Abstract
During infections, positive-strand RNA tombusviruses transcribe two subgenomic (sg) mRNAs that allow for the expression of a subset of their genes. This process is thought to involve an unconventional mechanism involving the premature termination of the virally encoded RNA-dependent RNA polymerase while it is copying the virus genome. The 3′ truncated minus strands generated by termination are then used as templates for sg mRNA transcription. In addition to requiring an extensive network of long-distance RNA-RNA interactions (H.-X. Lin and K. A. White, EMBO J. 23:3365-3374, 2004), the transcription of tombusvirus sg mRNAs also involves several additional RNA structures. In vivo analysis of these diverse RNA elements revealed that they function at distinct steps in the process by facilitating the formation or stabilization of the long-distance interactions, modulating minus-strand template production, or promoting the initiation of sg mRNA transcription. All of the RNA elements characterized could be readily incorporated into a premature termination model for sg mRNA transcription. Overall, the analyses revealed a complex system that displays a high level of structural integration and functional coordination. This multicomponent RNA-based control system may serve as a useful paradigm for understanding related transcriptional processes in other positive-sense RNA viruses.
Most positive-strand RNA genomes that are polycistronic rely on the transcription of smaller genome-derived mRNAs, termed subgenomic (sg) mRNAs, to mediate the translation of their 3′ proximally positioned genes (17). Although these viruses use different mechanisms to produce sg mRNAs, the involvement of their virally encoded RNA-dependent RNA polymerase (RdRp) is common to all (1). In viruses such as Brome mosaic virus, sg mRNAs are generated by the internal initiation of transcription by the RdRp on full-length negative strands of the viral genomes (16). Alternatively, corona- and arteriviruses transcribe their sg mRNAs from noncontiguous, minus-strand RNA templates that are produced by discontinuous RdRp copying of their genomes (10, 24, 25, 30, 36, 38). A third mechanism, suggested by studies of Red clover necrotic mosaic virus (RCNMV), involves the premature termination (PT) of the RdRp while copying viral RNA genomes and the subsequent use of the 3′ truncated minus strands as templates for sg mRNA transcription (33). This PT mechanism has also been proposed to function in a variety of viruses (e.g., Torovirus, Nodavirus, Closterovirus, and Tombusvirus) (7, 14, 27, 35, 39, 41, 48). Despite its prevalence and importance, many mechanistic aspects of PT-based sg mRNA transcription are poorly understood and comprehensive functional models that include all of the cis- and trans-acting factors involved are lacking (41).
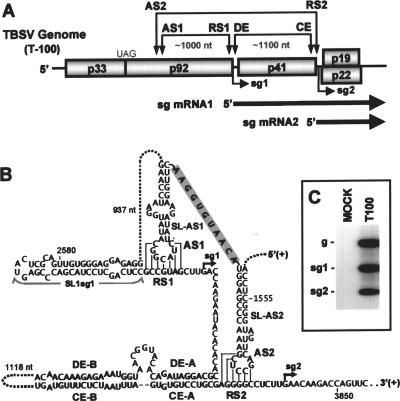
Tombusviruses represent one of the most advanced systems for studying viral RNA synthesis and gene expression (43). The prototype of this genus, Tomato bushy stunt virus (TBSV), possesses a small, 4.8-kb, positive-strand RNA genome that carries five genes (8) (Fig. 1A). The 5′ proximally encoded p33 and p92 are involved in viral RNA synthesis; the latter is a read-through product of the former and is the RdRp (21). Both of these proteins are translated directly from the genome by a mechanism involving a 3′ cap-independent translational enhancer element (3′CITE) located in the 3′ untranslated region (UTR) (5, 6, 44) (Fig. 1A). The more 3′ proximal open reading frames (ORFs) encode the coat protein (p41) and proteins involved in cell-to-cell movement (p22) and the suppression of gene silencing (p19) (28, 29, 31). These 3′ encoded proteins are translated from two sg mRNAs that are transcribed during infections, both of which can be readily detected by Northern blot analysis (Fig. 1A and C). The transcription of TBSV sg mRNAs is proposed to occur via a PT mechanism, and long-distance RNA-RNA interactions involved in this process have been identified (Fig. 1A and B) (2, 3, 13, 47). The transcription of sg mRNA1 requires the formation of the activator sequence 1/receptor sequence 1 (AS1/RS1) interaction that spans ∼1,000 nucleotides (nt), whereas sg mRNA2 transcription requires the formation of both the distal element A/core element A (DE-A/CE-A) interaction and the AS2/RS2 interaction, spanning ∼1,100 and ∼2,100 nt, respectively (Fig. 1A and B) (2, 3, 13, 47). For both sg mRNAs, the AS/RS interactions were shown to form and operate in the plus strand of the viral genome (3, 13). Moreover, these AS/RS interactions map just upstream from their respective transcriptional initiation sites and function as key structural components of the attenuation signals that promote the termination of the RdRp during minus-strand RNA synthesis (Fig. 1B) (3, 13).
