Abstract
Monocytes and macrophages are the cell types most commonly associated with the innate immune response against Candida albicans infection. Interactions between the host immune system and Candida organisms have been investigated for planktonic Candida cells, but no studies have addressed these interactions in a biofilm environment. In this study, for the first time, we evaluated the ability of C. albicans to form biofilms in the presence or absence of adherent peripheral blood mononuclear cells (PBMCs; enriched for monocytes and macrophages by adherence). Our analyses using scanning electron and confocal scanning laser microscopy showed that the presence of PBMCs enhanced the ability of C. albicans to form biofilms and that the majority of PBMCs were localized to the basal and middle layers of the biofilm. In contrast to the interactions of PBMCs with planktonic C. albicans, where PBMCs phagocytose fungal cells, PBMCs did not appear to phagocytose fungal cells in biofilms. Furthermore, time-lapse laser microscopy revealed dynamic interactions between C. albicans and PBMCs in a biofilm. Additionally, we found that (i) only viable PBMCs influence Candida biofilm formation, (ii) cell surface components of PBMCs did not contribute to the enhancement of C. albicans biofilm, (iii) the biofilm-enhancing effect of PBMCs is mediated by a soluble factor released into the coculture medium of PBMCs with C. albicans, and (iv) supernatant collected from this coculture contained differential levels of pro- and anti-inflammatory cytokines. Our studies provide new insight into the interaction between Candida biofilm and host immune cells and demonstrate that immunocytes may influence the ability of C. albicans to form biofilms.
The use of central venous catheters in current therapeutic practice has been found to be responsible for more than 70% of bloodstream and deep-tissue infections (48). Candida species have emerged as the fourth most common cause of nosocomial bloodstream infections (50), with a significantly high attributable mortality (49% to 71%) (14, 15). Most of these infections involve colonization of microorganisms on catheter surfaces where they eventually become embedded in a biofilm. These biofilms are associated with high antifungal resistance and can persist on infected sites, acting as a nidus of infection (11).
Candidiasis is most commonly manifested among immunocompromised patients, and components of the host immune response mechanisms play an important role in Candida pathogenesis. The ability of a fungal pathogen like Candida to cause infection is dependent on its ability to overcome the host immune response and is regulated by modulating the levels of pro- and anti-inflammatory cytokines (6, 12, 22, 29, 30).
Biofilms represent an entirely distinct ecological niche for Candida, with a resistance and growth phenotype that is drastically more altered than its planktonic (free-floating) form. The field of study of C. Candida albicans biofilms has focused mostly on model development and characterization, together with antifungal drug resistance, with little attention to the effects on immunity. Since biofilms that form on indwelling medical devices (e.g., intravascular catheters) are the predominant growth form on these catheters and since these biofilms are constantly exposed to serum factors and host immune cells, the interactions occurring between immune cells and Candida biofilms are expected to be unique or substantially different from the corresponding interactions with planktonic cells.
Candida spp. have been shown to have immunomodulatory activity mediated by fungal factors, including mannan and glycoproteins, mannoprotein constituents of the cell wall, and surface antigens (5, 9, 40, 47). Although interactions occurring between the host immune system and Candida have been investigated in some detail for planktonically grown Candida cultures (reviewed in references 31, 33, 34, 35, and 38), no information is currently available for such interactions in a biofilm environment.
Monocytes/macrophages are the cell types most commonly associated with the innate immune response against C. albicans infection, and they produce a variety of soluble factors, including cytokines and chemokines, in response to microorganisms (43). Therefore, we hypothesized that adherent peripheral blood mononuclear cells (PBMCs; enriched for monocytes and macrophages by adherence) influence the ability of C. albicans to form biofilms. To test this hypothesis, we evaluated the ability of C. albicans to form biofilms in the presence or absence of adherent PBMCs using scanning electron microscopy (SEM) and confocal scanning laser microscopy (CSLM) techniques. We also tested whether the interaction between Candida biofilm and PBMCs is dependent on the cell surface or on secretory soluble factors. Finally, we investigated whether interactions between Candida and PBMCs lead to a differential production of cytokines by PBMCs. Our studies demonstrated that (i) interactions of Candida biofilms with PBMCs are unique to this growth mode compared to those of planktonically grown Candida cells, (ii) the ability of PBMCs to enhance C. albicans biofilm formation is associated with a soluble factor(s) present in the biofilm-PBMC culture supernatant, and (iii) this supernatant contains differentially expressed levels of pro- and anti-inflammatory cytokines.
MATERIALS AND METHODS
Organisms.
A green fluorescent protein (GFP)-tagged C. albicans strain (a generous gift from B. Cormack, Johns Hopkins University) was used in this study. This system is based upon the plasmid pGFP, which contains the yeast-enhanced green fluorescent protein (yEGFP) cloned from the basal C. albicans ADH1 (CaADH1) promoter and the Saccharomyces cerevisiae CYC1 (ScCYC1) terminator on an integrating vector (10). C. albicans cells were grown overnight at 37°C in yeast-peptone-dextrose medium (Difco Laboratories, Detroit, MI) supplemented with 50 to 100 μg/ml uridine. Cells were harvested, washed with phosphate-buffered saline, and standardized to 1 × 107 cells/ml.
Biofilm formation.
Biofilms were formed on silicone elastomer (SE) discs using our catheter-based in vitro model described previously (7). Briefly, SE discs (12-mm diameter) were precoated with fetal bovine serum (FBS) and immersed in 4 ml of a standardized C. albicans cell suspension (1 × 107 cells/ml) for 90 min to allow adhesion of fungal cells to the substrate. The catheter disc containing adhered Candida cells was then placed in fresh yeast nitrogen base medium in a 12-well tissue culture plate and allowed to form mature biofilms. Biofilms were quantified by a tetrazolium-salt-based assay {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay} as described previously (7). Planktonic cells were grown similarly in tissue culture plates with the difference that no catheter discs were present.
Isolation of adherent human PBMCs.
To examine the interactions between host immune cells and Candida biofilms, PBMCs were extracted from peripheral blood as described previously (39, 40). Briefly, the collected blood was diluted (1:1) with Hanks' balanced salt solution (HBSS; Mediatech Inc., Herndon, VA), and 30 ml of this diluted blood was layered on a Histopaque density gradient (Sigma-Aldrich, St. Louis, MO) and centrifuged at 1,500 rpm for 40 min at 24°C. The buffy coat containing PBMCs was collected in HBSS supplemented with 1% FBS (HBSS/1% FBS) and centrifuged for 10 min at 4°C (1,500 rpm). The cell pellet was washed twice with HBSS/1% FBS by centrifugation at 1,500 rpm. Washed cell pellets were resuspended in complete medium containing RPMI medium and FBS supplemented with penicillin, streptomycin, and l-glutamine (Mediatech Inc., Herndon, VA) and incubated at 37°C for 60 min in a 5% CO2 incubator. Nonadherent cells (mainly T cells) were removed, and all adherent cells were gently scraped to harvest the cells. These cells were added to HBSS/1% FBS and washed twice as described above. Cells were counted, and 2 × 106 cells were used for mixed yeast-PBMC culture studies.
