Abstract
Francisella tularensis is a gram-negative intracellular bacterium that is considered to be a potential category A biological weapon due to its extreme virulence. Although vaccination with the attenuated live vaccine strain (LVS) of F. tularensis can protect against lethal challenge, use of inactivated or subunit forms as vaccine candidates for induction of protective antibody responses has not been fully evaluated. In the present study, we examined whether immune protection in the lung could be stimulated by intranasal administration of inactivated LVS together with interleukin-12 (IL-12) as an adjuvant. LVS was inactivated by heat, paraformaldehyde treatment, or exposure to UV, and inactivation of the preparations was confirmed by assessing bacterial growth and the survival of mice after direct inoculation. We found that mucosal vaccination with inactivated LVS provided 90 to 100% protection in mice after lethal intranasal challenge with 104 CFU of LVS, and this protection was dependent on inclusion of exogenous IL-12 during vaccine administration. Survival of vaccinated mice after live bacterial challenge was correlated with reduced bacterial burden, decreased pulmonary inflammation, increased serum antibody titers, and lower levels of gamma interferon (IFN-γ), tumor necrosis factor alpha, and IL-6 in the lungs, livers, and spleens. Whereas NK cells were primarily responsible for the production of IFN-γ in unvaccinated, challenged animals, vaccinated mice had increased levels of lung IFN-γ+ CD4+ T cells after challenge. Significantly, mice genetically deficient in immunoglobulin A (IgA) expression were unable to survive lethal challenge after vaccination. These results are the first results to demonstrate that IgA-mediated protection against lethal respiratory tularemia occurs after mucosal vaccination with inactivated F. tularensis LVS.
Francisella tularensis is a gram-negative bacterium that replicates within macrophages in mammalian hosts, and F. tularensis subspecies tularensis, also known as type A, is the most virulent F. tularensis for humans, having a lethal dose by the inhalation route of less than 10 CFU. Although the number of reported cases of naturally occurring tularemia is relatively low in the United States, the threat of use of this organism as a biological weapon has stimulated the search for safe and efficacious vaccines. The live vaccine strain (LVS), a type B biovar of F. tularensis, is attenuated in humans and has been used for vaccination, but it is no longer approved for use due to its reactogenicity and due to the lack of information regarding the genetic basis for its attenuation (24).
The use of inactivated or subunit forms of F. tularensis would be a safe alternative for vaccination, but the potential effectiveness of this approach has not been fully explored. In one early study, mice were immunized intraperitoneally (i.p.) with various doses of irradiated LVS or F. tularensis type A strain Schu S4 and then challenged i.p. 2 weeks later with virulent organisms (15). However, this immunization scheme provided only limited protection, and a maximum of 20% of the vaccinated animals survived challenge. Purified F. tularensis LVS lipopolysaccharide injected intradermally has been shown to protect against subsequent intradermal LVS challenge, but it was able to extend the mean time to death by only approximately 2 days after respiratory challenge (6). One potential reason for the lack of efficacy observed after vaccination with inactivated bacteria or bacterial subunits is the fact that F. tularensis is an intracellular pathogen. Although antibodies are known to protect against intradermal forms of tularemia (8, 27), their potential role in pulmonary infection has not been determined. It has been shown in other respiratory infection models that immunoglobulin A (IgA) is necessary to inhibit bacterial colonization (25). One goal of the current study was to determine the potential role of IgA in an intranasal (i.n.) vaccination strategy for use against F. tularensis infection.
We have previously shown that i.n. inoculation of interleukin-12 (IL-12) alone can induce protective innate immunity in the lungs against acute respiratory tularemia (9). In other experimental infection models, we have also employed i.n. administration of exogenous IL-12 in the presence of vaccine candidates to enhance long-term adaptive immunity in the respiratory tract, and the protection obtained was found to be dependent upon IgA antibody (2, 22, 31). Other workers (1, 19) have similarly reported the efficacy of IL-12 as an mucosal adjuvant for inducing protective adaptive immune responses against pulmonary bacterial pathogens. Here we investigated the ability of inactivated LVS given i.n. as a vaccine preparation in combination with IL-12 to stimulate adaptive immune protection against pulmonary tularemia. Our results show that inactivated F. tularensis LVS can indeed be used for effective i.n. vaccination and that the vaccine-mediated protection is dependent on IgA antibody. Our findings suggest that ultimately it should be possible to identify subunit vaccines for use against respiratory tularemia that induce protective mucosal antibody expression.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice that were 5 to 8 weeks old were obtained from Taconic (Germantown, NY). IgA−/− mice with a C57BL/6 × 129 background (17) were bred at Albany Medical College. Wild-type C57BL/6 × 129 F1 mice used as controls were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were maintained at Albany Medical College, and all experimental procedures were approved by the institutional animal care and use committee.
F. tularensis LVS inactivation.
Frozen stocks of F. tularensis LVS (kindly provided by Karen Elkins, U.S. Food and Drug Administration, Bethesda, MD) were inoculated at a concentration of 107 CFU/ml into Mueller-Hinton broth and incubated under stationary conditions for 2 h at 37°C with 5% CO2 and then with shaking for 13 h at room temperature. Bacterial CFU were enumerated by serial dilution on chocolate agar plates. Inactivation was performed by fixation with 2% paraformaldehyde for 1 h at room temperature, heating for 16 h at 60°C, or UV irradiation with 7,500 μJ/cm3 at room temperature. After inactivation, the bacteria were washed twice with sterile phosphate-buffered saline (PBS) and resuspended to the original volume. The sterility of all inactivated bacterial preparations was verified by plating on chocolate agar plates and by performing in vivo bacterial burden and survival studies with mice after i.n. or i.p. challenge (see Results). Inactivated bacteria corresponding to 5 × 109 CFU/ml were frozen in 1-ml aliquots at −80°C.