FIG. 1.
The wt TBSV genome (T100) and long-distance RNA-RNA interactions that mediate sg mRNA transcription. (A) Linear representation of the TBSV RNA genome showing its coding organization. The relative positions of interacting RNA elements involved in sg mRNA transcription are shown above the genome and are indicated by double-headed arrows. Initiation sites for sg mRNA transcription are labeled sg1 and sg2, and corresponding structures of the two sg mRNAs are represented by bold arrows below the genome. (B) The long-distance RNA-RNA interactions that regulate sg mRNA transcription in TBSV are shown in detail. Relevant sequences of the TBSV genome are presented with corresponding genomic coordinates. The AS1/RS1 base-pairing interaction is essential for the efficient transcription of sg mRNA1 (3), while the AS2/RS2 and DE-A/CE-A base-pairing interactions facilitate sg mRNA2 transcription (2, 13, 47). The proposed stem-loop structures, which encompass the AS1 and AS2 elements, are separated by an 11-nt-long sequence (shaded gray). A putative structure, SL1-sg1, located immediately 5′ to RS1 is also shown. Initiation sites for the two sg mRNAs are indicated by small arrows. (C) Northern blot analysis of TBSV viral RNAs. A representative example of viral RNA accumulation in TBSV-infected cucumber protoplasts after a 22-h incubation at 22°C. The positions of the TBSV genome (g) and its two subgenomic mRNAs (sg1 and sg2) are indicated to the left. TBSV- and mock-infected lanes are labeled T100 and MOCK, respectively. Viral RNAs were detected by Northern blotting using a 32P-labeled oligonucleotide probe complementary to the 3′ terminal sequence of the TBSV genome.
Although the long-distance AS/RS interactions have been characterized for both sg mRNAs, the roles of other associated RNA elements in the transcriptional process have not been investigated. For example, the AS elements are predicted by the mfold computer program to reside in terminal loops of stem-loop (SL) structures; however, it is not known whether such structures are functionally relevant (Fig. 1B) (3, 13). Similarly, the potential significance of a predicted SL, SL1-sg1, which is positioned immediately 5′ to RS1, has not been explored (Fig. 1B) (3). Furthermore, linear structures, such as the spacer segments between the RS elements and transcriptional initiation sites or the corresponding downstream sequences predicted to contain transcriptional promoter elements, remain uncharacterized (Fig. 1B). Accordingly, this study investigated the roles of these diverse RNA elements in the process of sg mRNA transcription. The analyses revealed that optimal sg mRNA transcription in TBSV requires the coordinated activity of several distinct and highly integrated RNA elements that form a multicomponent RNA-based control system.
MATERIALS AND METHODS
Plasmid construction.
Most of the clones used in this study were generated using previously described constructs. These include T100, the wild-type (wt) TBSV genome construct (8); Psg1+1 (47) and Psg51D (2), modified genomic clones; and DI-72, a prototypical TBSV defective interfering (DI) RNA (42). The genome-DI chimera was generated by replacing the sg mRNA1 region in T100 with DI-72. RTD-23 is a TBSV genome-derived mutant in which the internal sequences downstream of the p92 ORF and upstream of the 3′CITE were deleted. DP1 is a TBSV genome in which the p19 and p22 ORFs were inactivated by the introduction of premature stop codons. Where relevant, detailed structures of the modifications introduced into these different base constructs are presented in the respective figures. All modifications were introduced using PCR-based mutagenesis and standard cloning techniques. The PCR-derived regions introduced into constructs were sequenced completely to ensure that only the intended modifications were present.
In vitro transcription and protoplast inoculation.
In vitro RNA transcripts of genomic and DI RNAs were generated using T7 RNA polymerase as described previously (42). The preparation and inoculation of cucumber protoplasts were carried out as described in the past (42). Briefly, isolated cucumber protoplasts (∼300,000) were prepared from cucumber cotyledons and inoculated with 3 μg of TBSV genomic transcript. Infected protoplasts were incubated at 22°C for 22 h prior to viral RNA isolation. For experiments involving the genome-DI RNA launcher and RTD-23, the corresponding amounts of transcripts used were 3 μg and 3 μg, respectively, and incubations were carried out at 28°C for 22 h.
Viral RNA analysis.
Total nucleic acid preparations isolated from virus-inoculated protoplasts were subjected to Northern blot analysis for the detection of plus- or minus-strand viral RNAs as described previously (2). Isolated nucleic acids were treated with glyoxal and separated in 1.4% agarose gels. Equal loading of lanes was confirmed after staining the gels with ethidium bromide. For Northern blot analysis, plus-strand viral RNAs were detected using a 32P-labeled DNA oligonucleotide probe (42), while minus strands were detected using a 32P-labeled RNA riboprobe (2). Relative levels of viral RNAs were determined by radioanalytical scanning of blots with an instant imager. RNA secondary structures were predicted using the mfold computer program (15, 49).