Staining of extracted PBMCs.
To visualize live, metabolically active PBMCs, the isolated PBMCs were stained with Mitotracker Deep Red 633 dye (Invitrogen, Carlsbad, CA), which targets the intracellular mitochondrial network and stains live cells red. We and others have demonstrated that this vital dye does not interfere with cell viability (17) and that this dye can be used in conjunction with phagocytosis assays.
After PBMC extraction, 2 × 106 cells were counted and washed with HBSS/1% FBS. Next, 100 nM dye was added to the washed PBMC pellet and incubated at 37°C for 1 h. After staining, cells were washed three times with HBSS/1% FBS and adjusted to a concentration of 2 × 106 cells/ml. PBMCs stained with Mitotracker dye retained the stain for up to 48 h, with no visible change of the cells or decreased cell viability (assessed by trypan blue exclusion [data not shown]). These prestained cells were used for coculture experiments with C. albicans biofilms. For experiments involving PBMC/biofilm coculture, C. albicans cells were allowed to adhere to SE discs for 90 min at 37°C and then incubated with 2 × 106 prestained PBMCs. This coculture system was then allowed to mature to a 48-h biofilm in 2 ml RPMI medium and 2 ml HBSS/1% FBS medium supplemented with 100 μg/ml uridine. In these experiments, PBMCs stained with Mitotracker appeared red, while the GFP-tagged Candida cells appeared green under confocal scanning laser microscopy. Our studies revealed that staining of PBMCs with this dye did not affect the viability (and hence the functionality) of PBMCs.
Gamma irradiation of PBMCs.
Gamma irradiation causes DNA damage to PBMCs, yet cells retain intact membrane proteins (3). Irradiated cells are no longer proliferative and do not produce de novo protein but otherwise remain functional. Because the cell membrane and mitochondria remain intact, the cells retain the Mitotracker dye. These gamma-irradiated PBMCs are commonly used as feeder cells in propagating growth of immune cells in vitro (16, 36). To study whether surface molecules on PBMCs affect the growth of C. albicans as biofilms, we irradiated PBMCs with 3,000 rads and then incubated them with C. albicans at the initiation of biofilm formation (adhesion phase). These biofilms were then allowed to grow to the mature phase as described above.
Scanning electron microscopy.
The effect of coculturing C. albicans and PBMCs on the surface topography of a biofilm was investigated using SEM as described previously (7, 8). Briefly, 2 × 106 PBMCs were added at the adhesion phase of the C. albicans culture, and the whole mixed culture was allowed to form a biofilm. Following maturation for 48 h, biofilms were prepared for SEM. SE disks with mature biofilms were fixed with 2% glutaraldehyde, followed by fixing with osmium tetraoxide, tannic acid, and uranyl acetate. Planktonically grown C. albicans cells grown in the presence or absence of PBMCs were prepared for SEM analysis using the same methodology. This was followed by a series of ethanol dehydration steps, and the prepared samples were sputter coated with Au-Pd (60/40 ratio) and viewed with a model XL3C ESEM Philips microscope.
Confocal scanning laser microscopy.
CSLM was used to evaluate the effect of PBMCs on the architecture and thickness of C. albicans biofilms. Biofilms were grown on SE discs in the presence or absence of PBMCs and placed on a 35-mm-diameter glass-bottom petri dish (MatTek Corp., Ashland, MA). To visualize Candida and PBMCs simultaneously, GFP-tagged Candida biofilm (GFP excitation wavelength, 488 nm; emission, 505-nm long-pass filter) and PBMCs prestained with Mitotracker Deep Red dye (excitation, 644 nm; emission, 665 nm) were used. The coculture was observed as described earlier (7) with a Zeiss LSM510 confocal scanning laser microscope equipped with argon and HeNe lasers and mounted on a Zeiss Axiovert100 M microscope (Carl Zeiss, Inc., Thornwood, NY). The objective used was a water immersion C-Apochromat lens (20×). Depth measurements were taken at regular intervals across the width of the device. To determine the effect of Candida-PBMC interactions on the structure of the biofilms, a series of horizontal (xy) optical sections were taken throughout the full length of the biofilm. Confocal images of green (GFP) and red (Mitotracker dye) fluorescence were conceived simultaneously using a multitrack mode. Planktonically grown C. albicans cells were used as comparators in these studies.
Time-lapse laser microscopy.
Time-lapse laser microscopy (TLLM) is a technique that can be used to capture real-time images of a single frame at specific time intervals, allowing temporal monitoring of the interactions occurring between Candida and PBMCs as biofilm matures. The images for the real-time interactions taking place between C. albicans biofilm and PBMCs were captured using TLLM. Subsequently, these captured images were combined in a time sequence, resulting in an animation depicting the sequence of events that occurred with the passage of time. Briefly, PBMCs were isolated and prestained with Mitotracker Deep Red 633 dye as described above and then added to the adhesion phase of GFP-tagged C. albicans. The discs with C. albicans and PBMCs were placed on a 35-mm-diameter glass-bottom petri dish (MatTek Corp., Ashland, MA). Then growth medium was added, and the whole petri dish was incubated at 37°C to allow the formation of biofilm in the presence of PBMCs. Images for this interaction were captured immediately from 0 h and followed for up to 44 h with a Zeiss LSM510 confocal scanning laser microscope. Confocal images of green and red fluorescence were conceived simultaneously using a multitrack mode as described above. To determine the structural changes in the maturing biofilm-PBMC coculture, a series of horizontal (xy) optical sections were obtained throughout the full length of the PBMC-associated biofilm.
Isolation of supernatants from C. albicans-PBMC cocultures.
To determine whether a soluble factor was responsible for the biofilm-enhancing activity of PBMCs, we isolated supernatants from the PBMC-biofilm coculture growing under different conditions, as follows: (i) C. albicans biofilms, (ii) C. albicans biofilms grown in the presence of PBMCs, (iii) medium only, (iv) PBMCs grown in monocyte-specific medium, or (v) lipopolysaccharide (LPS)-activated PBMCs. For each condition, supernatant was collected after 48 h, centrifuged, and filter sterilized using 0.22-μm-pore-size filters. The filtered supernatants were added to C. albicans biofilm at the adhesion phase, and the biofilm was allowed to grow for 24 h. One set was used as a control in which no supernatant was added. After 24 h, C. albicans biofilm formation was analyzed using CSLM and quantified by XTT assay as described previously (7).
