Abstract
Variant surface antigens (VSA) on the surface of Plasmodium falciparum-infected red blood cells play a major role in the pathogenesis of malaria and are key targets for acquired immunity. The best-characterized VSA belong to the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family. In areas where P. falciparum is endemic, parasites causing severe malaria and malaria in young children with limited immunity tend to express semiconserved PfEMP1 molecules encoded by group A var genes. Here we investigated antibody responses of Tanzanians who were 0 to 19 years old to PF11_0008, a group A PfEMP1. PF11_0008 has previously been found to be highly transcribed in a nonimmune Dutch volunteer experimentally infected with NF54 parasites. A high proportion of the Tanzanian donors had antibodies against recombinant PF11_0008 domains, and in children who were 4 to 9 years old the presence of antibodies to the PF11_0008 CIDR2β domain was associated with reduced numbers of malaria episodes. These results indicate that homologues of PF11_0008 are present in P. falciparum field isolates and suggest that PF11_0008 CIDR2β-reactive antibodies might be involved in protection against malaria episodes.
Variant surface antigens (VSA) appear to play a major role in malaria pathogenesis and to be key targets for acquired immunity (10, 13, 17, 46). Moreover, severe disease in children is associated with parasites expressing a restricted subset of VSA (VSASM), and children possessing antibodies against these antigenic types seem to be protected against severe, noncerebral malaria (7, 32, 33). In line with this, a mathematical model based on actual data (42) estimated that immunity to severe, noncerebral malaria is apparent after a few disease episodes and is essentially complete before the age of 5 years, whereas immunity to mild disease takes many years to develop (18). Furthermore, Plasmodium falciparum isolates associated with severe malaria and young host age are recognized by human plasma immunoglobulin G (IgG) in areas where malaria is endemic more frequently than isolates associated with uncomplicated malaria are recognized (7, 9, 32). The best-characterized VSA, P. falciparum erythrocyte membrane protein 1 (PfEMP1), mediates cytoadherence and antigenic variation (4, 41, 44). PfEMP1 is encoded by 50 to 60 var genes per haploid parasite genome (15, 39, 44, 45), but only one or a few genes are expressed by each parasite at any given time (12, 14, 34, 45, 48). The availability of the complete genome sequence of P. falciparum laboratory clone 3D7 (16) and the ability to select 3D7 for VSASM-like expression (43) have made it possible to identify the group A var genes likely to code for VSASM types (21, 25, 28). Group A var genes belong to three major groups (groups A, B, and C) of 3D7 var gene sequences that have been categorized on the basis of chromosomal location and transcription direction, domain structure of the encoded proteins, and sequence similarities in coding and noncoding regions (25, 28). Unlike the members of the other groups, group A var genes encode large PfEMP1 variants, in agreement with the finding that parasites causing (cerebral) malaria as opposed to nonsevere malaria express high-molecular-weight PfEMP1 (5). Moreover, group A var genes possess structural features (e.g., DBL1α sequences lacking cysteine residues) linked to severe malaria (6, 24). Such structural features may confer better cytoadherence and rosetting, two main phenomena associated with the pathogenesis of severe malaria (11, 26, 36, 37).
We have previously shown that a group A var gene, PF11_0008, was transcribed at increased levels in an NF54 (isogenic line of 3D7) isolate from a naïve human host compared with the levels of expression of this gene in the parental isolate prior to infection (27). Such a naïve human host is comparable to young children living in areas where malaria is endemic who have not yet acquired immunity. In this study, we characterized the antibody response to PF11_0008 since parasites causing severe malaria seem to be dominant early in life in individuals living in areas where malaria is endemic.
MATERIALS AND METHODS
Expression of recombinant PF11_0008 domains.
The DBL1α, DBL2γ, and CIDR2β domains of PF11_0008 were PCR amplified from 3D7 genomic DNA and cloned into the pBAD-TOPO vector (Invitrogen) using the following primers: DBL1α-FW (5′-GAATTCTGTTATGGCAGACAAGCAA-3′), DBL1α-RV (5′-GTATTTATTTTTTTGTTTATCTAATTCATTTTC-3′), DBL2γ-FW (5′-GAATTCTGTAATCCAAAAAAGGAT-3′), DBL2γ-RV (5′-TGGTTTATTCTGACTTTTATCAATATC-3′), CIDR2β-FW (5′-GAATTCAAAAAACAAGAAAAACTATAT-3′), and CIDR2β-RV (5′-ACATGGATTTGCTGGAACA-3′).