RESULTS
SL-AS1 facilitates sg mRNA1 transcription.
The AS1 element is predicted to reside in the terminal loop of an SL structure termed SL-AS1 (3) (Fig. 1B). Interestingly, depending on the parameters used, three different conformations for SL-AS1 are predicted by mfold (Fig. 2A) (3). However, the variability between these structures is limited to alternative base pairing in their upper stems. These differences left open the possibility that one or more of these RNA conformations could be involved in facilitating the transcription of sg mRNA1. The standard approach for investigating such structures is to introduce mutations into the TBSV genome that would disrupt and then restore base pairing of the upper stems in SL-AS1. Corresponding sg mRNA1 levels would then be monitored in cell infections to determine whether stem stability influenced accumulation. Unfortunately, this approach was not possible in this case because SL-AS1 resides in the p92 coding region and none of the base pairs in the various upper stems are present in directly opposing degenerate codon positions. Consequently, an alternative approach was developed to assess the potential importance of SL-AS1 in a context independent of its coding role.
FIG. 2.
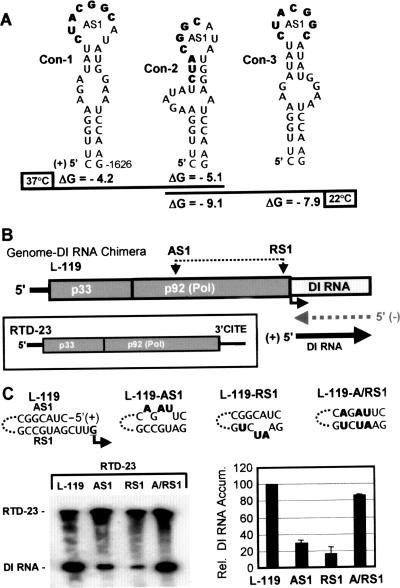
Analysis of the AS1/RS1 interaction in a genome-DI RNA chimera. (A) Alternative SL-AS1 structures. The AS1 elements are presented in bold within three different possible conformations (con-1 through con-3) predicted using different mfold parameters (3). (B) Schematic representation of the genome-DI RNA chimera. The AS1 and RS1 elements in the genome-DI RNA chimera and the proposed “launching” scheme for the production of DI RNA are shown above and below the chimera, respectively. In the boxed inset, the general structure of RTD-23, used as a source of p33 and p92 in cotransfections, is depicted. The functional 3′CITE at the 3′ end of RTD-23 is labeled. (C) Functional analysis of mutant genome-DI chimeras. The substitutions (in bold) in the AS1 and RS1 elements in the genome-DI RNA chimeras are shown at the top. Northern blot analysis was carried out with viral RNAs isolated from protoplasts cotransfected with RTD-23 and the different genome-DI RNA chimeras. The positions of DI RNA and RTD-23 in the blot are indicated, and the RNAs present in each infection are indicated above each lane. The corresponding relative (Rel.) levels of accumulation (Accum.) of the DI RNAs were determined from radioanalytical scanning of blots and are presented graphically on the right. The values represent means with standard deviations (error bars) from three independent experiments.
The system created involved the use of a TBSV DI RNA as a reporter for sg mRNA1 transcriptional activity. DI RNAs are small, noncoding, virally derived replicons that are amplified efficiently when the TBSV replication proteins p33 and p92 are provided in trans (40). In the system devised, a DI RNA was used to replace the sequence corresponding to sg mRNA1 in the TBSV genome, creating the genome-DI RNA chimera L-119 (Fig. 2B). Notably, this chimera is not translationally active because it lacks an essential portion (region 3.5) of the 3′CITE that is normally present in the 3′ UTR of the viral genome (5, 6, 44). Therefore, any RNA synthesis involving the chimera requires that p33 and p92 be provided in trans and a second viral RNA, RTD-23, was used to fulfill this role. RTD-23 is an internally truncated form of the TBSV genome, lacking the p41 and p19/22 ORFs but maintaining the 3′CITE, and encodes and expresses both p33 and p92 (Fig. 2B, inset). Thus, in cotransfections of protoplasts with the genome-DI RNA chimera and RTD-23, the latter provides p33 and p92 for the former. Importantly, since the genome-DI RNA chimera also contained the AS1 and RS1 elements required for sg mRNA1 transcription, it was anticipated that base pairing of these sequences would act to “launch” the replication of the DI RNA in a manner analogous to their roles in the production of sg mRNA1 (Fig. 2B). Consequently, if the system operated as anticipated, it would provide an indirect relative measure of AS1/RS1-dependent sg mRNA1 transcriptional activity.