F. tularensis LVS immunization and lung infection.
Mice anesthetized with ketamine HCl (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (Phoenix Scientific, St. Joseph, MO) were vaccinated i.n. with 40 μl of PBS containing inactivated F. tularensis LVS that corresponded to 108 CFU. Mice were also inoculated i.n. at the time of vaccination with 1 μg of murine recombinant IL-12 (kindly provided by Wyeth Vaccines, Pearl River, NY) in 25 μl of PBS containing 1% normal mouse serum as a vehicle. The same amount of IL-12 was given i.n. for an additional three consecutive days following the primary immunization. Control animals received PBS-1% normal mouse serum alone. The results of preliminary experiments indicated that 1 μg of IL-12 given i.n. was optimal for vaccination efficacy, and no toxicity was observed. Mice received booster i.n. inoculations of inactivated F. tularensis LVS corresponding to 108 CFU plus 1 μg IL-12 on days 14 and 28 after the primary immunization. The mice were then infected i.n. at different times (2 weeks or 2 months after the final boost) with 40 μl of Ringer's solution containing 104 CFU of F. tularensis LVS; sham-infected mice received Ringer's solution alone. The numbers of CFU inoculated were confirmed by plating aliquots on horse blood-cysteine agar plates at the time of infection.
Bacterial burden and cytokine measurement.
The numbers of bacteria in the lungs, spleens, and livers of mice were determined on days 1, 3, 5, 7, 9, and 30 after i.n. infection. The organs were removed aseptically and mechanically homogenized with a Mini-BeadBeater-8 (BioSpec Products Inc., Bartlesville, OK) in 500 μl of PBS-protease inhibitor cocktail. Fifty microliters of each homogenate was plated on chocolate agar, and this was followed by enumeration 3 days later. Bacterial loads were expressed as log10 means ± standard errors of the means for three to six mice per group. To measure expression of gamma interferon (IFN-γ), tumor necrosis factor alpha, and IL-6, homogenates were centrifuged at 10,000 rpm for 20 min, and the supernatants were analyzed by the cytometric bead array method using a FACSArray (BD Biosciences, San Jose, CA). Cytokine production was expressed in pg/ml (mean ± standard errors of the means for three to six mice per group).
Histology.
Lungs, spleens, livers, and kidneys from mice sacrificed at different times (three mice per group) were collected in 10% buffered formalin. The tissues were prepared by preparing 5-μm paraffin-embedded sections by standard methods. The sections were stained with hematoxylin and eosin and were analyzed in a blinded fashion. The lungs were assessed at different times to determine the extent of peribronchovascular inflammation, the amount of bronchial luminal exudate, the percentage of bronchi and blood vessels affected, and parenchymal pneumonia. Liver sections were evaluated to determine the degenerative or necrotic changes in hepatic lobules, the infiltration in sinusoids and portal triads, and the presence, distribution, and cellular composition of granulomatous lesions. Spleen sections were evaluated to determine the activation and infiltration of follicles, lymphoproliferation, marginal zone thickening, red pulp infiltration, and granuloma formation. Kidneys were assessed to determine the presence of inflammatory infiltrates and tubule integrity.
Lung lymphocyte isolation and staining.
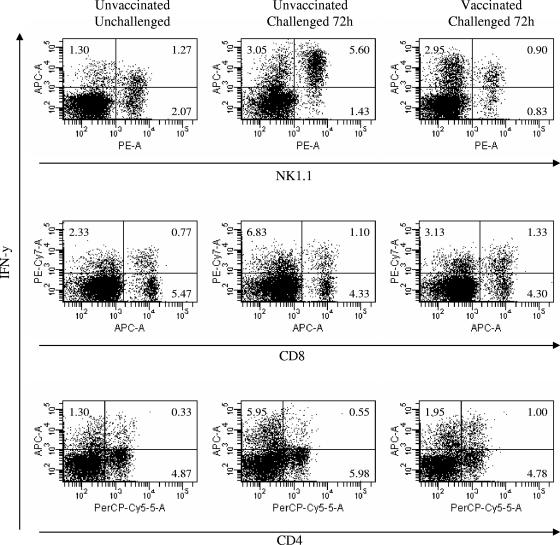
Mice used for ex vivo studies were sacrificed 2 weeks after the final vaccination and 72 h after challenge with 104 CFU of LVS. The lungs were excised after perfusion with sterile PBS, cut into small pieces, and digested for 1 h at 37°C using 2 mg/ml collagenase D, 0.25 mg/ml DNase I, and 10 mM MgCl2. The digests were pressed through a 40-μm cell strainer (BD Falcon, Bedford, MA), and the resulting cell suspensions were filtered through a nylon-cotton wool column. The cell suspensions were centrifuged at 500 × g for 10 min at 4°C, the cell pellets were resuspended in RPMI 1640 containing 10% fetal calf serum, 300 mg/liter glutamine, 25 mM HEPES buffer, 100 IU/liter penicillin, and 100 μg/liter streptomycin, and the lymphocytes were isolated by centrifugation on Lympholyte M (Cedarlane, Hornby, Ontario, Canada) at 600 × g for 20 min at room temperature. The cells were washed and stimulated in 24-well plates in the presence of phorbol myristate acetate (50 ng/ml), ionomycin (500 ng/ml), and 10 μg/ml Brefeldin A (Epicenter Technology, Madison, WI) for 5 h at 37°C. Intracellular IFN-γ expression and cell surface markers were analyzed as described previously (21) using the following monoclonal antibodies: phycoerythrin-conjugated anti-mouse NK1.1, Alexa Fluor 647-conjugated anti-mouse CD8α and anti-mouse IFN-γ, and phycoerythrin-Cy7-conjugated anti-mouse IFN-γ, all obtained from BD Pharmingen (San Diego, CA). We also used tricolor-conjugated anti-mouse CD4 (Caltag, Burlingame, CA) and fluorescein isothiocyanate-conjugated anti-mouse B220 (Southern Biotech, Birmingham, AL). The stained cells were stored in the dark at 4°C and analyzed within 24 h with a FACSCanto flow cytometer (BD Biosciences). The data were evaluated using the FacsDiva software.