Determination of the cytokine profile of different supernatants.
To determine whether growth in C. albicans biofilms induces a change in the cytokine profile of PBMCs, we measured the levels of different cytokines in biofilms formed in the presence or absence of PBMCs. As a comparator, we also evaluated the cytokine released by PBMCs when interacting with planktonically grown C. albicans cells. Briefly, 2 × 106 PBMCs were added to C. albicans cells adhering to SE discs, and biofilm was allowed to form. Discs incubated with only PBMCs or only growth medium served as controls. After 48 h, supernatant was collected, centrifuged, and filter sterilized using 0.22-μm filters. Human cytokine antibody arrays V and 5.1 (Ray Biotech, Inc., Norcross, GA) were used for the detection of multiple cytokines in these supernatants (18-21). Each membrane was placed in the eight-well tray and blocked with blocking buffer according to the manufacturer's instructions. The membrane was incubated with 2 ml of supernatant obtained from the biofilm or the planktonic Candida cells, followed by biotin-conjugated anticytokine antibody treatment. Next, membranes were washed and incubated with horseradish peroxidase-conjugated streptavidin, and the signals were captured using a chemiluminescent phosphorimager (VersaDoc; Bio-Rad Laboratories, CA). Cytokine spots were quantified using a densitometer (VersaDoc; Bio-Rad Laboratories, CA), and relative values were calculated as percentages with respect to internal positive controls (used as 100%) from each cytokine array membrane.
Statistical analyses.
Each experiment was performed three times on separate days. All statistical analyses were performed using StatView (version 4.5; Abacus Concepts Inc., Berkeley, CA). One-way analysis of variance was performed to compare means of multiple groups, and a two-tailed Student's t test was used for analysis of two groups. Results with a P value of less than 0.05 were considered statistically significant.
RESULTS
C. albicans forms hypha-rich biofilms in the presence of adherent PBMCs.
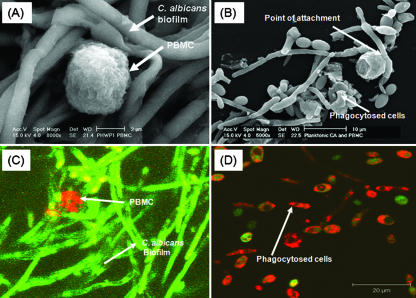
Since C. albicans biofilms formed in the clinical setting on indwelling catheters are likely to be exposed to host immune cells, we hypothesized that immune cells (e.g., adherent PBMCs) will influence the ability of C. albicans to form biofilms. To test our hypothesis, we used SEM to visualize the surface topology of C. albicans biofilms formed in the presence of adherent PBMCs isolated from healthy volunteers. Our analyses showed that C. albicans formed hypha-rich biofilms in the presence of PBMCs, with very few monocytes visible on the surfaces of the biofilms (Fig. 1A). High-magnification images revealed that the monocytes were intact and were localized close to the biofilm hyphae (Fig. 1A). Furthermore, these monocytes/macrophages did not appear to phagocytose Candida cells. In contrast, PBMCs incubated with planktonic C. albicans for the same amount of time clearly demonstrate phagocytosis of the fungal cells (Fig. 1B).
FIG. 1.
Effect of PBMCs on the surface topography of C. albicans biofilms and planktonic cells. SEM analyses of the effect of PBMCs on surface topography of C. albicans grown as (A) biofilms or (B) planktonic cells. (A) C. albicans formed hypha-rich biofilms in the presence of PBMCs, with very few monocytes visible on the surfaces of the biofilms. SEM images revealed that the PBMCs were intact and appeared close to biofilm hyphae. The monocytes/macrophages do not appear to have engulfed Candida. (B) In contrast, PBMCs incubated with planktonic C. albicans for the same amount of time clearly demonstrate phagocytosis of the fungal cells. (C) CSLM revealed the attachment of only a few PBMCs to the top slices of fungal biofilm (magnification, ×40). Interestingly, the adherent PBMCs do not appear to have any phagocytosing processes extended into the Candida biofilm. (D) CSLM images of planktonic C. albicans cells grown in the presence of PBMCs, where phagocytosis was clearly visible. All experiments were performed three times on separate days. Magnification, ×40.
To determine the architecture and morphological composition and organization of live, hydrated biofilms, we used CSLM, which revealed few PBMCs in the top layers of biofilm (Fig. 1C). Interestingly, the adherent PBMCs did not appear to have any phagocytosing processes extended into the Candida biofilm, in contrast to planktonic cells where phagocytosis was visible as indicated by green-stained Candida cells within the red-stained PBMCs (Fig. 1D). Our findings show that the interactions between PBMCs and Candida differ depending on the growth form (biofilm versus planktonic) of this fungus. Importantly, these results demonstrated that staining of PBMCs with Mitotracker dye did not alter their functionality because prestained PBMCs were able to phagocytose planktonically grown Candida cells.
PBMCs induce enhanced biofilm formation by C. albicans and are localized to the basal layers.
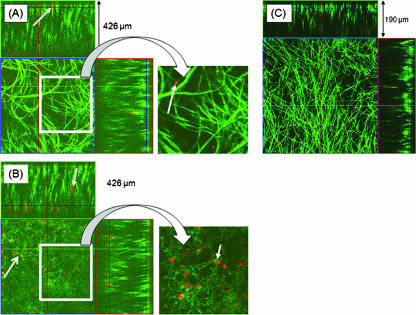
Since our SEM analyses revealed the presence of only a few PBMCs on the surface of the C. albicans biofilm, we hypothesized that the adherent PBMCs may have localized to the basal and middle layers of the biofilm. To test this hypothesis, we evaluated the architecture of biofilms using CSLM, which unlike SEM, can provide information about the three-dimensional localization of cells as well as their spatial arrangement (7). We performed CSLM analyses using GFP-tagged C. albicans and prestained PBMCs. Orthogonal analyses of the top layers of PBMC-containing fungal biofilms revealed the presence of profuse hypha-rich biofilms with very few PBMCs, confirming our observations from SEM analyses (Fig. 2A). In contrast, orthogonal analyses of the basal and middle layers showed that PBMCs were present chiefly in these layers of C. albicans biofilm (Fig. 2B). Biofilms formed in the presence of adherent PBMCs were significantly thicker than those formed in their absence (thickness, 426 ± 11 μm versus 190 ± 17 μm, respectively; P = 0.0014) (Fig. 2). These studies demonstrated that C. albicans formed enhanced and thicker biofilms in the presence of PBMCs and that these immune cells are localized in the basal and middle layers of biofilms.