The genome-DI RNA chimera L-119 was cotransfected into protoplasts with RTD-23, and viral RNA accumulation was assessed following a 22-h incubation (Fig. 2C). No viral RNAs were detected in mock or L-119-only transfections (data not shown). In cotransfections, RTD-23 replicated and accumulated to detectable levels, as did the DI RNA (Fig. 2C). L-119 did not accumulate, consistent with previous findings that large D RNAs of TBSV are not replicated efficiently in trans (42). Similar levels of DI RNA were observed in cotransfections of RTD-23 and a L-119 derivative containing frameshift-inactivated p33/p92 ORFs (data not shown). This control showed that DI RNA launching utilized p33/p92 provided in trans from RTD-23 and confirmed that the modifications introduced into SL-AS1 in L-119 would indeed be translation independent. To test whether the DI RNA was being launched by the AS1/RS1 interaction, mutations were introduced into these sequences that were predicted to disrupt and then restore base pairing (Fig. 2C). Disruptions in L-119-AS1 or L-119-RS1 reduced DI RNA accumulation levels to ∼20 to 30%, while the restoration of the AS1/RS1 interaction in L-119-A/RS1 led to recovered levels of ∼85% (Fig. 2C). This result indicated that the chimeric system was AS1/RS1 responsive and validated its use as a surrogate for assessing the importance of AS1 for transcription.
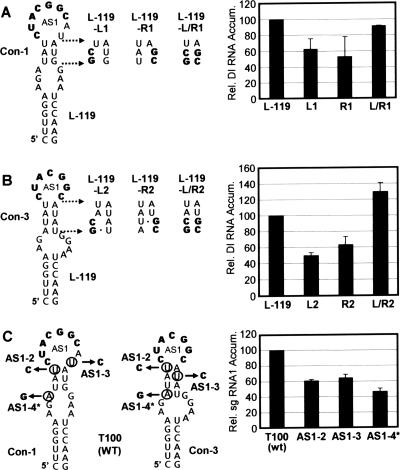
The genome-DI RNA chimera system was then used to investigate the potential roles of the different conformations of SL-AS1. Mutations were introduced into L-119 that would disrupt and then restore the predicted upper stems in conformation 1 (con-1) and con-3 (Fig. 3A and B). Con-2 could not be assessed in this manner because its upper stem included the AS1 element that needed to be maintained in its wt form (Fig. 2A). For both con-1 and con-3, reductions in DI RNA accumulation to ∼50 to 60% of that for L-119 were observed when their upper stems were destabilized, but near wt or greater levels of accumulation were seen upon restoration of the stems (Fig. 3A and B). These data suggest that the structural context of AS1, namely the adjacent upper stem, can facilitate DI RNA launching and, by extension, sg mRNA1 transcription. Mutant L-119-L/R1 was designed to favor the reformation of con-1 and to not form either con-2 or con-3, whereas L-119-L/R2 was designed to favor the reformation of con-3 and to not form either con-1 or con-2 (Fig. 3A and B). Based on the relatively high levels of activation observed for these two compensatory mutants, it can be deduced (i) that either con-1 or con-3 is able to function well independently and (ii) that con-2 is not necessary for efficient activity.
FIG. 3.
Analysis of SL-AS1 in the genome-DI RNA chimera and TBSV genome. (A) Compensatory mutational analysis of the upper stem in con-1. Mutations introduced into the genome-DI RNA chimera are indicated to the right of the structure with substitutions in bold. Relative levels of DI RNA accumulation are presented graphically on the right. (B) Compensatory mutational analysis of the upper stem in con-3 using the genome-DI RNA chimera. Relative levels of DI RNA accumulation are presented graphically on the right. For panels A and B, the relative DI RNA accumulation levels were calculated as described in the legend for Fig. 2. (C) Analysis of SL-AS1 in the wt TBSV genome (T100). Mutations introduced into the genome at degenerate positions are mapped onto both con-1 and con-3. The nucleotides circled were substituted with those indicated by the arrows. Note that AS1-4* has all three of the substitutions shown. Mutant genomes were transfected into protoplasts, and viral RNAs were analyzed by Northern blotting following a 22-h incubation. The corresponding relative (Rel.) levels of accumulation (Accum.) of sg mRNA1 were determined from radioanalytical scanning of blots. The relative sg mRNA1 accumulation levels correspond to means with standard deviations (error bars) from three independent experiments and represent the ratios of sg mRNA1 levels to their corresponding genomic RNA levels, all normalized to that for T100.
Next, in an attempt to corroborate these results in a more biologically relevant context, mutations were introduced into the upper stem of SL-AS1 in the wt TBSV genome (T100) (Fig. 3C). As explained previously, a complete set of compensatory mutations could not be constructed; however, substitutions predicted to destabilize the predicted stems could be introduced. Mutant AS1-2 contained a U-to-C substitution that would reduce the stability of the upper stem in con-1 and con-3, while mutant AS1-3 had a U-to-C substitution that would interfere with the formation of the upper stem in only con-3 (Fig. 3C). Mutant AS1-4* was a triple mutant that contained both of the aforementioned substitutions as well as an A-to-G substitution that would be disruptive to the upper stem in con-3 (Fig. 3C). In all cases, the modifications led to substantial decreases in sg mRNA1 levels in protoplast infections (∼40 to 55%), with the triple mutant being most detrimental (Fig. 3C). These genome-based findings are consistent with the results from the genome-DI RNA chimeric system and support a role for the SL1-AS1 conformations in mediating sg mRNA1 transcription. Additionally, in this natural context, a dominant role for con-3 is suggested by (i) the reduced activity observed for the AS1-3 mutant in which con-3 (but not con-1) was destabilized and (ii) the added negative effect seen in AS1-4*, where only con-3 was further destabilized (Fig. 3C).