Antibody measurement.
Anti-LVS antibody expression in mouse serum was quantified by an enzyme-linked immunosorbent assay. To do this, microtiter plates were coated with 100 μl of F. tularensis LVS (7 × 107 CFU/ml) in carbonate buffer for 2 h at 37°C and then used immediately or incubated overnight at 4°C. The plates were washed with PBS containing 0.05% Tween 20 and blocked for 2 h with 200 μl of PBS containing 10% bovine serum albumin. Serial dilutions of sera were added to the plates (100 μl/well) and incubated for 90 min at 37°C. After washing, biotin-conjugated goat anti-mouse antibodies specific for IgM, IgG1, IgG2a, or IgA (Caltag, Burlingame, CA) were added and incubated for 1 h at 37°C. The plates were washed, and then 100 μl/well of streptavidin conjugated to horseradish peroxidase (Biosource, Camarillo, CA) was added. The plates were incubated for 20 min, which was followed by addition of 100 μl of TMB peroxidase substrate (KPL, Gaithersburg, MD). After 20 min, the reaction was stopped, and the plates were read at 450 nm using a PowerWave HT microplate reader (BioTek Instruments, Winooski, VT). The results were expressed as endpoint dilution titers (means ± standard errors of the means) for three mice per group.
Statistics.
Kaplan-Meier log rank analyses of survival were performed with the GraphPad Prism 4 software (San Diego, CA). Differences were considered significant if the P values were <0.05. Data for bacterial burdens, cytokine levels, flow cytometric staining, and antibody levels were expressed as means ± standard errors of the means, and groups were compared using the Student t test. A P value of <0.05 was considered significant.
RESULTS
Efficacy of F. tularensis inactivation.
Since the purpose of the study was to assess our ability to use inactivated bacteria rather than live bacteria for i.n. vaccination, it was important to determine whether any viable bacteria remained in the inactivated inoculum. Indeed, after in vivo challenge, we found that while all mice succumbed to 25 CFU of live LVS injected i.p., all mice survived i.p. injection of 108 CFU equivalents of UV-inactivated LVS (UV-LVS). Similarly, i.n. challenge of mice with 103 CFU of LVS resulted in the presence of more than 107 bacteria in the lungs 3 days later, whereas no live bacteria were detected in mice that received 108 CFU equivalents of UV-LVS. Therefore, these two experiments demonstrated that no live bacteria were present in the inactivated LVS preparation.
Inactivated LVS given i.n. together with IL-12 induces protection against respiratory tularemia.
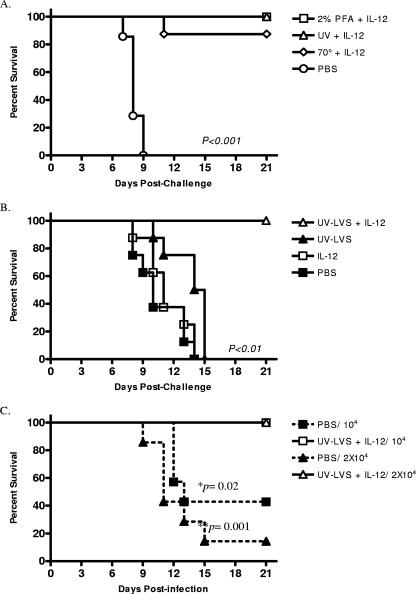
Gordon et al. (15) previously reported that i.p. vaccination with irradiated LVS provided little protection against subsequent F. tularensis challenge. Since this could have been related to a lack of an appropriate Th1-like response, it was of interest to determine if addition of IL-12 as an adjuvant during vaccination with inactivated LVS provided better protection. Additionally, we chose to vaccinate mice i.n. since this is the route that confers optimal protection against respiratory tularemia (7, 13). C57BL/6 mice were inoculated i.n. with amounts of inactivated LVS preparations corresponding to 108 CFU of LVS together with 1 μg of IL-12. The animals were boosted i.n. with the same doses of inactivated LVS plus IL-12 on days 14 and 28. Two weeks after the final booster, the mice were challenged i.n. with a lethal dose of LVS (104 CFU), and survival was monitored for 21 days postchallenge. We found that all unvaccinated mice succumbed to the infection, and the median survival time was 8 days (Fig. 1A). However, every animal vaccinated with UV-LVS or paraformaldehyde-fixed LVS plus IL-12 survived the bacterial challenge (P < 0.001); seven of eight mice vaccinated with heat-treated LVS plus IL-12 also survived the challenge (P < 0.001).
FIG. 1.
Intranasal vaccination with inactivated F. tularensis LVS plus IL-12 protects against respiratory tularemia. (A) C57BL/6 mice were vaccinated i.n. with inactivated LVS and IL-12 and then challenged i.n. with 104 CFU of F. tularensis LVS 2 weeks after the final boost. Survival was monitored daily beginning on day 1 postchallenge. (B) Mice were immunized in the presence or absence of IL-12 adjuvant and challenged i.n. with 104 CFU of F. tularensis LVS 2 weeks after the final boost. Survival was monitored daily beginning on day 1 postchallenge. (C) Mice were immunized with F. tularensis LVS plus IL-12 and challenged i.n. with 104 or 2 × 104 CFU of F. tularensis LVS 2 months after the final boost. Survival was monitored for 21 days beginning on day 1 postchallenge, and log rank tests were used to assess statistical significance. In all cases, animals vaccinated with UV-LVS plus IL-12 showed better survival after challenge than unvaccinated mice or mice inoculated with UV-LVS alone or IL-12 alone (P < 0.01). There were eight mice per group for each experiment. PFA, paraformaldehyde.