FIG. 2.
Confocal scanning laser microscopic analyses of biofilms formed by C. albicans in the presence of PBMCs. PBMCs were prestained with Mitotracker Deep Red 633 dye (small white arrows), incubated with the adhesion phase of GFP-tagged C. albicans biofilms, and allowed to mature. Orthogonal images of (A) the upper layers of biofilm formed by C. albicans in the presence of PBMCs, showing the presence of a few PBMCs; (B) the basal and middle layers of biofilm formed by C. albicans in the presence of PBMCs, showing numerous PBMCs in these layers (biofilm thickness, 426 μm); and (C) Candida biofilm formed in the absence of PBMCs (biofilm thickness, 190 μm). All experiments were done in triplicate on separate days. Magnification, ×20. Parts of panels A and B are enlarged (insets) to show PBMCs.
Time-lapse microscopy reveals dynamic interactions between PBMCs and C. albicans during biofilm formation.
To evaluate the real-time interactions between PBMCs and C. albicans biofilms that occur during biofilm formation, we used time-lapse video confocal microscopy. Images of a single area of the Candida biofilm-PBMC coculture were obtained beginning with biofilm adhesion and followed at regular intervals up to the mature phase at 48 h (see Video S1 in the supplemental material). Our time-lapse analyses revealed that PBMCs interact with C. albicans biofilms over time. Active PBMC migration/movement was observed within localized areas of the biofilm. In contrast to PBMCs cocultured with planktonic C. albicans, where phagocytosis was clearly evident, our analyses with C. albicans biofilms did not reveal any occurrence of phagocytosis, as evidenced by the lack of green-stained Candida elements within the red-stained PBMCs. Interestingly, by the time C. albicans biofilms reached the mature phase, Mitotracker Red-stained PBMCs appeared diffuse, suggesting an increase in the thick biofilm extracellular material embedding the PBMCs.
Proliferating PBMCs are needed to enhance biofilm formation by C. albicans.
The above findings showed that C. albicans biofilm formation is increased in the presence of PBMCs. To examine whether this effect on Candida biofilms is mediated only by proliferating monocytes and whether cell surface molecules of PBMCs contribute to this activity, we determined the effect that gamma-irradiated PBMCs had on the ability of C. albicans to form biofilms. Gamma-irradiated PBMCs maintain intact cell membranes and associated cell surface molecules but have decreased de novo production of soluble factors and are commonly used as feeder cells in propagating the growth of immune cells in vitro (16, 36). PBMCs prepared in this manner are viable but irreversibly inactivated (27). We chose to use gamma irradiation as the method to treat PBMCs because this treatment will stop PBMC proliferation and maintain cell surface molecules while inhibiting de novo cytokines (or other soluble factors) from being produced. Our analyses revealed that following irradiation, the biofilm enhancement observed with nonirradiated PBMC-Candida mixed cultures was reversed, suggesting that the factor(s) produced by PBMCs contributes to biofilm augmentation and that gamma irradiation reversed the production of these required “factors.” Furthermore, the C. albicans biofilms formed in the presence of actively proliferating PBMCs were significantly thicker than those formed in the presence of gamma-irradiated PBMCs (426 ± 11 μm versus 210 ± 10 μm, respectively; P = 0.0002) (Fig. 3B and C). The thickness of the C. albicans biofilm formed in the presence of gamma-irradiated PBMCs was similar to that of the control C. albicans biofilms with no PBMCs (Fig. 3A and C; P > 0.05). To test the possibility that gamma irradiation may render PBMCs nonviable, we assessed cellular viability by trypan blue exclusion. We found that dye exclusion was not affected by gamma irradiation (data not shown), demonstrating that the PBMC membranes remained intact and functional. Taken together, these results showed that the biofilm-enhancing effect is dependent on proliferating, active PBMCs. Furthermore, because gamma-irradiated PBMCs retain intact cell surface molecules, our results also indicate that the formation of enhanced biofilm in the presence of PBMCs is not dependent upon interactions between PBMC surface proteins and C. albicans cells.
FIG. 3.

Effect of gamma-irradiated PBMCs on the ability of C. albicans to form biofilms. Side-view image of biofilms formed by C. albicans in (A) the absence of PBMCs (thickness, 190 μm), (B) the presence of normal, active PBMCs (thickness, 426 μm), and (C) the presence of gamma-irradiated PBMCs (thickness, 210 μm). As can be clearly seen, gamma-irradiated PBMCs were unable to enhance biofilm formation by C. albicans. All experiments were done in triplicate on separate days. Magnification, ×20.
The biofilm-enhancing effect of PBMCs on C. albicans is mediated by a secretory factor(s).
Since our studies showed that enhancement of C. albicans biofilm in the presence of PBMCs is not dependent on cell surface components of PBMCs, we hypothesized that this effect is mediated by an extracellular factor(s). To examine this hypothesis, we tested the ability of supernatants obtained under different culture conditions to influence C. albicans biofilm formation. We found that biofilms formed in the presence of supernatant obtained from C. albicans-PBMC biofilms had significantly higher metabolic activity than those formed in the presence of supernatant obtained from C. albicans biofilms grown in the absence of PBMCs (P = 0.0013) (Table 1). Furthermore, biofilms formed in the presence of supernatant obtained from medium alone or from PBMCs alone were not significantly different from the biofilm formed in the presence of supernatant obtained from C. albicans grown alone (P > 0.18) (Table 1). CSLM analyses confirmed these findings and showed that biofilms formed by C. albicans in the presence of supernatant obtained from C. albicans-PBMC biofilm coculture contained profuse hyphal elements and were significantly thicker than those formed in the presence of supernatant from C. albicans biofilm grown alone (P = 0.0013) (Fig. 4). The thicknesses of biofilms formed in the presence of supernatant obtained from C. albicans biofilm formed alone were similar to those obtained from medium or from PBMCs grown alone (P < 0.05), demonstrating that the biofilm-enhancing activity was present only in the supernatant obtained from the coculture of C. albicans-PBMC biofilms.
TABLE 1.