For production of a carboxy-terminal V5 epitope and histidine-tagged protein, the DBL1α and DBL2γ inserts were excised by EcoRI and PmeI digestion and subcloned into the EcoRI and blunt-ended BglII sites of the Baculovirus transfer vector pAcGP67-A (BD Biosciences). The CIDR2β domain sequence, which had a PmeI site, was PCR amplified from pBAD-TOPO-CIDR2β to introduce EcoRI and NotI sites and then subcloned into the EcoRI and NotI sites of pAcGP67-A. Recombinant Baculovirus was generated by cotransfection of the pAcGP67-A-PF11_0008 domain construct and Bsu36I-linearized Bakpak6 Baculovirus DNA (BD Biosciences) into insect Sf9 cells. Recombinant PF11_0008 domains were expressed by infection of insect High Five cells with recombinant Baculovirus, purified from culture supernatants or pellets on Co2+-metal chelate agarose columns, and eluted with 25 mM HEPES-KOH (pH 7.6), 0.5 mM MgCl2, 0.5 mM dithiothreitol, 100 mM NaCl, 10% glycerol, and 100 mM imidazole.
Plasma donors.
To determine the human antibody reactivity to PF11_0008 domains, we used 60 plasma samples collected in April 2001 as part of a longitudinal study from each of three villages in the Tanga region in northeastern Tanzania. The villages are located close to each other but are markedly different in terms of malaria transmission and endemicity due to differences in altitude. The lowland village Mgome is subject to holoendemic transmission, Ubiri at 1,200 m above sea level is characterized by seasonal and mesoendemic transmission, and Magamba at 1,700 m is located in an area where endemicity is low (29). The plasma samples represented the following age groups: 2 to 4 years (n = 15), 5 to 9 years (n = 15), 10 to 14 years (n = 15), and 15 to 19 years (n = 15).
Correlations of PF11_0008 antibody levels in human plasma with protection from malaria episodes were assessed using 225 plasma samples collected in March 2004 as part of an on-going longitudinal study in Mkokola. Mkokola is a village situated in an area where there is a high level of malaria transmission and is in the region described above (30). Parasite density and hemoglobin levels were determined at the time of enrollment. Anemia was defined as a hemoglobin level of <11.0 g/dl. The 225 individuals were 0 to 19 years old, and 65 of them had at least one malaria episode, as defined by a parasite-positive slide and a history of fever or temperature of ≥37.5°C, while 160 did not have a malaria episode during the 7-month follow-up. Informed consent was obtained from all individuals studied and/or their parents. Ethical clearance was granted by the Ministry of Health and the Ethics Committee of the National Institute for Medical Research in Tanzania.
Enzyme-linked immunosorbent assay (ELISA) of PF11_0008 domains.
Dilutions (1:100) of plasma samples were incubated in 96-well MaxiSorp plates (Nunc, Denmark) precoated with recombinant PF11_0008 domains at a concentration of 1 or 2 μg/ml (depending on the point of signal saturation using antihistidine antibodies) in 100 μl coating buffer (0.1 M glycine HCl, pH 2.75) and processed as described previously (22). Antibody responses were expressed in arbitrary units calculated as follows: (optical density of sample − optical density of background)/(optical density of positive control sample − optical density of background). A Tanzanian plasma pool known to react strongly with recombinant PfEMP1 domains was used as a positive control. The mean optical density plus 2 standard deviations obtained with plasma from 20 healthy Danish donors who had not been exposed to malaria was used as a negative cutoff value. Individuals having plasma antibody levels above this negative cutoff value were considered to have responded positively to the domain tested.
Statistical analysis.