SL-AS2 influences sg mRNA2 accumulation.
AS2 is also predicted to be located in the terminal loop of an SL structure, SL-AS2 (13) (Fig. 1B). Fortunately, only a single RNA conformation was predicted in this case and its upper stem contained a central base pair with both partners in degenerate codon positions. Thus, this upper stem could be disrupted and restored in the wt TBSV genome and the effect on sg mRNA2 levels could be assessed directly. An analysis of a set of compensatory mutants in the upper stem (AS2-1A, AS2-1B, and AS2-1C) by protoplast infection revealed that both disruptions caused a boost (25 to 50%) in sg mRNA2 levels, while stem restoration led to an almost twofold increase (Fig. 4B). Additional analyses of these mutants at higher temperatures or in different host cells revealed similar results (not shown). These data contrast those for SL-AS1, where the destabilization of the upper stem was detrimental to sg mRNA1 accumulation (Fig. 3C). Nonetheless, the findings do indicate that the immediate context of AS2 can influence sg mRNA2 accumulation levels.
FIG. 4.
Analysis of SL-AS2 in the TBSV genome. (A) Compensatory mutations introduced into the genome (T100) at degenerate positions are indicated to the right of the structure. (B) Relative (Rel.) levels of sg mRNA1 are presented graphically. Analysis and quantification of sg mRNA1 levels were carried out as described in the legend for Fig. 3. Error bars indicate standard deviations. Accum., accumulation.
SL1-sg1 facilitates sg mRNA1 transcription.
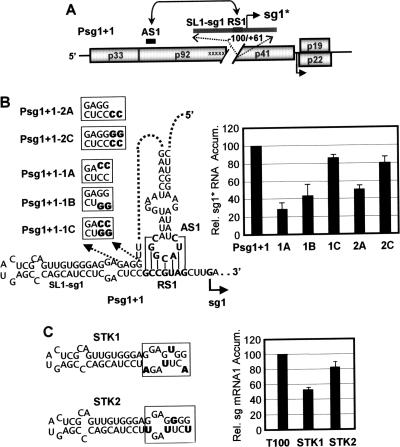
Having investigated the role of the contexts of the two AS elements in sg mRNA transcription, we next focused our attention on the contexts of the RS elements. RS1 is predicted to be located directly adjacent to a conserved upstream SL structure termed SL1-sg1 (Fig. 1B) (3). Due to this proximity, it was proposed that this structure could be important for sg mRNA1 transcription. One possible role suggested for SL1-sg1 is that it could coaxially stack with the AS1/RS1 helix, thereby stabilizing the AS1/RS1 interaction (3). Unfortunately, mutational analysis of SL1-sg1 in the wt TBSV genome was complicated by the fact that it resides in the 3′ coding region for p92. To circumvent this coding restriction, a mutant TBSV genome that was constructed and analyzed previously was utilized (47). Psg1+1 contains five substitutions in SL1-sg1 and RS1 at degenerate codon positions and has a segment downstream of the p92 ORF deleted (Fig. 5A). These modifications completely inactivate sg mRNA1 transcription from this mutant genome (47). Additionally, Psg1+1 contains an inserted segment (−100/+61 relative to the sg mRNA1 transcription initiation site) that contains wt versions of SL1-sg1, RS1, and downstream sequences. This segment was previously shown to be transcriptionally active at this ectopic site and to direct the synthesis of an sg mRNA1-like RNA termed sg1* (47) (Fig. 5A). Importantly, sg1* transcription was also shown to be dependent on the AS1/RS1 interaction (47). Psg1+1 was used to assess the role of SL1-sg1 in the transcription of sg1* in a context free of translational constraints.
FIG. 5.
Analysis of SL1-sg1 in Psg1+1 and the TBSV genome. (A) Schematic representation of the Psg1+1 mutant genome (47). Each substitution at a degenerate position in the p92 coding region is indicated by an “x,” and the gap represents the region deleted downstream of this ORF. At the top, the inserted segment containing wt versions of SL1-sg1, RS1, and associated downstream sequences is shown. The functional AS1/RS1 interaction in this mutant genome is represented by the double-headed arrow connecting these elements, and the initiation site for sg1* is indicated by the solid arrow. (B) Mutations (in bold) that were introduced into SL1-sg1 in Psg1+1 are indicated in the boxes, and corresponding sg1* levels are shown to the right. (C) Substitutions (in bold) introduced into the wt genome (T100) at degenerate positions are indicated in the boxes, and corresponding sg mRNA1 levels are shown to the right. Analysis and quantification were carried out as described in the legend for Fig. 3. Error bars indicate standard deviations. Rel., relative; Accum., accumulation.