Metabolic activity of C. albicans biofilms formed in the presence of supernatants from six different conditionsa
| Supernatant | Optical density at 592 nm
|
P value | |
|---|---|---|---|
| Mean | SD | ||
| C. albicans biofilm | 0.122 | 0.022 | |
| PBMC + C. albicans biofilm | 0.196 | 0.018 | 0.0013 |
| Medium alone | 0.142 | 0.013 | 0.2978 |
| PBMC alone | 0.163 | 0.027 | 0.2883 |
| LPS + PBMC | 0.178 | 0.029 | 0.1037 |
Metabolic activity is presented as means ± standard deviations (SD) of optical density at 592 nm. P value compared to supernatant obtained from biofilm formed by C. albicans.
FIG. 4.
Enhancement of C. albicans biofilm in the presence of PBMCs is mediated by a secretory extracellular factor(s). Supernatants (sup) were obtained from C. albicans biofilms (CA), C. albicans biofilms grown in the presence of PBMCs, medium only, PBMCs grown in monocyte-specific medium, and lipopolysaccharide-activated PBMCs. Supernatants were added to C. albicans at the adhesion phase of biofilm development, and the biofilms were allowed to develop to mature phase. Biofilm formations in the presence of different supernatants were evaluated by CSLM, and their thicknesses were determined. *, P < 0.05. All experiments were done in triplicate on separate days.
Augmentation of Candida biofilm formation is specific to biofilm-activated PBMCs.
To determine whether the biofilm-enhancing effect of PBMCs is specifically associated with the activation of these immune cells by C. albicans biofilms, we tested whether PBMCs activated by a nonbiofilm source can also enhance biofilm formation. The activation method selected was exposure to LPS, a method which has previously been used to activate PBMCs (4, 40). Adherent PBMCs were activated with LPS, and the ability of this LPS-induced PBMC supernatant to influence the ability of C. albicans to form biofilm was then determined. Our results showed that supernatant from LPS-activated PBMCs did not significantly alter C. albicans biofilm formation; however, there was a trend toward increased metabolic activity (Table 1), as well as a slight increase in C. albicans biofilm thickness (P > 0.05 in each case; Fig. 4). These results demonstrated that the biofilm-enhancing secretory factor(s) activity of PBMCs is specifically induced by C. albicans biofilms.
C. albicans biofilms induce differential production of cytokines and chemokines by PBMCs.
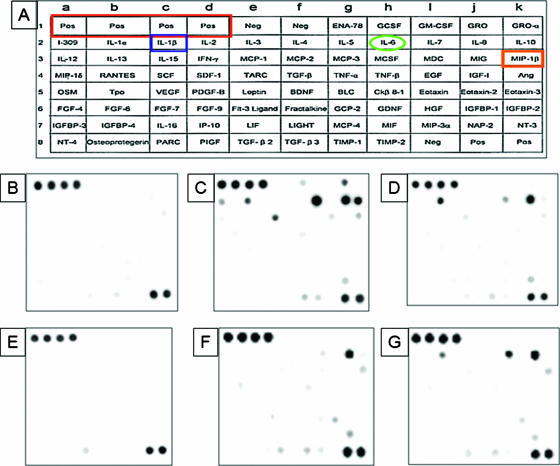
Our studies revealed that the biofilm-enhancing activity of PBMCs involves a secretory factor(s). Since cytokines are common factors secreted by PBMCs, we tested whether supernatant obtained from a biofilm of cocultured PBMCs and C. albicans contained differential levels of specific cytokines, compared with those present in supernatant from PBMCs alone or from PBMC incubated with planktonic Candida cells. As shown in Fig. 5 and Table 2, compared to the level in supernatant obtained from PBMCs grown alone (Fig. 5C), the level of proinflammatory cytokine interleukin-1β (IL-1β) was significantly up-regulated in supernatant obtained from the PBMC-Candida biofilm coculture (P = 0.0211), while six cytokines (IL-6, IL-10, monocyte chemoattractant protein 1 [MCP-1], I-309, tumor necrosis factor alpha [TNF-∝], and chemokine macrophage inflammatory protein 1β [MIP1β]) were significantly down-regulated in this biofilm-PBMC supernatant (Fig. 5D). Interestingly, the level of proinflammatory cytokine IL-8 appeared unchanged in both of the supernatants (Table 2).
FIG. 5.
Cytokine profile of supernatants obtained from PBMCs exposed to C. albicans biofilms or planktonic cells. PBMCs were grown in the absence or presence of C. albicans biofilms or planktonic cells for 48 h, their supernatants were collected, and the cytokines present in these supernatants were identified using the preprinted human cytokine antibody arrays V and 5.1 (Ray Biotech, Inc.). (A) Location of different cytokines on the preprinted array. Samples were obtained from medium or cells incubated with catheter discs (B through D) or incubated without any substrate (E through G) in tissue culture plates. Supernatants were obtained from (B and E) medium alone, (C and F) PBMCs grown alone, (D) PBMCs grown in the presence of C. albicans biofilms, and (G) PBMCs grown in the presence of planktonic Candida cells. The proinflammatory cytokine IL-1β was up-regulated in supernatant obtained from PBMC-Candida coculture, while six cytokines (IL-6, IL-10, MCP-1, I-309, TNF-∝, and chemokine MIP1β) were down-regulated in this supernatant (D), compared to that in supernatant obtained from PBMCs grown alone (C). Furthermore, cytokines produced by PBMCs cocultured with planktonic C. albicans (G) showed different levels compared to PBMCs cocultured with biofilms (D). All experiments were done in triplicate on separate days.
TABLE 2.
Relative cytokine/chemokine levelsa
| Cytokine or chemokine | % Fluorescence intensity level (mean ± SD) of C. albicans grown asb:
|
|||||
|---|---|---|---|---|---|---|
| Biofilm
|
Planktonic cells
|
|||||
| Biofilm + PBMCs | PBMCs alone | P value | Planktonic + PBMCs | PBMCs alone | P value | |
| IL-1β | 112 ± 11.0 | 66 ± 1.0 | 0.021 | 38 ± 0.6 | 0.4 ± 0.4 | 0.001 |
| IL-6 | 39 ± 4.0 | 129 ± 3.0 | 0.002 | 62 ± 3.0 | 10 ± 1.4 | 0.001 |
| IL-8 | 131 ± 33.0 | 127 ± 25.0 | 0.756 | 117 ± 2.0 | 84 ± 1.7 | 0.003 |
| IL-10 | 6 ± 0.4 | 105 ± 10.0 | 0.003 | 0.4 ± 0.1 | 4 ± 0.3 | 0.004 |
| I-309 | 0 ± 0.0 | 32 ± 4.0 | 0.007 | 0.007 ± 0.01 | 0.5 ± 0.05 | 0.006 |
| MCP-1 | 10 ± 0.7 | 57 ± 2.0 | 0.000 | 4.3 ± 0.9 | 2 ± 0.8 | 0.010 |
| MCP-3 | 0.98 ± 0.9 | 0 ± 0.0 | 0.199 | 0 ± 0.0 | 3 ± 0.3 | 0.002 |
| MIP-1β | 8 ± 0.6 | 25 ± 4.0 | 0.014 | 14.2 ± 0.6 | 16 ± 3.0 | 0.293 |
| TNF-α | 0 ± 0.0 | 6 ± 0.8 | 0.005 | 1.4 ± 0.09 | 7 ± 1.0 | 0.008 |
| Eotaxin-2 | 0 ± 0.0 | 0.8 ± 0.7 | 0.168 | 0.1 ± 0.03 | 0.2 ± 0.1 | 0.007 |
Relative cytokine/chemokine levels were determined as the mean percentages of intensity ± standard deviations (SD) in supernatant obtained from PBMCs cultured in the presence or absence of C. albicans grown as biofilm or as planktonic cells. Relative intensity values were calculated as percentages with respect to internal positive controls (used as 100%) on each cytokine array membrane.