Logistic and Cox regression models were used to determine associations between PF11_0008 IgG levels and the incidence of malaria episodes. A two-sample t test with equal variances was used to determine the significance of differences between PF11_0008 IgG levels. Regression models were used to determine the relationship between PF11_0008 IgG levels and hemoglobin (linear regression model) or anemia (logistic regression model) at the time of enrollment. The Stata/SE 8.2 software (StataCorp, Texas; http://www.stata.com) was used for statistical analysis. A P value of <0.05 was considered statistically significant.
RESULTS
PF11_0008 antibody levels depend on age and transmission intensity.
To compare the acquisition of PF11_0008 antibodies in areas where the malaria transmission intensities are different, we tested plasma collected in three villages in northeastern Tanzania by ELISA using three recombinant PF11_0008 domains as catching antigens. Age group-specific responder frequencies and IgG levels were related to transmission intensity (Fig. 1). In the high-transmission village (Mgome), all donors carried plasma IgG that reacted with one or more of the PF11_0008 domains, and the antibody levels were generally high even in children as young as 2 years old. In the moderate-transmission village (Ubiri), the antibody prevalence and levels were high in individuals more than 10 years old, whereas the antibody levels were low and the occurrence was sporadic in all age groups in the village with low transmission (Magamba).
FIG. 1.
Point prevalence (circles and error bars showing 95% confidence intervals) and plasma levels (bars and error bars showing geometric means and 95% confidence intervals) of IgG antibodies to PF11_0008 DBL1α, DBL2γ, and CIDR2β domains. Plasma was collected from individuals who were 2 to 19 years old living in Mgome (high malaria transmission) (A), Ubiri (moderate malaria transmission) (B), or Magamba (low malaria transmission) (C).
Since antibody levels were already high in children who were 2 years old in Mgome, we performed a more detailed analysis of the age-specific acquisition of antibodies in Mkokola, another village in the area characterized by high and perennial malaria transmission (Fig. 2). In this setting, antibody reactivities to all three recombinant domains were acquired early in life, and more than 40% of the 1-year-old children exhibited a detectable response. The antibody levels also increased sharply in the first years of life and seemed to plateau around the age of 5 years.
FIG. 2.
Point prevalence (circles and error bars showing 95% confidence intervals) and plasma levels (bars and error bars showing geometric means and 95% confidence intervals) of IgG antibodies reacting to DBL1α (A), DBL2γ (B), and CIDR2β (C) of PF11_0008. Plasma was collected from individuals who were 0 to 19 years old living in Mkokola, a village where the level of malaria transmission is high.
These results show that the plasma levels of IgG to the recombinant PF11_0008 domains increase with age and transmission intensity and that the antibodies are acquired early in life in areas where the levels of malaria transmission is high. The observation that recombinant PF11_0008 domains were recognized by most individuals who had been exposed to malaria indicates that homologues of PF11_0008 are present in P. falciparum field isolates.
Antibodies against CIDR2β of PF11_0008 correlate with protection from malaria.
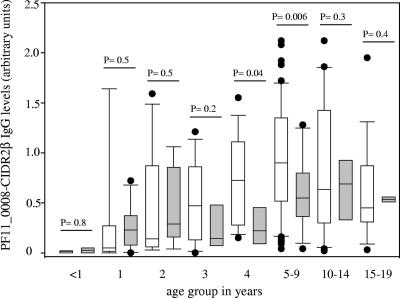
Malaria morbidity was monitored in the village of Mkokola, and this enabled us to analyze whether the levels of antibodies to the PF11_0008 domains were associated with reduced risk of developing disease or other parasitological parameters. Plasma collected at the initiation of the study was available from a total of 225 children and adolescents who completed the clinical monitoring. The mean parasite density at the time of enrollment, as well as the risk of malaria episodes during the follow-up period, had a tendency to decrease with age (Fig. 3), consistent with what would be expected in an area in which there is holoendemic transmission of P. falciparum (29, 47). Logistic regression models were used to determine the effect of PF11_0008 IgG