2000. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J. Immunol. 165:5062-5068. [DOI] [PubMed] [Google Scholar]
- 22.Iwai, L. K., M. A. Juliano, J. Kalil, and E. Cunha-Neto. 2005. T-cell molecular mimicry in Chagas disease: identification and partial structural analysis of multiple cross-reactive epitopes between Trypanosoma cruzi B13 and cardiac myosin heavy chain. J. Autoimmun. 24:111-117. [DOI] [PubMed] [Google Scholar]
- 23.Lenschow, D. J., A. I. Sperling, M. P. Cooke, G. Freeman, L. Rhee, D. C. Decker, G. Gray, L. M. Nadler, C. C. Goodnow, and J. A. Bluestone. 1994. Differential up-regulation of the B7-1 and B7-2 co-stimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 153:1990-1997. [PubMed] [Google Scholar]
- 24.Lindsten, T., K. P. Lee, E. S. Harris, B. Petryniak, N. Craighead, P. J. Reynolds, D. B. Lombard, G. J. Freeman, L. M. Nadler, and G. S. Gray. 1993. Characterization of CTLA-4 structure and expression on human T cells. J. Immunol. 151:3489-3499. [PubMed] [Google Scholar]
- 25.Linsley, P. S., and J. A. Ledbetter. 1993. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 11:191-212. [DOI] [PubMed] [Google Scholar]
- 26.Linsley, P. S., J. Bradshaw, J. Greene, R. Peach, K. L. Bennett, and R. S. Mittler. 1996. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 4:535-543. [DOI] [PubMed] [Google Scholar]
- 27.Linsley, P. S., J. L. Greene, W. Bradley, J. Bajorth, J. A. Ledbetter, and R. Peach. 1994. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1:793-801. [DOI] [PubMed] [Google Scholar]
- 28.Martins, G. A., A. P. Campanelli, R. B. Silva, C. E. Tadokoro, M. Russo, F. Q. Cunha, L. V. Rizzo, and J. S. Silva. 2004. CD28 is required for T cell activation and IFN-gamma production by CD4+ and CD8+ T cells in response to Trypanosoma cruzi infection. Microbes Infect. 6:1133-1144. [DOI] [PubMed] [Google Scholar]
- 29.Martins, G. A., C. E. Tadokoro, R. B. Silva, J. S. Silva, and L. V. Rizzo. 2004. CTLA-4 blockage increases resistance to infection with the intracellular protozoan Trypanosoma cruzi. J. Immunol. 172:4893-4901. [DOI] [PubMed] [Google Scholar]
- 30.Menezes, C. A., M. O. Rocha, P. E. Souza, A. C. Chaves, K. J. Gollob, and W. O. Dutra. 2004. Phenotypic and functional characteristics of CD28+ and CD28− cells from chagasic patients: distinct repertoire and cytokine expression. Clin. Exp. Immunol. 137:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morato, M. J., Z. Brener, J. R. Cançado, R. M. Nunes, E. Chiari, and G. Gazzinelli. 1986. Cellular immune responses of chagasic patients to antigens derived from different Trypanosoma cruzi strains and clones. Am. J. Trop. Med. Hyg. 35:505-511. [DOI] [PubMed] [Google Scholar]
- 32.Planelles, L., M. C. Thomas, C. Maranon, M. Morell, and M. C. López. 2003. Differential CD86 and CD40 co-stimulatory molecules and cytokine expression pattern induced by Trypanosoma cruzi in APC from resistant or susceptible mice. Clin. Exp. Immunol. 131:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qadoumi, M., I. Becker, N. Donhauser, M. Röllinghoff, and C. Bogdan. 2002. Expression of inducible nitric oxide synthase in skin lesions of patients with American cutaneous leishmaniasis. Infect. Immun. 70:4638-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reis, D. A., E. M. Jones, S. Tostes, Jr., E. R. Lopes, G. Gazzinelli, D. G. Colley, and T. L. McCurley. 1993. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-α+ cells and dominance of granzime A+, CD8+ lymphocytes. Am. J. Trop. Med. Hyg. 48:637-644. [DOI] [PubMed] [Google Scholar]
- 35.Riley, J. L., M. Mao, S. Kobayashi, M. Biery, J. Burchard, G. Cavet, B. P. Gregson, C. H. June, and P. S. Linsley. 2002. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc. Natl. Acad. Sci. USA 99:11790-11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocha, M. O., A. L. Ribeiro, and M. M. Teixeira. 2003. Clinical management of chronic Chagas cardiomyopathy. Front. Biosci. 8:44-54. [DOI] [PubMed] [Google Scholar]
- 37.Ropert, C., and R. T. Gazzinelli. 2004. Regulatory role of Toll-like receptor 2 during infection with Trypanosoma cruzi. J. Endotoxin Res. 10:425-430. [DOI] [PubMed] [Google Scholar]
- 38.Russo, D. M., M. Barral-Netto, A. Barral, and S. G. Reed. 1993. Human T cell responses in Leishmania infections. Prog. Clin. Parasitol. 3:119-144. [DOI] [PubMed] [Google Scholar]
- 39.Saito, T., and S. Yamasaki. 2003. Negative feedback of T cell activation through inhibitory adapters and costimulatory receptors. Immunol. Rev. 192:143-160. [DOI] [PubMed] [Google Scholar]
- 40.Santoro, G., G. Anastasi, D. Saverino, C. Puri, D. Zarcone, C. Tacchetti, E. Ciccone, and C. E. Grossi. 2000. Molecules that inhibit T-cell functions: cytochemical localization and shuttling. Eur. J. Histochem. 44:89-99. [PubMed] [Google Scholar]
- 41.Silva, J. S., J. C. Aliberti, G. A. Martins, M. A. Souza, J. T. Souto, and M. A. Padua. 1998. The role of IL-12 in experimental Trypanosoma cruzi infection. Braz. J. Med. Biol. Res. 31:111-115. [DOI] [PubMed] [Google Scholar]
- 42.Souza, P. E. A., M. O. C. Rocha, E. Rocha-Vieira, C. A. S. Menezes, A. C. L. Chaves, K. J. Gollob, and W. O. Dutra. 2004. Monocytes from patients with indeterminate and cardiac forms of Chagas' disease display distinct phenotypic and functional characteristics associated with morbidity. Infect. Immun. 72:5283-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spits, H., and R. de Waal Malefyt. 1992. Functional characterization of human IL-10. Int. Arch. Allergy Immunol. 99:8-15. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira, M. M., R. T. Gazzinelli, and J. S. Silva. 2002. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 18:262-265. [DOI] [PubMed] [Google Scholar]
- 45.Vago, A. R., L. O. Andrade, A. A. Leite, D. d'Ávila Reis, A. M. Macedo, S. J. Adad, S. Tostes, Jr., M. C. Moreira, G. B. Filho, and S. D. Pena. 2000. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am. J. Pathol. 156:1805-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walunas, T. L., D. J. Lenschow, C. Y. Bakker, P. S. Linsley, G. J. Freeman, J. M. Green, C. B. Thompson, and J. A. Bluestone. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1:405-413. [DOI] [PubMed] [Google Scholar]
- 47.Wang, S., and L. Chen. 2004. T lymphocyte co-signaling pathways of the B7-CD28 family. Cell. Mol. Immunol. 1:37-42. [PubMed] [Google Scholar]
- 48.Yssel, H., R. de Waal Malefyt, M. G. Roncarolo, J. S. Abrams, R. Lahesmaa, H. Spits, and J. E. de Vries. 1992. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J. Immunol. 149:2378-2384. [PubMed] [Google Scholar]