P values compared to PBMC alone for C. albicans cultured as biofilm or as planktonic cells.
Next, we compared the cytokine profile of biofilm-PBMC supernatant with that obtained from planktonic-PBMC supernatant. Our studies revealed that IL-1β, IL-10, and MCP-1 were significantly up-regulated in biofilm-PBMC supernatant compared to that in planktonic-PBMC supernatant (P = 0.0067, 0.0015, and 0.023, respectively) (Fig. 6). In contrast, IL-6 and MIP1β were down-regulated in supernatant obtained from PBMC-biofilm coculture compared to that obtained from planktonic-PBMC cocultures (P = 0.013 and 0.008, respectively). These studies demonstrated that biofilms induce PBMCs to secrete differential levels of cytokines, with IL-1β as the cytokine most highly overexpressed. However, the link between these differentially expressed cytokines and the biofilm-enhancing activity of the PBMC-biofilm culture supernatant is not known.
FIG. 6.
Percentage levels of cytokines from PBMCs grown in the presence of C. albicans biofilms or planktonic cultures. Cytokine spots were quantified using a densitometer (VersaDoc; Bio-Rad, CA), and relative values were calculated as percentages with respect to internal positive controls (used as 100%) from each preprinted cytokine array membrane. These analyses revealed that IL-1β, IL-10, and MCP-1 were significantly up-regulated in PBMCs incubated with Candida biofilms, compared to those incubated with planktonic Candida cells. In contrast, IL-6 and MIP-1β were down-regulated in PBMCs exposed to Candida biofilms, compared to those exposed to planktonic fungal cells. *, P value < 0.05. All experiments were done in triplicate on separate days.
DISCUSSION
In this study, we demonstrated that a coculture of C. albicans with PBMCs enhances the ability of this pathogen to form biofilms and that this activity is mediated by a soluble factor present in biofilm-PBMC coculture supernatant. Our observation that only supernatant obtained from biofilm-PBMC coculture enhanced biofilm formation, while supernatant obtained from LPS-activated PBMC did not exhibit this effect, clearly showed that this enhancing activity is biofilm specific. Dynamic interactions between PBMCs and fungal biofilm were also clearly evident from the real-time images obtained by time-lapse confocal microcopy.
An important finding in regard to the immunosuppressive effect of fungal biofilms was the lack of PBMC-mediated phagocytosis of fungal elements in biofilms. In contrast, and similar to findings from previous studies (28, 32, 44), planktonically grown Candida cells were actively phagocytosed by immune cells. The immunoprotective effect of fungal biofilms observed in our studies is similar to that reported previously for bacterial biofilms (23, 26, 45, 46). In this regard, Leid et al. (26) showed that the exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from gamma interferon (IFN-γ)-mediated macrophage killing. In another study, Walker et al. (46) reported that bacterial biofilm formation is increased by 2- to 3.5-fold in the presence of neutrophils. Vuong et al. (45) showed that polysaccharide intercellular adhesin protects the common skin commensal Staphylococcus epidermidis against phagocytosis and killing by human polymorphonuclear leukocytes and major antibacterial peptides of human skin (e.g., human beta-defensin 3, LL-37, and dermcidin). In an earlier study, Jesaitis et al. (23) also showed that host defense mechanisms are compromised in the presence of P. aeruginosa biofilms. These investigators showed that neutrophils exposed to P. aeruginosa biofilms become phagocytically engorged, partially degranulated, immobilized, and rounded and also cause an increase in oxygen consumption of the system. As a result of these changes, the host defense becomes compromised as biofilm bacteria are able to ward off the effects of the immobilized neutrophils. To demonstrate that the PBMCs used in our assays that were prestained with Mitotracker dye did not exhibit any loss of functionality, we examined unstained PBMCs and demonstrated that (i) these unstained cells also resulted in biofilm enhancement, as shown by XTT assay and SEM analyses, and that (ii) prestained PBMCs were able to phagocytose planktonically grown C. albicans cells.
In contrast to these studies, others have shown that immune cells can also produce components that inhibit biofilm formation. One example of such an inhibitory molecule is lactoferrin, a common secretory component of human neutrophils, which has been shown to inhibit P. aeruginosa biofilm production (37). The role of lactoferrin in microbial biofilms has been suggested to be modulated by scavenging and protease- or oxygen radical-mediated degradation by P. aeruginosa and neutrophils (1, 2, 49).
An important characteristic of biofilms formed in the presence of immune cells was the asymmetric localization of these cells within the biofilm. We found that inside a biofilm, monocytes were localized mostly to the basal layers and were unable to escape the biofilm milieu. Heterogeneous bilayer architecture (basal yeast cells and top hyphal elements) is characteristic of fungal biofilms formed on catheter surfaces (7), and this architecture was retained even in the presence of monocytes. Whether this bilayer architecture of the biofilm dictates and influences the distribution of PBMCs within fungal biofilms is unknown.