plasma levels at the time of enrollment on the risk of having a malaria attack. We found that an increase in the PF11_0008 CIDR2β IgG level of 1 arbitrary unit decreased the risk of having a malaria episode by 60% (95% confidence interval, 19 to 82%; P = 0.012), with adjustment for the effects of age and bednet use (Table 1). Several models were used to adjust for age. All of them produced similar results and P values, indicating that the models were robust (data not shown). Using the Cox regression model considering the occurrence of a malaria episode through time, the PF11_0008 CIDR2β IgG levels were found to predict the incidence of malaria. An increase in the PF11_0008 CIDR2β IgG level of 1 arbitrary unit significantly decreased the risk of getting malaria by 53% (95% confidence interval, 32 to 88%; P = 0.014), after we controlled for age and bednet use. In order to detect whether the associations between PF11_0008 CIDR2β antibodies and malaria risk were similar in all age groups, we compared the plasma PF11_0008 CIDR2β IgG levels of individuals who got malaria and individuals who did not get malaria in the different age groups (Fig. 4). Interestingly, the PF11_0008 CIDR2β IgG levels were higher for protected individuals who were between 3 and 9 years old, and the differences were statistically significant for the 4-year-old children (P = 0.04) and for children between 5 and 9 years old (P = 0.006). For two age groups (0 to 2 and 10 to 19 years), the mean PF11_0008 CIDR2β IgG levels were slightly higher in individuals who had a clinical attack than in individuals who did not, but the differences were not statistically significant (Fig. 4). The levels of plasma IgG to other recombinant domains (PF11_0008 DBL1α or PF11_0008 DBL2γ) were not associated with a subsequent risk of having a malaria attack after adjustment for the effects of age and bednet use (Table 1).
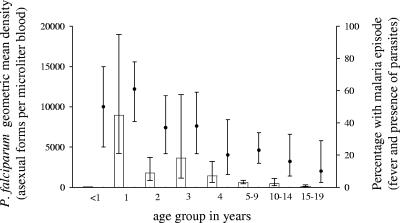
FIG. 3.
P. falciparum geometric mean density (bars and error bars showing 95% confidence intervals) and percentage of children and adults with a malaria episode in a high-malaria-transmission village (Mkokola) as defined by fever and the presence of parasites (circles and error bars showing 95% confidence intervals).
TABLE 1.
Logistic regression model showing the influence of PF11_0008 IgG levels on the risk of having a malaria episodea
| Variable | Unadjusted odds ratio
|
Adjusted odds ratiob
|
||
|---|---|---|---|---|
| Ratio (95% confidence interval) | P value | Ratio (95% confidence interval) | P value | |
| PF11_0008 DBL1α IgG level | 0.63 (0.40-0.99) | 0.044 | 0.81 (0.52-1.28) | 0.368 |
| PF11_0008 DBL2γ IgG level | 0.36 (0.17-0.77) | 0.009 | 0.63 (0.30-1.33) | 0.223 |
| PF11_0008 CIDR2β IgG level | 0.26 (0.14-0.49) | <0.001 | 0.40 (0.19-0.82) | 0.012 |
The risk of having a malaria episode is expressed as an odds ratio. The study was conducted with 225 individuals from Mkokola, a high-malaria-transmission village.
Adjusted for the effect of the square root of age and bednet use.
FIG. 4.
PF11_0008 CIDR2β IgG levels in individuals with (shaded box plots) or without (open box plots) a malaria episode in different age groups in Mkokola. The IgG levels are indicated by box plots with the median and the 25th and 75th percentiles; the error bars indicate the 10th and 90th percentiles, and the circles indicate outliers. P values calculated using the two-sample t test with equal variances are indicated.
Models used to test for associations between PF11_0008 IgG levels and hemoglobin levels by linear regression or between PF11_0008 IgG levels and anemia by logistic regression did not reveal any statistically significant associations when we controlled for the confounding effects of age, bednet use, and sex (data not shown). The linear regression model revealed that after age correction, every 1-arbitrary unit increase in PF11_0008 CIDR2β IgG levels was predicted to decrease parasitemia at the time of enrollment by 2,400 parasites/μl blood (95% confidence interval, 589 to 5,325 parasites/μl blood), but this effect was not statistically significant (P = 0.1).
These results indicate that high IgG levels against the CIDR2β domain of PF11_0008 protect children who are 4 to 9 years old against malaria.