The sequence at the bottom of SL1-sg1 was targeted for compensatory mutational analysis in mutants Psg1+1-1A, Psg1+1-1B, and Psg1+1-1C (Fig. 5B). In protoplast infections, disruptions in SL1-sg1 resulted in reduced sg1* accumulation, while restoration caused relative two- to threefold levels of recovery (Fig. 5B). These results indicate that the base of SL1-sg1 contributes to sg1* production. Next, a 2-nt CC spacer, which would presumably leave SL1-sg1 intact, was inserted between SL1-sg1 and RS1. This modification caused an ∼50% reduction in sg1* accumulation (Fig. 5B), suggesting that a key feature for optimal sg1* production is the location of SL1-sg1 directly adjacent to RS1. The importance of such positioning was further supported by the enhancement of sg1* levels when a corresponding tandem GG was added (which would restore the bottom of an extended SL1-sg1 directly adjacent to RS1) (Fig. 5B).
To further substantiate the importance of SL1-sg1 in a more natural viral context, mutations in degenerate positions that were predicted to destabilize the base of SL1-sg1 to greater or lesser degrees were introduced into the wt TBSV genome (Fig. 5C). For STK1, the introduction of a UU mismatch, two GA mismatches, and a GU wobble pair in this region caused sg mRNA1 levels to drop by ∼50%, whereas a more modest ∼20% decrease was seen for STK2, in which all four positions were converted to GU wobble pairs (Fig. 5C). These results are consistent with those generated from the analysis of Psg1+1 and support a role for SL1-sg1 in sg mRNA1 transcription. Moreover, the relatively strong level of activity observed for STK2, containing multiple GU base pairs, supports SL1-sg1 functioning in the plus strand (as these pairs would correspond to disruptive CA mismatches in the minus strand).
The local context of the DE-A/CE-A interaction does not enhance sg mRNA2 transcription.
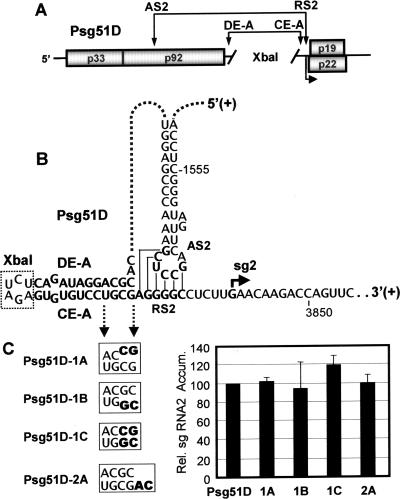
The long-distance DE-A/CE-A interaction has been shown to be important for the recruitment of AS2 to RS2 in the context of the TBSV genome (2). Interestingly, the position of the DE-A/CE-A interaction relative to the AS2/RS2 interaction is comparable to the location of SL1-sg1 relative to the AS1/RS1 interaction (Fig. 1B). This correspondence in structure and relative positioning prompted us to ask whether the “local” DE-A/CE-A interaction could participate in facilitating sg mRNA2 transcription in a manner analogous to that shown for SL1-sg1 (Fig. 5). To address this question, the long-distance recruiting function of the DE-A/CE-A interaction needed to be suppressed. This was accomplished by using a genome mutant, Psg51D, in which the ∼1,100-nt-long sequence between DE-A and CE-A was deleted and replaced by a 6-nt-long XbaI restriction enzyme site (2) (Fig. 6A). The absence of this long intervening sequence in Psg51D eliminated the long-distance recruiting function of the DE-A/CE-A interaction, but maintained possible local functions (Fig. 6B). When the base of the DE-A/CE-A interaction was either disrupted or restored or a 2-nt-long spacer was inserted between it and RS2, no major differences in sg mRNA2 accumulation were observed (Fig. 6C), suggesting no appreciable function for the DE-A/CE-A interaction in this local context.
FIG. 6.
Analysis of a localized DE-A/CE-A interaction in Psg51D. (A) Schematic representation of the Psg51D mutant TBSV genome (2). The AS2/RS2 and DE-A/CE-A interactions are indicted by double-headed arrows. The ∼1,100-nt-long sequence between DE-A and CE-A was deleted (indicated by the space between diagonal lines) and replaced by sequence corresponding to an XbaI restriction enzyme site. (B) RNA sequence and secondary structure of relevant portions of Psg51D. The XbaI restriction enzyme site is enclosed by a dotted box. (C) Mutations (in bold) introduced into DE-A and/or CE-A in Psg51D are indicated in the boxes, and relative sg mRNA2 levels are shown to the right. Analysis and quantification were carried out as described in the legend for Fig. 3. Error bars indicate standard deviations. Rel., relative; Accum., accumulation.