Planktonically grown Candida cells can also induce time-dependent differential expression of specific cytokines/chemokines from human monocytes (24). Our studies showed that the supernatant from a biofilm-PBMC coculture is responsible for biofilm enhancement and that this supernatant contains increased levels of the proinflammatory cytokine IL-1β but decreased levels of IL-6, IL-10, MCP-1, I-309, TNF-∝, and the chemokine MIP1β. The down-regulated cytokines included both proinflammatory (e.g., TNF-α) as well as anti-inflammatory (e.g., IL-6 and IL-10) cytokines, suggesting that C. albicans biofilms and PBMCs undergo multiple interactions mediated by different cytokines. Differential production of cytokines by leukocytes in the presence of bacterial biofilms was also reported by Leid et al. (25), who demonstrated that under conditions mimicking physiological shear, leukocytes can attach, penetrate, and produce cytokines in response to mature Staphylococcus aureus biofilm. van der Graaf et al. (42) showed that Candida blastoconidia stimulated large amounts of IFN-γ production by human PBMCs or murine splenic lymphocytes, while Candida hyphae did not exhibit such stimulation. This response is similar to our findings, in which up-regulated hypha formation associated with mature biofilm formation did not result in any change in IFN-γ level. Similar results were also reported for interactions between dendritic cells and C. albicans yeast and hyphae (13) and the role of C. albicans in the differentiation of monocytes to dendritic cells (41).
As our results demonstrate, the cytokine profile of PBMCs following coculture with planktonic versus biofilm C. albicans was vastly different. Cytokines produced by PBMCs cocultured with planktonic C. albicans showed that IL-1β, IL-10, and MCP-1 levels were significantly down-regulated compared to those produced by biofilm-PBMC coculture. Our results suggest that PBMCs elicit a strong inflammatory response when exposed to biofilm. However, concurrently, the induction of anti-inflammatory cytokines (e.g., IL-10), potentially via autoregulatory pathways, may contribute to the modulation of the inflammatory response. It is possible that biofilm formation may be a defense mechanism by which C. albicans protects itself against human immune cells such as monocytes.
In conclusion, this is the first report that has investigated the interactions between C. albicans biofilm and host immune cells. We showed that live PBMCs enhance the biofilm-forming ability of C. albicans and that this enhancing activity is dependent on proliferating PBMCs and is mediated by a soluble factor(s) present in the biofilm-PBMC coculture supernatant. Characterization of the soluble factor and testing its activity in vivo may lead to the identification of biomolecules regulating interactions of C. albicans biofilms with host immune cells and may help develop novel strategies to manage and treat biofilm-related infections.
Supplementary Material
Acknowledgments
We thank Wendy Goodman for technical support.
This work was supported by funds from the NIH (R01 AI035097-10) and by a Bristol-Myers Squibb Freedom to Discover award to M.A.G. and an American Heart Association (Scientist Development Grant 0335313N) award to P.K.M. Assistance for the Confocal Scanning Laser Microscopy Core Facility (NCI grant P30CA43703-12) at Case Western Reserve University is gratefully acknowledged.
Editor: A. Casadevall
Footnotes
Published ahead of print on 5 March 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Britigan, B. E., and B. L. Edeker. 1991. Pseudomonas and neutrophil products modify transferrin and lactoferrin to create conditions that favor hydroxyl radical formation. J. Clin. Investig. 88:1092-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britigan, B. E., J. S. Serody, M. B. Hayek, L. M. Charniga, and M. S. Cohen. 1991. Uptake of lactoferrin by mononuclear phagocytes inhibits their ability to form hydroxyl radical and protects them from membrane autoperoxidation. J. Immunol. 147:4271-4277. [PubMed] [Google Scholar]
- 3.Bromelow, K. V., W. Hirst, R. L. Mendes, A. R. Winkley, I. E. Smith, M. E. O'Brien, and B. E. Souberbielle. 2001. Whole blood assay for assessment of the mixed lymphocyte reaction. J. Immunol. Methods 247:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Byrne, A., and D. J. Reen. 2002. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 168:1968-1977. [DOI] [PubMed] [Google Scholar]
- 5.Carrow, E. W., and J. E. Domer. 1985. Immunoregulation in experimental murine candidiasis: specific suppression induced by Candida albicans cell wall glycoprotein. Infect. Immun. 49:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall, A., and L. A. Pirofski. 2003. Microbial virulence results from the interaction between host and microorganism. Trends Microbiol. 11:157-158. [DOI] [PubMed] [Google Scholar]
- 7.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 9.Chinen, T., M. H. Qureshi, Y. Koguchi, and K. Kawakami. 1999. Candida albicans suppresses nitric oxide (NO) production by interferon-gamma (IFN-γ) and lipopolysaccharide (LPS)-stimulated murine peritoneal macrophages. Clin. Exper. Immunol. 115:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cormack, B. P., G. Bertram, M. Egerton, N. A. Gow, S. Falkow, and A. J. Brown. 1997. Yeast-enhanced green fluorescent protein (yEGFP) a reporter of gene expression in Candida albicans. Microbiology 143:303-311. [DOI] [PubMed] [Google Scholar]
- 11.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 12.Dongari-Bagtzoglou, A., and P. L. Fidel, Jr. 2005. The host cytokine responses and protective immunity in oropharyngeal Candidiasis. J. Dent. Res. 84:966-977. [DOI] [PubMed] [Google Scholar]
- 13.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falagas, M. E., K. E. Apostolou, and V. D. Pappas. 2006. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur. J. Clin. Microbiol. Infect. Dis. 25:419-425. [DOI] [PubMed] [Google Scholar]
- 15.Gudlaugsson, O., S. Gillespie, K. Lee, B. J. Vande, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 16.Hallan, E., H. K. Blomhoff, E. B. Smeland, and J. Lomo. 1997. Involvement of ICE (caspase) family in gamma-radiation-induced apoptosis of normal B lymphocytes. Scand. J. Immunol. 46:601-608. [DOI] [PubMed] [Google Scholar]
- 17.Hodge, S., G. Hodge, R. Scicchitano, P. N. Reynolds, and M. Holmes. 2003. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 81:289-296. [DOI] [PubMed] [Google Scholar]
- 18.Huang, R. P. 2004. Cytokine protein arrays. Methods Mol. Biol. 264:215-231. [DOI] [PubMed] [Google Scholar]
- 19.Huang, R. P. 2001. Detection of multiple proteins in an antibody-based protein microarray system. J. Immunol. Methods 255:1-13. [DOI] [PubMed] [Google Scholar]