To determine whether inclusion of IL-12 was necessary for the vaccine-mediated protection observed, groups of mice were inoculated with UV-LVS alone, IL-12 alone, UV-LVS plus IL-12, or the vehicle and then challenged with lethal doses of LVS as described above. Administration of UV-LVS alone or IL-12 alone did not significantly improve survival (the median survival time was 12 days, compared to 11 days for unvaccinated mice) (Fig. 1B). Thus, the protective innate immune response that can be induced by IL-12 (9) was not effective at later times after bacterial challenge, such as the times used in the current study. However, 100% of the mice vaccinated with inactivated LVS plus IL-12 survived the LVS challenge (P < 0.01). Together, these results show that i.n. vaccination with inactivated LVS using IL-12 as a mucosal adjuvant can protect mice against respiratory tularemia. Since the various inactivated bacterial preparations provided similar levels of protection, UV-LVS was used for all subsequent studies.
To assess the generation of protective memory after vaccination, C57BL/6 mice were vaccinated as described above and then 2 months after the final boost were challenged with either 104 or 2.0 × 104 CFU of F. tularensis LVS. The survival of the mice was monitored for 21 days. We found that all vaccinated mice that were challenged with either dose of LVS survived the infection, whereas unvaccinated mice remained highly susceptible (Fig. 1C) Therefore, i.n. vaccination with UV-LVS plus IL-12 induces long-term memory and protection from lethal respiratory doses of F. tularensis LVS.
Vaccination with inactivated LVS augments bacterial clearance.
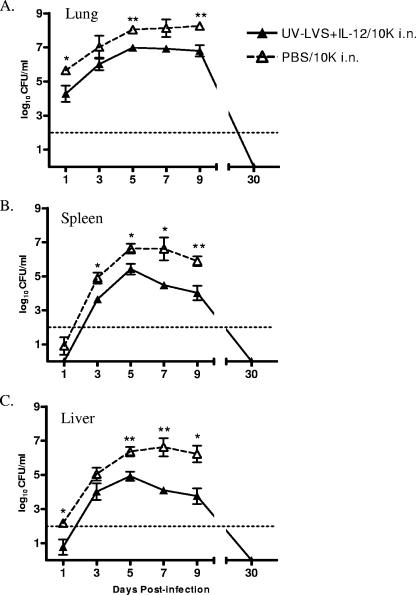
Since UV-LVS inoculated i.n. protected mice against death from respiratory tularemia, the efficacy of this vaccination regimen for actual clearance of bacteria from the target organs was determined. Groups of mice were vaccinated i.n., challenged i.n. 2 weeks after the last booster, and then sacrificed at various times. The bacterial CFU were enumerated in the lungs, livers, and spleens. One day after challenge with 104 LVS, the lungs of vaccinated mice harbored essentially the amount that was originally inoculated (∼104 CFU), while the lungs of naïve mice contained approximately 50- to 100-fold-higher numbers of bacteria (P < 0.05) (Fig. 2A). After this the numbers of bacteria in the lungs of naïve mice increased exponentially, reaching approximately 108 CFU by day 9, when the animals began to succumb to infection. The numbers of bacteria in the lungs of vaccinated mice increased to a maximum of approximately 106 CFU on day 5, plateaued, and then began to decrease on day 9. In general, 50- to 100-fold reductions in the numbers of bacteria were observed in the lungs of vaccinated mice compared to the numbers of bacteria in the lungs of naïve mice during the first 9 days following infection. No bacteria were detected in the lungs of vaccinated mice at day 30 postchallenge. Similar trends were observed for the spleens and livers of infected mice. The numbers of bacteria in the spleens of naïve mice increased exponentially after challenge and reached approximately 107 CFU by day 5 (Fig. 2B). In contrast, the spleens of vaccinated mice contained significantly fewer bacteria on days 3 and 5 postchallenge. The numbers of bacteria began to decrease by day 7, and bacteria were not detectable on day 30 postchallenge. In the liver, bacteria were detected as early as day 1 postchallenge in naïve mice but not in vaccinated mice. The numbers of bacteria increased exponentially in naïve mice and reached 107 CFU before the animals succumbed to infection. However, the numbers of bacteria in the livers of vaccinated mice were 50- to 100-fold less than the numbers of bacteria in the livers of naïve mice on days 3 through 9, and bacteria were not detectable by day 30 postchallenge (Fig. 2C). These data indicate that i.n. vaccination with UV-LVS plus IL-12 aids in protection against a lethal challenge by limiting colonization and enhancing clearance of bacteria from the lungs, spleen, and liver.
FIG. 2.
Intranasal vaccination with UV-LVS combined with IL-12 enhances bacterial clearance from the lungs, spleens, and livers of infected mice. C57BL/6 mice were vaccinated i.n. with UV-LVS plus IL-12 or with PBS and then challenged i.n. with 104 CFU of F. tularensis LVS (10K) 2 weeks after the final boost. The numbers of CFU (means ± standard errors of the means) in the lungs (A), spleens (B), and livers (C) were determined on days 1, 3, 5, 7, 9, and 30 after infection. There were three to six mice per group. The asterisks indicate P values (one asterisk, P < 0.05; two asterisks, P < 0.01).
Vaccinated mice exhibit less severe pathology in response to LVS infection.