Spacer length and identity modulate sg mRNA2 transcription.
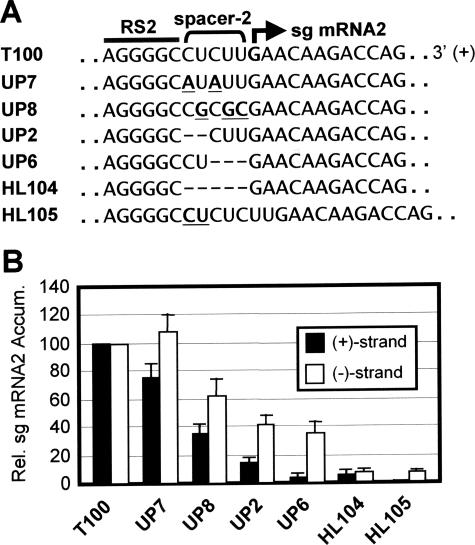
Having examined the RNA elements 5′ to the RS elements, we next turned our attention to the sequences downstream of the RS elements. The sequence between RS and its initiation site, termed the spacer, is CUU for sg mRNA1 or CUCUU for sg mRNA2 (Fig. 1B). The spacer for sg mRNA2, spacer 2, was selected for analysis as it is not present in a coding region (unlike that for sg mRNA1) and thus could be studied in a wt TBSV genome context. The location of spacer 2 between the AS2/RS2 attenuation structure and the site of sg mRNA2 initiation suggested that it could contribute to the activity of one or both of these elements. For this reason, both plus and minus sg mRNA strands were monitored by Northern blotting (see Fig. S1 in the supplemental material). First, the composition of spacer 2 was modified in UP7 or UP8 from CUCUU to AUAUU or CGCGC, respectively (substitutions are underlined) (Fig. 7B). These changes altered sg mRNA2 levels in UP7 and UP8 to ∼75 and ∼35%, respectively, for plus strands and ∼105 and ∼60%, respectively, for minus strands (Fig. 7B). Next, genomes with deletions of some or all of the spacer 2 sequence were analyzed. The removal of the first 2 or last 3 nt in UP2 and UP6 resulted in levels of ∼15 and ∼5%, respectively, for plus strands, and ∼40 and ∼35%, respectively, for minus strands (Fig. 7B). The deletion of the entire spacer 2 caused very low levels for both plus and minus strands (∼5%). When the size of spacer 2 was increased by the insertion of CU at its 5′ end, plus strands were undetectable and minus strands accumulated to ∼5% (Fig. 7B). Taken together, these results show that both the length and composition of spacer 2 affect sg mRNA2 levels, with the effect of the former being more pronounced.
FIG. 7.
Analysis of the spacer 2 element in the TBSV genome. (A) Mutations introduced into the spacer 2 element (delineated by the bracket) that is located between RS2 (bar) and the site of initiation (bold arrow). Substitutions and an insertion are in bold and underlined, while deleted nucleotides are represented by dashes. (B) Relative accumulation levels of plus (+) and minus (−) strands of sg mRNA2 are presented graphically. Analysis and quantification were carried out as described in the legend for Fig. 3. Error bars indicate standard deviations. Rel., relative; Accum., accumulation.
Defining the promoter for sg mRNA2 transcription.
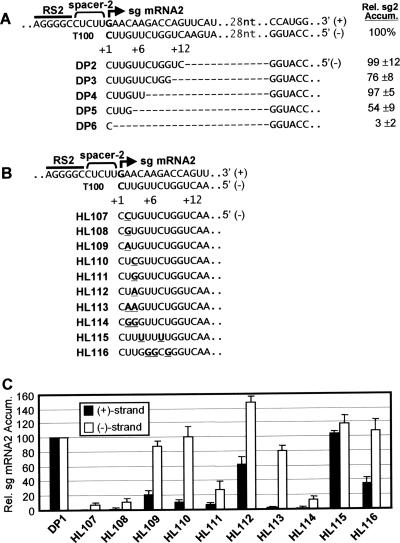
The PT model proposes that the initiation site and adjacent downstream sequence in the plus strand correspond to the promoter for sg mRNA transcription in the minus strand. As a first step in defining the promoter for sg mRNA2, termed promoter 2, a set of nested deletions were introduced into the TBSV genome and the mutants were tested in protoplast infections (Fig. 8A). Deletions that mapped up to position +7 (relative to the transcription initiation site, defined as +1 in the minus-strand template) did not dramatically effect sg mRNA2 levels, while those that mapped to +5 or +2 exhibited more significant defects (i.e., ∼54 of ∼3%, respectively) (Fig. 8A). These findings suggest that the core promoter sequence may be small (i.e., +1 to +4); however, other downstream sequences also contribute to the activity.
FIG. 8.