DISCUSSION
Individuals living in areas where malaria is endemic develop immunity against severe malaria early in life (18), and parasites causing severe malaria tend to express a restricted, semiconserved subset of VSA (VSASM) that is recognized by antibodies from exposed individuals better than the VSA expressed by parasites causing uncomplicated malaria (7, 9, 32, 33). In a previous study, we analyzed var gene expression in malaria-naïve human volunteers who were experimentally infected with P. falciparum NF54 (an isogenic line of 3D7) sporozoites (19, 27). The largest var transcription changes and highest growth rates were observed in isolates from two of five volunteers. The levels of transcription of PF11_0008, a group A var gene, increased the most (∼50-fold) when isolates from earlier and later days during infection were compared in one of the volunteers. In the present study, we investigated the antibody response to PF11_0008 in areas where malaria is endemic.
We found that the levels of IgG against recombinant PF11_0008 domains increased with transmission intensity and age. In the two high-transmission villages a very high percentage of individuals carried antibodies against the three PF11_0008 domains. The presence of these antibodies might have been the result of widespread serological cross-reactivity between PfEMP1 domains. The PF11_0008 gene exhibits some similarity with another well-studied var gene, var4 (PFD1235w) (21). Both of these var genes belong to group A (28), both genes are transcribed at high levels in P. falciparum isolates from nonimmune human hosts (27), antibodies against recombinant domains encoded by both genes start to appear early in life in children living in areas where P. falciparum is endemic, and antibodies against the CIDR1α domain of VAR4 are associated with a reduced risk of malaria and anemia (30). However, antibodies against the PF11_0008 domains did not show significant cross-reactivity with VAR4 in competition ELISA experiments (22a) indicating that the PF11_0008 antibodies were not induced by parasites expressing VAR4. In general, cross-reactivity between recombinant 3D7 domains seems to be limited (22a), and we favor the explanation that the PF11_0008 antibodies were induced by parasites expressing PfEMP1 molecules having domains homologous to the PF11_0008 domains. Four var gene subfamilies have been identified: var1 (28, 38), var2csa (40), var3 (25), and var4 (21). These genes are carried by most parasites and exhibit similarity in large parts of the genome. So far, PF11_0008 has been identified only in laboratory clone 3D7 and the isogenic isolate NF54. The PF11_0008 gene could belong to a var subgene family, but perhaps more likely, sequences encoding domains homologous to PF11_0008 domains could be present as individual “building blocks” in different parasite genomes, probably due to recombination of var genes. We are currently producing affinity-purified human antibodies specific for PF11_0008 domains to be tested against field isolates to investigate this possibility.
There have been several studies which have indicated the importance of VSA-specific IgG in mediating acquired protective immunity to P. falciparum malaria (1, 8, 10, 13, 17, 23, 31, 35). In our study, we found that higher plasma levels of PF11_0008 CIDR2β antibodies were associated with a lower risk of developing malaria. Such an immune response seems to be relevant since in another study using the same plasma set, levels of antibodies to merozoite surface protein 1 constructs and a control CIDR1α domain were found not to be associated with morbidity protection (30). The control CIDR1α domain is encoded by PF08_107, which encodes a group C PfEMP1 with a four-domain structure predicted not to be involved in the pathogenesis of severe malaria (28). The probable mechanism by which PfEMP1 antibodies protect against malarial disease is by reducing tissue-specific sequestration and inflammation and by reducing the parasite burden as the nonbinders are destroyed in the spleen (13). The evidence which supports this conclusion includes the ability of PfEMP1 antibodies to block adhesion to certain host receptors (2, 3, 41). Our data indicate that the antibodies may have played a biological role in the 4- to 9-year-old children. The expression of PfEMP1 is probably not random but depends on the relative growth rates of parasites expressing different PfEMP1 types and the ability of antibodies to dampen this growth. In a host with limited or no immunity, the parasite preferentially expresses var genes coding for VSASM types that give the parasite a selective advantage through high growth rates as a result of increased survival due to effective cytoadhesion (20, 21, 27, 28). Following the production of antibodies that curb the growth rates of parasites expressing these early and virulent variant types, infections are dominated by parasites expressing PfEMP1 types that result in slightly lower growth rates than the most virulent types. Parasites expressing these types are probably still quite pathogenic, causing malaria in slightly older children, and PF11_0008 CIDR2β antibodies could play a role in protection against such parasites.