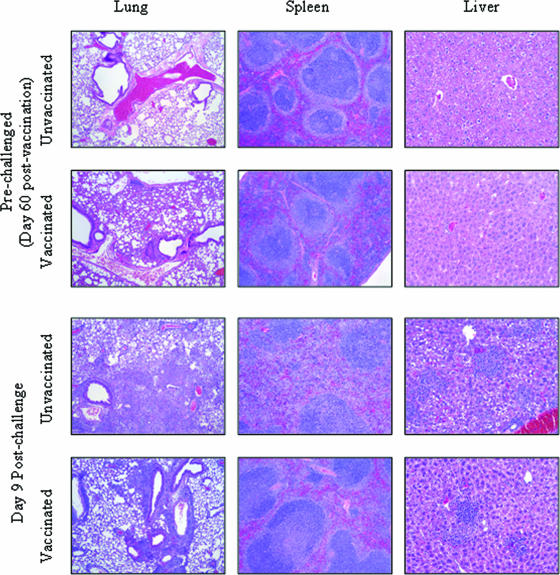
Histological analyses were performed with vaccinated and unvaccinated mice both before and after challenge (Fig. 3), and we focused on day 9 in particular, when unvaccinated mice begin to succumb to infection. Unvaccinated mice exhibited no obvious inflammation in any organ prior to challenge; however, 5 days after challenge the lungs showed moderate peribronchovascular infiltration of mononuclear cells (activated macrophages and a few lymphocytes). In addition, the lung parenchyma showed severe consolidation with necrotizing, diffuse to multifocal, neutrophilic to pyogranulomatous bronchopneumonic lesions with occasional clumps of bacteria and many apoptotic cells. The septal cells of the alveoli were hypertrophied, and the lumens of bronchioles and alveoli were filled with neutrophils in protein-rich exudates. The changes described above became more pneumonic and less necrotizing on days 7 and 9, and the infiltrates contained activated macrophages, lymphocytes, and fibroblasts, along with apoptotic cells. The spleens on day 5 had multifocal discrete neutrophilic to pyogranulomatous lesions involving the white pulp, marginal zones, and red pulp, along with many apoptotic cells. By day 7, the changes described above had become more diffuse and less necrotizing, causing disruption of the splenic architecture. By day 9, the spleens contained increased numbers of lymphoblasts. The livers on day 5 after challenge had discrete multifocal pyogranulomas (>10 to 20 pyogranulomas per ×10 field) containing 10 to >100 cells. In addition, there were two to four apoptotic cells per ×40 field that were adjacent to portal triads and the central vein and in the parenchyma. Few hepatic cells showed necrosis. On day 7 after challenge, hepatitis was severe, and the livers showed increased sinusoidal infiltration with activated macrophages and lymphocytes. The number of granulomas increased to about 50 per ×10 field, and the granulomas consisted of macrophages, lymphocytes, and many apoptotic cells. On day 9 after challenge, the changes became more acute and involved many lymphocytes and apoptotic cells.
FIG. 3.
Histological analysis of mice after challenge. C57BL/6 mice were vaccinated as described in the text and then challenged i.n. with 104 CFU of F. tularensis LVS 2 months after the final boost. Mice were sacrificed before challenge or on day 9 after challenge for histological analysis. Lungs, spleens, and livers were stained with hematoxylin and eosin and analyzed in a blinded fashion. There were three mice per group, and the sections are representative tissue sections. Magnifications, ×40 for lungs and spleens and ×100 for livers.
In contrast to the lungs of unvaccinated mice, the lungs of vaccinated mice prior to challenge contained moderate peribronchovascular lymphoid aggregates (plasma cells, lymphoblasts, and macrophages) that formed occasional bronchus-associated lymphoid tissue-like structures and resulted in mild alveolar wall thickening. Five days after challenge, the lymphoid aggregates were more intense, and many bronchus-associated lymphoid tissue-like structures were visible. The bronchopneumonic lesions were less severe than the lesions in unvaccinated mice. On day 7 after challenge, the changes were characterized by more discrete and coalescing lymphoid tissue that contained many plasma cells, lymphocytes, and macrophages. By day 9 after challenge, the pneumonic changes had resolved, and by day 30 only mild cellular infiltrates in peribronchovascular and interalveolar septa were visible (data not shown). The spleens of vaccinated mice before challenge showed only mild macrophage infiltration compared to the spleens of unvaccinated controls. On days 5 and 7 after challenge, the pyogranulomatous changes seen in unvaccinated mice were less severe, and by day 9 the granulomatous reactions had subsided and the pulp showed more lymphoproliferation. By day 30 after challenge, the spleens showed histology that was comparable to that of unchallenged mice (data not shown). The livers of vaccinated mice prior to challenge had few mononuclear cells in the sinusoids and few nondiscrete multiple granulomas (two to five granulomas per ×10 field). By days 5, 7, and 9 after challenge, the livers contained fewer granulomas (2 to 15 granulomas per ×10 field) than were contained in the livers of unvaccinated controls. By day 30 after challenge, in the livers there were few mononuclear cells around triads and occasional minute granulomas (macrophages and lymphocytes) in the parenchyma (data not shown). Kidneys did not show any appreciable changes in any of the treatment groups, as determined by the absence of inflammatory infiltrates and tubule integrity.
Levels of proinflammatory cytokines after lethal infection.
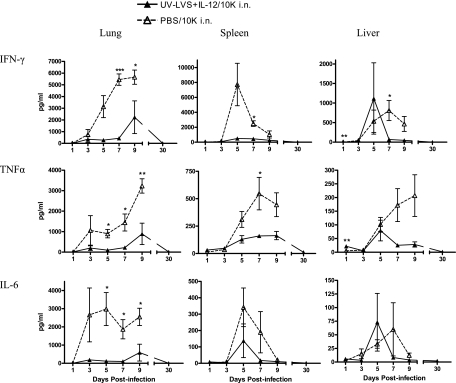
Since the histopathological analysis revealed significant reductions in inflammation in the lungs, spleens, and livers of vaccinated mice compared to the inflammation in naïve mice following challenge, the levels of proinflammatory cytokines in these organs at the same times were determined. Groups of mice were vaccinated with UV-LVS plus IL-12 and challenged with 104 CFU as described above. Analysis of tissue homogenates showed that the levels of cytokines after bacterial challenge were significantly lower in the lungs of vaccinated mice than in the lungs of naïve mice (Fig. 4). Significantly decreased cytokine levels were also observed in livers and spleens of vaccinated mice compared to the levels in unvaccinated mice. In vaccinated mice, after initial modest increases for the first 9 days postchallenge, the levels of all cytokines returned to the baseline levels by day 30 postinfection. However, in unvaccinated mice there was a sharp increase in cytokine expression immediately after the challenge, and cytokine expression continued to increase until the mice succumbed to the infection. The uncontrolled production of cytokines observed in unvaccinated mice is in agreement with the results of other studies in which researchers noted increased production of cytokines, particularly of IFN-γ, following lethal F. tularensis challenge (20). The results show that vaccinated mice have a subdued cytokine response following lethal challenge with F. tularensis, which correlates with the limited histopathological changes observed in these mice.