- 20.Huang, R. P. 2001. Simultaneous detection of multiple proteins with an array-based enzyme-linked immunosorbent assay (ELISA) and enhanced chemiluminescence (ECL). Clin. Chem. Lab. Med. 39:209-214. [DOI] [PubMed] [Google Scholar]
- 21.Huang, R. P., R. Huang, Y. Fan, and Y. Lin. 2001. Simultaneous detection of multiple cytokines from conditioned media and patient's sera by an antibody-based protein array system. Anal. Biochem. 294:55-62. [DOI] [PubMed] [Google Scholar]
- 22.Huffnagle, G. B., and G. S. Deepe. 2003. Innate and adaptive determinants of host susceptibility to medically important fungi. Curr. Opin. Microbiol. 6:344-350. [DOI] [PubMed] [Google Scholar]
- 23.Jesaitis, A. J., M. J. Franklin, D. Berglund, M. Sasaki, C. I. Lord, J. B. Bleazard, J. E. Duffy, H. Beyenal, and Z. Lewandowski. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171:4329-4339. [DOI] [PubMed] [Google Scholar]
- 24.Kim, H. S., E. H. Choi, J. Khan, E. Roilides, A. Francesconi, M. Kasai, T. Sein, R. L. Schaufele, K. Sakurai, C. G. Son, B. T. Greer, S. Chanock, C. A. Lyman, and T. J. Walsh. 2005. Expression of genes encoding innate host defense molecules in normal human monocytes in response to Candida albicans. Infect. Immun. 73:3714-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leid, J. G., M. E. Shirtliff, J. W. Costerton, and P. Stoodley. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70:6339-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leid, J. G., C. J. Willson, M. E. Shirtliff, D. J. Hassett, M. R. Parsek, and A. K. Jeffers. 2005. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-γ-mediated macrophage killing. J. Immunol. 175:7512-7518. [DOI] [PubMed] [Google Scholar]
- 27.Liegler, T. J., W. Hyun, T. S. Yen, and D. P. Stites. 1995. Detection and quantification of live, apoptotic, and necrotic human peripheral lymphocytes by single-laser flow cytometry. Clin. Diag. Lab. Immunol. 2:369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Netea, M. G., J. W. Van der Meer, and B. J. Kullberg. 2006. Role of the dual interaction of fungal pathogens with pattern recognition receptors in the activation and modulation of host defense. Clin. Microbiol. Infect. 12:404-409. [DOI] [PubMed] [Google Scholar]
- 29.Pirofski, L. A., and A. Casadevall. 2002. The meaning of microbial exposure, infection, colonisation, and disease in clinical practice. Lancet Infect. Dis. 2:628-635. [DOI] [PubMed] [Google Scholar]
- 30.Poulain, D., and T. Jouault. 2004. Candida albicans cell wall glycans, host receptors and responses: elements for a decisive crosstalk. Curr. Opin. Microbiol. 7:342-349. [DOI] [PubMed] [Google Scholar]
- 31.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 32.Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4:1-13. [DOI] [PubMed] [Google Scholar]
- 33.Romani, L. 2000. Innate and adaptive immunity in Candida albicans infections and saprophytism. J. Leukoc. Biol. 68:175-179. [PubMed] [Google Scholar]
- 34.Romani, L., F. Bistoni, and P. Puccetti. 2003. Adaptation of Candida albicans to the host environment: the role of morphogenesis in virulence and survival in mammalian hosts. Curr. Opin. Microbiol. 6:338-343. [DOI] [PubMed] [Google Scholar]
- 35.Romani, L., P. Puccetti, and F. Bistoni. 1997. Interleukin-12 in infectious diseases. Clin. Microbiol. Rev. 10:611-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy, A., E. Krzykwa, R. Lemieux, and S. Neron. 2001. Increased efficiency of gamma-irradiated versus mitomycin C-treated feeder cells for the expansion of normal human cells in long-term cultures. J. Hematother. Stem Cell Res. 10:873-880. [DOI] [PubMed] [Google Scholar]
- 37.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Welsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552-555. [DOI] [PubMed] [Google Scholar]
- 38.Stevens, D. A., T. J. Walsh, F. Bistoni, E. Cenci, K. V. Clemons, G. Del Sero, C. Fe d'Ostiani, B. J. Kullberg, A. Mencacci, E. Roilides, and L. Romani. 1998. Cytokines and mycoses. Med. Mycol. 36:174-182. [PubMed] [Google Scholar]
- 39.Takahara, M., K. Kang, L. Liu, Y. Yoshida, T. S. McCormick, and K. D. Cooper. 2003. iC3b arrests monocytic cell differentiation into CD1c-expressing dendritic cell precursors: a mechanism for transiently decreased dendritic cells in vivo after human skin injury by ultraviolet B. J. Investig. Dermatol. 120:802-809. [DOI] [PubMed] [Google Scholar]
- 40.Tang, N., L. Liu, K. Kang, P. K. Mukherjee, M. Takahara, G. Chen, T. S. McCormick, K. D. Cooper, and M. Ghannoum. 2004. Inhibition of monocytic interleukin-12 production by Candida albicans via selective activation of ERK mitogen-activated protein kinase. Infect. Immun. 72:2513-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torosantucci, A., G. Romagnoli, P. Chiani, A. Stringaro, P. Crateri, S. Mariotti, R. Teloni, G. Arancia, A. Cassone, and R. Nisini. 2004. Candida albicans yeast and germ tube forms interfere differently with human monocyte differentiation into dendritic cells: a novel dimorphism-dependent mechanism to escape the host's immune response. Infect. Immun. 72:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Graaf, C. A., M. G. Netea, I. Verschueren, J. W. van der Meer, and B. J. Kullberg. 2005. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 73:7458-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Torres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vonk, A. G., C. W. Wieland, M. Versteegen, I. C. Verschueren, M. G. Netea, L. A. Joostent, P. E. Verweij, and B. J. Kullberg. 2005. Influence of endogenous pro-inflammatory cytokines on neutrophil-mediated damage of Candida albicans pseudohyphae, quantified in a modified tetrazolium dye assay. Med. Mycol. 43:551-557. [DOI] [PubMed] [Google Scholar]
- 45.Vuong, C., J. M. Voyich, E. R. Fischer, K. R. Braughton, A. R. Whitney, F. R. Deleo, and M. Otto. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 6:269-275. [DOI] [PubMed] [Google Scholar]
- 46.Walker, T. S., K. L. Tomlin, G. S. Worthen, K. R. Poch, J. G. Lieber, M. T. Saavedra, M. B. Fessler, K. C. Malcolm, M. L. Vasil, and J. A. Nick. 2005. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect. Immun. 73:3693-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, Y., S. P. Li, S. A. Moser, K. L. Bost, and J. E. Domer. 1998. Cytokine involvement in immunomodulatory activity affected by Candida albicans mannan. Infect. Immun. 66:1384-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wenzel, R. P., and M. B. Edmond. 1999. The evolving technology of venous access. N. Engl. J. Med. 340:48-50. [DOI] [PubMed] [Google Scholar]
- 49.Wilderman, P. J., A. I. Vasil, Z. Johnson, M. J. Wilson, H. E. Cunliffe, I. L. Lamont, and M. L. Vasil. 2001. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun. 69:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in U.S. hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.