In conclusion, we found that homologues of PF11_0008, a group A PfEMP1 expressed early during experimental infection of a naïve individual, elicit antibodies that are acquired early in life. The fact that the presence of PF11_0008 IgG is associated with protection of children who are 4 to 9 years old indicates that parasites expressing homologues of PF11_0008-like PfEMP1s or domains are responsible for malaria in individuals who have survived the first malaria attacks and made antibodies against the most virulent PfEMP1 types.
Acknowledgments
We are very grateful to all study participants and their parents or guardians, as well as village helpers and health management teams in the Tanga region for providing the plasma samples used in this study. Lotte Bram and Susanne Pedersen are thanked for excellent technical assistance.
We received financial support from Danish International Development Assistance (grant 104.DAW.8.L.312), the Danish Council for Health Science Research (grant 271-05-0427), and the Commission of the European Communities (grant QLK2-CT-2002-01197, EUROMALVAC). P.M. and J.L. were supported by the Gates Malaria Partnership. A.T.R.J. was supported by the Howard Hughes Medical Institute.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Baruch, D. I., B. Gamain, J. W. Barnwell, J. S. Sullivan, A. Stowers, G. G. Galland, L. H. Miller, and W. E. Collins. 2002. Immunization of Aotus monkeys with a functional domain of the Plasmodium falciparum variant antigen induces protection against a lethal parasite line. Proc. Natl. Acad. Sci. USA 99:3860-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruch, D. I., J. A. Gormely, C. Ma, R. J. Howard, and B. L. Pasloske. 1996. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 93:3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruch, D. I., X. C. Ma, B. Pasloske, R. J. Howard, and L. H. Miller. 1999. CD36 peptides that block cytoadherence define the CD36 binding region for Plasmodium falciparum-infected erythrocytes. Blood 94:2121-2127. [PubMed] [Google Scholar]
- 4.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 5.Bian, Z., G. Wang, X. Tian, and J. Fan. 1999. Expression of Plasmodium falciparum-infected erythrocyte membrane protein from cerebral malaria patients. Zhongguo Jiehe Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17:359-362. [Chinese.] [PubMed] [Google Scholar]
- 6.Bull, P. C., M. Berriman, S. Kyes, M. A. Quail, N. Hall, M. M. Kortok, K. Marsh, and C. I. Newbold. 2005. Plasmodium falciparum variant surface antigen expression patterns during malaria. PLoS Pathog. 1:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bull, P. C., M. Kortok, O. Kai, F. Ndungu, A. Ross, B. S. Lowe, C. I. Newbold, and K. Marsh. 2000. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182:252-259. [DOI] [PubMed] [Google Scholar]
- 8.Bull, P. C., B. S. Lowe, N. Kaleli, F. Njuga, M. Kortok, A. Ross, F. Ndungu, R. W. Snow, and K. Marsh. 2002. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J. Infect. Dis. 185:1688-1691. [DOI] [PubMed] [Google Scholar]
- 9.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson, J., H. Helmby, A. V. Hill, D. Brewster, B. M. Greenwood, and M. Wahlgren. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336:1457-1460. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Q., V. Fernandez, A. Sundstrom, M. Schlichtherle, S. Datta, P. Hagblom, and M. Wahlgren. 1998. Developmental selection of var gene expression in Plasmodium falciparum. Nature 394:392-395. [DOI] [PubMed] [Google Scholar]