FIG. 4.
Mice vaccinated with UV-LVS plus IL-12 had reduced levels of proinflammatory cytokines in their lungs, spleens, and livers after infection. Mice were vaccinated as described in the text and challenged i.n. with 104 CFU of F. tularensis LVS (10K) 2 weeks after the final boost. All homogenates from vaccinated mice and unvaccinated mice were then assayed to determine the levels of the cytokines IFN-γ, tumor necrosis factor alpha (TNFα), and IL-6 on different days after the challenge. The levels of cytokines (means ± standard errors of the means) were determined by using a cytometric bead array. There were five mice per group.
Vaccination alters the relative ratios of IFN-γ+ T and NK cells.
Our previous studies demonstrated that there is a dramatic influx of IFN-γ+ NK cells in mouse lungs 3 days after i.n. LVS infection (21). To determine if vaccination with inactivated LVS influenced the pulmonary infiltration of IFN-γ-secreting NK cells, lungs were excised 72 h after infection with 104 CFU of LVS, and an intracellular cytokine analysis was performed. In agreement with our previous findings (21), we found that the percentage of NK1.1+ cells producing IFN-γ was significantly increased in unvaccinated animals 72 h after infection (5.6% IFN-γ+ NK cells in the lungs of infected mice, compared with 1.3% IFN-γ+ NK cells in the lungs of uninfected mice) (Fig. 5). Additionally, NK cells stained more intensely for IFN-γ than the cells of unchallenged, naïve mice stained (mean fluorescence intensity [MFI] at 72 h after challenge, 2.2 × 104; MFI without challenge, 3 × 103). However, in vaccinated mice there was no detectable increase in the percentage of lung IFN-γ+ NK cells (0.9%) following infection, nor was there an increase in staining intensity (MFI, 5 × 103) compared to the staining intensity for uninfected mice. A significant increase (P < 0.05) in the percentage of IFN-γ+ CD4+ cells was observed in the lungs of vaccinated and challenged mice compared to the percentage in the lungs of unchallenged mice. Thus, vaccination resulted in decreased numbers of lung IFN-γ+ NK cells and increased numbers of IFN-γ+ CD4+ T cells after bacterial challenge.
FIG. 5.
Vaccination results in changes in IFN-γ-expressing cells in the lung after infection. Mice were vaccinated as described in the text and challenged i.n. with 104 CFU of F. tularensis LVS 2 weeks after the final boost. Lung lymphocytes from vaccinated and unvaccinated C57BL/6 mice were isolated 72 h after infection with 104 CFU of LVS using collagenase digestion and Lympholyte M gradient separation. Cells were then cultured in the presence of phorbol myristate acetate, ionomycin, and Brefeldin A for 5 h and stained to visualize surface markers and intracellular IFN-γ. Samples were analyzed with a FACSCanto flow cytometer. There were three mice per group, and the mean cell percentages in each group are shown.
IgA is required for protection of vaccinated animals.
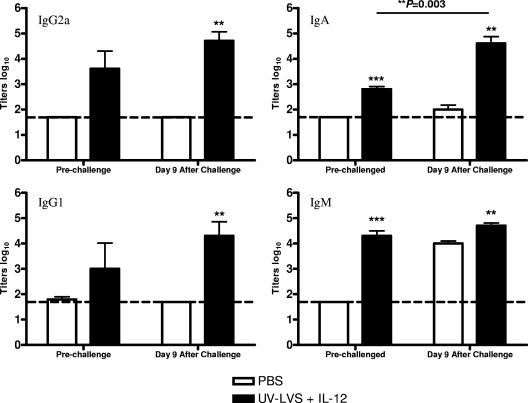
It has been shown that antibodies contribute to protection against lethal intradermal Francisella infection (8, 27), but little is known about the role of humoral immunity following pulmonary infection. The presence of IgM, IgG1, IgG2a, and IgA anti-LVS antibodies in sera collected from vaccinated mice either before challenge or 9 days after challenge was determined by an enzyme-linked immunosorbent assay. Vaccinated mice were found to produce specific antibodies of all isotypes prior to challenge, whereas unvaccinated mice expressed no detectable antibodies (Fig. 6). After challenge, unvaccinated mice produced IgM antibody but no other antibodies, indicating that there was a lack of isotype switching. However, in vaccinated animals, the LVS-specific antibody titers of all isotypes increased significantly.
FIG. 6.
Serum antibody titers are higher following i.n. vaccination with UV-LVS plus IL-12. Mice were vaccinated as described in the text and challenged i.n. with 104 CFU of F. tularensis LVS 2 weeks after the final boost. Sera were collected from vaccinated and unvaccinated controls prior to challenge and 9 days after challenge. The bars indicate mean log10 titers, and the error bars indicate standard errors of the means for three mice per group. The asterisks indicate P values (two asterisks, P < 0.01; three asterisks, P < 0.001).
To determine the importance of IgA in the protection observed in vaccinated mice, IgA+/+ wild-type and IgA−/− deficient mice were vaccinated with UV-LVS plus IL-12 as described above and challenged i.n. with LVS 2 weeks after the final boost. The results showed that while IgA+/+ mice vaccinated with UV-LVS plus IL-12 survived a lethal bacterial challenge, nearly all IgA−/− mice succumbed to infection by day 9 (Fig. 7). Previous studies have shown that IgA−/− mice express normal levels of serum IgM and IgG (except for lower levels of IgG3) (3, 17). After immunization, we found that IgA+/+ and IgA−/− mice expressed similar levels of serum IgM, IgG1, IgG2a, IgG2b, and IgG3 LVS-specific antibodies, and in fact, IgA−/− mice tended to produce higher levels of these antibodies than IgA+/+ mice produced, presumably to compensate for the lack of IgA (data not shown). All IgA+/+ and IgA−/− mice that received IL-12 alone succumbed to infection; the mean time to death was 8 days for IgA+/+ mice and 6 days for IgA−/− mice. Thus, IgA is required for protection against respiratory tularemia following mucosal vaccination with UV-LVS. However, it should be noted that the results do not address whether IgA by itself is sufficient for protection.
FIG. 7.
IgA is required for the protection induced by vaccination with UV-LVS plus IL-12. IgA−/− mice and wild-type controls were inoculated i.n. with UV-LVS plus IL-12 or with IL-12 alone as described in the legend to Fig. 2 and challenged i.n. with 104 CFU of LVS 2 weeks later. Survival was monitored daily for 21 days.
DISCUSSION
In this study we focused on investigating approaches for producing a mucosal inactivated vaccine for the prevention of tularemia. We found that i.n. vaccination of mice with inactivated F. tularensis LVS in combination with IL-12 conferred >90% protection after a lethal respiratory challenge with LVS. This protection was correlated with enhanced bacterial clearance, reduced lung cytokine expression, decreased tissue inflammatory changes, and increased serum IgG and IgA antibody responses. In addition, vaccination resulted in a shift in the IFN-γ-producing cells in the lung, from significant infiltration of IFN-γ+ NK cells after challenge of unvaccinated animals to a dominant IFN-γ+ CD4 population in vaccinated mice. Of particular interest, the protection against respiratory tularemia required IgA antibody. The use of inactivated LVS as a mucosal vaccine has significant implications for the ultimate development of a safe and effective subunit vaccine for use in humans.
We demonstrated that vaccination with inactivated LVS plus IL-12 induced a strong adaptive immune response that protected against delayed challenge such that mice were able to survive a lethal dose of respiratory LVS 2 months after the final vaccine boost. Similar results were obtained with both C57BL/6 and BALB/c mice (unpublished observations). In the majority of previous mucosal vaccination experiments examining protection against tularemia, workers have focused on low-dose inoculation of active LVS (7, 33). Unfortunately, LVS for vaccination of humans is no longer approved by the U.S. Food and Drug Administration (12). The use of inactivated LVS as a mucosal vaccine, however, has yet to be fully examined. Investigators employing the so-called “Foshay” tularemia vaccine containing killed organisms reported limited protection after challenge with type A F. tularensis in both intracutaneous (29) and respiratory infection models (28). Also, in one study the workers showed that subcutaneous inoculation of the O antigen of F. tularensis LVS lipopolysaccharide could protect against an aerosol LVS challenge, but the protection observed was only partial; the time to death was delayed by only 2 days, and there was no effect on the overall survival (6).
Since it has been shown that vaccination with as little as 200 CFU of live LVS can protect mice against a lethal challenge with virulent strains of F. tularensis (33), it was important to confirm the absence of any live LVS in the vaccine preparation by in vivo challenge experiments. The results indicated that the vaccine inoculum did not contain live bacteria. However, we did find that IL-12 was absolutely required as an adjuvant to induce protection with UV-LVS. We have previously shown that IL-12 can induce a protective innate immune response against respiratory tularemia (9), but this activity is lost within 2 weeks. Although IL-12 alone was found to be ineffective in the current vaccination study, when given together with inactivated LVS i.n., this cytokine was able to drive induction of an effective specific adaptive immune response in the lungs. IL-12 is known to enhance IFN-γ production by T cells and NK cells and IgG2a antibody production by B cells (23, 32), and it has been shown to enhance humoral immune responses in other pulmonary infection models (2, 4, 22). In addition, we found that i.n. administration of recombinant IL-12 is significantly less toxic than inoculation by the parenteral route, while still generating protective immunity (18).
Bacterial burden studies showed that vaccinated mice did become infected, albeit with fewer bacteria than unvaccinated animals, and continued to have significantly decreased bacterial loads in the lungs, spleens, and livers after challenge until finally, all organs showed complete clearance of bacteria by day 30 postinfection. Thus, the protection provided by vaccination with inactivated LVS may be associated with increased clearance and decreased dissemination of infection from the lung to the spleen and liver. It has been proposed that in naïve animals, dissemination of bacteria to the spleen and liver, rather initial pulmonary infection, is responsible for the mortality (7). This is in agreement with our histological analyses, which revealed extreme damage in the spleen and liver at the time of death and lesions that were significantly decreased in vaccinated mice. Our results are in agreement with other studies showing that there were reductions in inflammation and associated tissue damage after challenge of i.n. vaccinated mice (33).
Vaccinated and challenged animals also had reduced levels of IFN-γ and other proinflammatory cytokines compared to the levels in unvaccinated mice. On the other hand, we have also found that IFN-γ−/− mice vaccinated with inactivated F. tularensis are not protected against lethal challenge (unpublished observations). In other studies workers have also demonstrated that IFN-γ is required for protective immunity to F. tularensis, including protection induced by aerosol LVS immunization (7, 9, 10). One study using iNOS−/− mice showed that increased levels of IFN-γ do not necessarily correlate with a reduced bacterial load (20). Flow cytometry analysis demonstrated that i.n. vaccination resulted in decreased IFN-γ production by NK cells and increased IFN-γ production by CD4+ T cells. Previous studies in our laboratory have shown that NK cells are a major source of lung IFN-γ at early times after infection (21). Other studies (11, 14) have also suggested that NK cells have a role in the production of IFN-γ during infection. At the same time, it has been found that T cells play an essential role in protection against F. tularensis (7). Together, the data are consistent with the concept that a switch in pulmonary IFN-γ expression from NK cells to CD4 T cells is related to the protection observed.
IgA has been shown to be important in various respiratory infections (3, 25, 31), but its role in protection against F. tularensis has not been determined previously. We found that vaccinated IgA−/− mice were unable to survive a lethal pulmonary challenge with F. tularensis LVS, whereas vaccinated wild-type IgA+/+ mice all survived. It has been shown that passive antibody transfer can aid in protection against F. tularensis LVS (30), although it not known yet whether IgA by itself is sufficient for protection.