- 13.Dodoo, D., T. Staalsoe, H. Giha, J. A. Kurtzhals, B. D. Akanmori, K. Koram, S. Dunyo, F. K. Nkrumah, L. Hviid, and T. G. Theander. 2001. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect. Immun. 69:3713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy, M. F., G. V. Brown, W. Basuki, E. O. Krejany, R. Noviyanti, A. F. Cowman, and J. C. Reeder. 2002. Transcription of multiple var genes by individual, trophozoite-stage Plasmodium falciparum cells expressing a chondroitin sulphate A binding phenotype. Mol. Microbiol. 43:1285-1293. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, K., P. Horrocks, M. Preuss, J. Wiesner, S. Wunsch, A. A. Camargo, and M. Lanzer. 1997. Expression of var genes located within polymorphic subtelomeric domains of Plasmodium falciparum chromosomes. Mol. Cell. Biol. 17:3679-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giha, H. A., T. Staalsoe, D. Dodoo, C. Roper, G. M. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 2000. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol. Lett. 71:117-126. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 19.Hermsen, C. C., S. J. de Vlas, G. J. van Gemert, D. S. Telgt, D. F. Verhage, and R. W. Sauerwein. 2004. Testing vaccines in human experimental malaria: statistical analysis of parasitemia measured by a quantitative real-time polymerase chain reaction. Am. J. Trop. Med. Hyg. 71:196-201. [PubMed] [Google Scholar]
- 20.Hviid, L., and T. Staalsoe. 2004. Malaria immunity in infants: a special case of a general phenomenon? Trends Parasitol. 20:66-72. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, A. T., P. Magistrado, S. Sharp, L. Joergensen, T. Lavstsen, A. Chiucchiuini, A. Salanti, L. S. Vestergaard, J. P. Lusingu, R. Hermsen, R. Sauerwein, J. Christensen, M. A. Nielsen, L. Hviid, C. Sutherland, T. Staalsoe, and T. G. Theander. 2004. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199:1179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen, A. T., H. D. Zornig, C. Buhmann, A. Salanti, K. A. Koram, E. M. Riley, T. G. Theander, L. Hviid, and T. Staalsoe. 2003. Lack of gender-specific antibody recognition of products from domains of a var gene implicated in pregnancy-associated Plasmodium falciparum malaria. Infect. Immun. 71:4193-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Joergensen, L., L. Turner, P. Magistrado, M. A. Dahlbäck, L. S. Vestergaard, J. P. Lusingu, M. Lemnge, A. Salanti, T. G. Theander, and A. T. R. Jensen. 2006. Limited cross-reactivity among domains of the Plasmodium falciparum clone 3D7 erythrocyte membrane protein 1 family. Infect. Immun. 74:6778-6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinyanjui, S. M., T. Mwangi, P. C. Bull, C. I. Newbold, and K. Marsh. 2004. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J. Infect. Dis. 190:1527-1533. [DOI] [PubMed] [Google Scholar]
- 24.Kirchgatter, K., and H. A. Portillo. 2002. Association of severe noncerebral Plasmodium falciparum malaria in Brazil with expressed PfEMP1 DBL1 alpha sequences lacking cysteine residues. Mol. Med. 8:16-23. [PMC free article] [PubMed] [Google Scholar]
- 25.Kraemer, S. M., and J. D. Smith. 2003. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 50:1527-1538. [DOI] [PubMed] [Google Scholar]
- 26.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707.:673-707. [DOI] [PubMed] [Google Scholar]
- 27.Lavstsen, T., P. Magistrado, C. C. Hermsen, A. Salanti, A. T. Jensen, R. Sauerwein, L. Hviid, T. G. Theander, and T. Staalsoe. 2005. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malar. J. 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavstsen, T., A. Salanti, A. T. Jensen, D. E. Arnot, and T. G. Theander. 2003. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar. J. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lusingu, J. P., L. S. Vestergaard, B. P. Mmbando, C. J. Drakeley, C. Jones, J. Akida, Z. X. Savaeli, A. Y. Kitua, M. M. Lemnge, and T. G. Theander. 2004. Malaria morbidity and immunity among residents of villages with different Plasmodium falciparum transmission intensity in North-Eastern Tanzania. Malar. J. 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lusingu, J. P. A., A. T. R. Jensen, L. S. Vestergaard, D. T. Minja, M. B. Dalgaard, S. Gesase, B. P. Mmbando, A. Y. Kitua, M. M. Lemnge, D. Cavanagh, L. Hviid, and T. G. Theander. 2006. Levels of plasma immunoglobulin G with specificity against the cysteine-rich interdomain regions of a semiconserved Plasmodium falciparum erythrocyte membrane protein 1, VAR4, predict protection against malarial anemia and febrile episodes. Infect. Immun. 74:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen, M. A., T. Staalsoe, J. A. Kurtzhals, B. Q. Goka, D. Dodoo, M. Alifrangis, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J. Immunol. 168:3444-3450. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen, M. A., L. S. Vestergaard, J. Lusingu, J. A. Kurtzhals, H. A. Giha, B. Grevstad, B. Q. Goka, M. M. Lemnge, J. B. Jensen, B. D. Akanmori, T. G. Theander, T. Staalsoe, and L. Hviid. 2004. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect. Immun. 72:3531-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noviyanti, R., G. V. Brown, M. E. Wickham, M. F. Duffy, A. F. Cowman, and J. C. Reeder. 2001. Multiple var gene transcripts are expressed in Plasmodium falciparum infected erythrocytes selected for adhesion. Mol. Biochem. Parasitol. 114:227-237. [DOI] [PubMed] [Google Scholar]
- 35.Ofori, M. F., D. Dodoo, T. Staalsoe, J. A. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringwald, P., F. Peyron, J. P. Lepers, P. Rabarison, C. Rakotomalala, M. Razanamparany, M. Rabodonirina, J. Roux, and J. Le Bras. 1993. Parasite virulence factors during falciparum malaria: rosetting, cytoadherence, and modulation of cytoadherence by cytokines. Infect. Immun. 61:5198-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowe, A., J. Obeiro, C. I. Newbold, and K. Marsh. 1995. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect. Immun. 63:2323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe, J. A., S. A. Kyes, S. J. Rogerson, H. A. Babiker, and A. Raza. 2002. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J. Infect. Dis. 185:1207-1211. [DOI] [PubMed] [Google Scholar]
- 39.Rubio, J. P., J. K. Thompson, and A. F. Cowman. 1996. The var genes of Plasmodium falciparum are located in the subtelomeric region of most chromosomes. EMBO J. 15:4069-4077. [PMC free article] [PubMed] [Google Scholar]
- 40.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. Jensen, M. P. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179-191. [DOI] [PubMed] [Google Scholar]
- 41.Smith, J. D., A. G. Craig, N. Kriek, D. Hudson-Taylor, S. Kyes, T. Fagen, R. Pinches, D. I. Baruch, C. I. Newbold, and L. H. Miller. 2000. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA 97:1766-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snow, R. W., J. A. Omumbo, B. Lowe, C. S. Molyneux, J. O. Obiero, A. Palmer, M. W. Weber, M. Pinder, B. Nahlen, C. Obonyo, C. Newbold, S. Gupta, and K. Marsh. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650-1654. [DOI] [PubMed] [Google Scholar]
- 43.Staalsoe, T., M. A. Nielsen, L. S. Vestergaard, A. T. Jensen, T. G. Theander, and L. Hviid. 2003. In vitro selection of Plasmodium falciparum 3D7 for expression of variant surface antigens associated with severe malaria in African children. Parasite Immunol. 25:421-427. [DOI] [PubMed] [Google Scholar]
- 44.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, H. M., S. A. Kyes, D. Harris, N. Kriek, and C. I. Newbold. 2000. A study of var gene transcription in vitro using universal var gene primers. Mol. Biochem. Parasitol. 105:13-23. [DOI] [PubMed] [Google Scholar]
- 46.Tebo, A. E., P. G. Kremsner, K. P. Piper, and A. J. Luty. 2002. Low antibody responses to variant surface antigens of Plasmodium falciparum are associated with severe malaria and increased susceptibility to malaria attacks in Gabonese children. Am. J. Trop. Med. Hyg. 67:597-603. [DOI] [PubMed] [Google Scholar]
- 47.Trape, J. F., P. Peelman, and B. Morault-Peelman. 1985. Criteria for diagnosing clinical malaria among a semi-immune population exposed to intense and perennial transmission. Trans. R. Soc. Trop. Med. Hyg. 79:435-442. [DOI] [PubMed] [Google Scholar]
- 48.Voss, T. S., J. Healer, A. J. Marty, M. F. Duffy, J. K. Thompson, J. G. Beeson, J. C. Reeder, B. S. Crabb, and A. F. Cowman. 2006. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature 439:1004-1008. [DOI] [PubMed] [Google Scholar]