Inactivated microbes have been used extensively as vaccines against both extracellular and intracellular pathogens, including polio virus and Salmonella enterica serovar Typhi (16, 26). In addition, workers have recently developed subunit vaccines against some important biothreat agents, such as Yersinia pestis and Bacillus anthracis (5). The results described in this paper suggest that it should be possible to develop a subunit mucosal vaccination approach for effective antibody-mediated protection against lethal respiratory tularemia.
Acknowledgments
We thank the Immunology Core Facility of the Center for Immunology and Microbial Disease at Albany Medical College for help with tissue processing and flow cytometry.
This work was supported by NIH grant PO1 AI056320 to D.W.M.
We have no conflict of interest.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Amemiya, K., J. L. Meyers, S. R. Trevino, T. C. Chanh, S. L. Norris, and D. M. Waag. 2006. Interleukin-12 induces a Th1-like response to Burkholderia mallei and limited protection in BALB/c mice. Vaccine 24:1413-1420. [DOI] [PubMed] [Google Scholar]
- 2.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity, J. Infect. Dis. 180:940-949. [DOI] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., R. H. Raeder, J. G. Nedrud, D. J. Bucher, J. Le, and D. W. Metzger. 2001. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J. Immunol. 166:226-231. [DOI] [PubMed] [Google Scholar]
- 4.Boyaka, P. N., M. Marinaro, R. J. Jackson, S. Menon, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. IL-12 is an effective adjuvant for induction of mucosal immunity. J. Immunol. 162:122-128. [PubMed] [Google Scholar]
- 5.Bramwell, V. W., J. E. Eyles, and H. Oya Alpar. 2005. Particulate delivery systems for biodefense subunit vaccines. Adv. Drug Delivery Rev. 57:1247-1265. [DOI] [PubMed] [Google Scholar]
- 6.Conlan, J. W., H. Shen, A. Webb, and M. B. Perry. 2002. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine 20:3465-3471. [DOI] [PubMed] [Google Scholar]
- 7.Conlan, J. W., H. Shen, R. KuoLee, X. Zhao, and W. Chen. 2005. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an αβT cell- and interferon gamma-dependent mechanism. Vaccine 23:2477-2485. [DOI] [PubMed] [Google Scholar]
- 8.Drabick, J. J., R. B. Narayanan, J. C. Williams, J. W. Leduc, and C. A. Nacy. 1994. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am. J. Med. Sci. 308:83-87. [DOI] [PubMed] [Google Scholar]
- 9.Duckett, N. S., S. Olmos, D. M. Durrant, and D. W. Metzger. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 73:2306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135-142. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, J., P. C. F. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallichan, W. S., and K. L. Rosenthal. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golovliov, I., G. Sandstrom, M. Ericsson, A. Sjostedt, and A. Tarnvik. 1995. Cytokine expression in the liver during the early phase of murine tularemia. Infect. Immun. 63:534-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon, M., D. M. Donaldson, and g. G. Wright. 1964. Immunization of mice with irradiated Pasteurella tularensis. J. Infect. Dis. 114:435-440. [DOI] [PubMed] [Google Scholar]
- 16.Groschel, D. H., and R. B. Hornick. 1981. Who introduced typhoid vaccination: Almroth Write or Richard Pfeiffer? Rev. Infect. Dis. 3:1251-1254. [DOI] [PubMed] [Google Scholar]
- 17.Harriman, G. R., M. Bogue, P. Rogers, M. Finegold, S. Pacheco, A. Bradley, Y. Zhang, and I. N. Mbawuike. 1999. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 162:2521-2529. [PubMed] [Google Scholar]
- 18.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801-809. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindgren, H., S. Stenmark, W. Chen, A. Tarnvik, and A. Sjostedt. 2004. Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect. Immun. 72:7172-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez, M. C., N. S. Duckett, S. D. Baron, and D. W. Metzger. 2004. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell. Immunol. 232:75-85. [DOI] [PubMed] [Google Scholar]
- 22.Lynch, J. M., D. E. Briles, and D. W. Metzger. 2003. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect. Immun. 71:4780-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger, D. W., J. M. Buchanan, J. T. Collins, T. L. Lester, K. S. Murray, V. H. Van Cleave, L. A. Vogel, and W. A. Dunnick. 1996. Enhancement of humoral immunity by interleukin-12. Ann. N. Y. Acad. Sci. 795:100-115. [DOI] [PubMed] [Google Scholar]
- 24.Oyston, P. C. F., and J. E. Quarry. 2005. Tularemia vaccine: past, present and future. Antonie Leeuwenhoek 87:277-281. [DOI] [PubMed] [Google Scholar]
- 25.Pilette, C., Y. Ouadrhiri, V. Godding, J. P. Vaerman, and Y. Sibille. 2001. Lung mucosal immunity: immunoglobulin-A revisited. Eur. Respir. J. 18:571-588. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin, S. A. 2002. Vaccines in the 21st century. Hybrid Hybrid. 21:135-145. [DOI] [PubMed] [Google Scholar]
- 27.Rhinehart-Jones, T. R., A. H. Fortier, and K. L. Elkins. 1994. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect. Immun. 62:3129-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:134-146. [DOI] [PubMed] [Google Scholar]
- 29.Saslaw, S., H. T. Eigelsbach, H. E. Wilson, J. A. Prior, and S. Carhart. 1961. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Intern. Med. 107:121-133. [DOI] [PubMed] [Google Scholar]
- 30.Stenmark, S., H. Lindgren, A. Tarnvik, and A. Sjostedt. 2003. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb. Pathog. 35:73-80. [DOI] [PubMed] [Google Scholar]
- 31.Sun, K., F. E. Johansen, L. Eckmann, and D. W. Metzger. 2004. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J. Immunol. 173:4576-4581. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 33.Wu, T. H., J. A. Hutt, K. A. Garrison, L. S. Berliba, Y. Zhou, and C. R. Lyons. 2005. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect. Immun. 73:2644-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]