Analysis of the promoter 2 element in the TBSV genome. (A) Internal deletion analysis of the promoter region for sg mRNA2. At the top, the complementary plus and minus strands that correspond to this region in the wt genome (T100) are shown. The RS2 element (bar), the spacer 2 element (bracket), and the site of sg mRNA2 initiation (bold arrow) are denoted. The numbering system used specifies nucleotides in the minus strand. Position +1 corresponds to the minus-strand nucleotide that templates initiation (i.e., C), and the increasing numbers correspond to adjacent minus-strand positions going in the 5′ direction. Other sequence that is not shown due to space constraints is represented by dots with the number of missing nucleotides indicated. Deletions (represented by dashes) that were introduced are shown in the minus strands below. Relative sg mRNA2 levels are indicated to the right. (B) Substitution analysis of the promoter region for sg mRNA2. Nucleotides that were substituted are in bold and underlined. (C) Relative accumulation levels of the plus (+) and minus (−) strands of sg mRNA2 are presented. DP1 is a TBSV genome in which the p19 and p22 ORFs were inactivated by the introduction of stop codons (allowing for the analysis of transcriptional activity independent of possible effects due to the perturbation of the sg mRNAs translational function). DP1 was used to construct the accompanying promoter mutants. Analysis and quantification were carried out as described in the legend for Fig. 3.
Previous studies revealed that the +1C which templates initiation is essential for plus- but not minus-strand sg mRNA2 synthesis (2). To examine the role of the +2U and +3U positions in the minus strand, each was substituted individually with C, G, or A (mutants HL107 to HL112) (Fig. 8B). Since these modifications would also be present in sg mRNA2 and could potentially affect translational activity, the base genome used for construction, DP1, contained inactivated p19 and p22 ORFs. The accumulation levels of sg mRNA2 plus and minus strands were monitored by Northern blot analysis (see Fig. S2 in the supplemental material) and quantified (Fig. 8C). All mutations, except for +3A (HL112), caused dramatic drops in plus-strand accumulation (to 0 through ∼20% of the level of the wt) (Fig. 8B and C). In contrast, three mutants containing +2A, +3C, and +3A (HL109, HL110, and HL112) showed near wt or greater levels of minus-strand accumulation (Fig. 8B and C). In HL113 and HL114, where +2 and +3 were simultaneously changed to AA and GG, respectively, plus strands were near undetectable for both, but the minus-strand levels of the former were around 80% (Fig. 8B and C). Finally, the sequence between +4 and +8 was examined. Making this template sequence U rich in HL115 resulted in essentially wt levels for both plus and minus strands, while converting it to a GC-rich sequence in HL116 yielded wt levels of minus strands, but reduced levels of plus strands (∼35%) (Fig. 8B and C). Collectively, the data indicate that the substitutions introduced within the +2 to +8 region have a greater relative overall effect on plus-strand accumulation (i.e., affect promoter activity); however, some of the changes in the +2 and +3 positions also influence minus-strand sg mRNA2 accumulation (i.e., affect the premature termination process).
DISCUSSION
SL-AS1 and SL-AS2: the role of structural contexts of AS elements.
One way to facilitate base pairing of two distally positioned cRNA sequences is to position one or both of the sequences in single-stranded regions within an RNA secondary structure (45). In the TBSV genome, two different functional RNA-RNA interactions involving sequences in internal or terminal loops have been described previously (43). These respective interactions operate to modulate TBSV genome replication (4, 18, 19, 26) or facilitate efficient cap-independent translation of viral proteins (5, 6). Considering these cases, it is not surprising to find that similar examples related to sg mRNA transcription also exist.
For the SL-AS1 conformations, two of the three possible conformations (con-1 and con-3) were shown to be capable of assisting transcriptional activation, with con-3 likely playing the more dominant role (Fig. 3). A probable function for SL-AS1 is to present AS1 in a context that promotes its single strandedness, thereby facilitating the formation of the AS1/RS1 interaction. An analogous role in sg mRNA transcription has also been suggested for a predicted SL in the related RCNMV (family Tombusviridae) (33). In RCNMV, this SL, termed the trans-activator (TA), is located in RNA2 and acts to induce sg mRNA transcription by base pairing with a TA-binding site (TABS) in RNA1 (33) (Fig. 9A). The TA/TABS interaction in RCNMV is thus analogous to the AS/RS interactions in TBSV, except that the former occurs intermolecularly. However, the proposed function for both of these interactions is the same, i.e., forming part of an attenuation signal that mediates RdRp termination during minus-strand synthesis. Currently, it is not known whether the secondary structure of the TA is important for its transcriptional function in vivo. However, this structure was found to be an essential cis-acting RNA replication element that is required for RNA2 accumulation in protoplasts (37). In contrast, none of the stem mutations in SL-AS1 or SL-AS2 notably affected TBSV genome accumulation levels (data not shown), indicating that both of these structures are transcription specific.
FIG. 9.
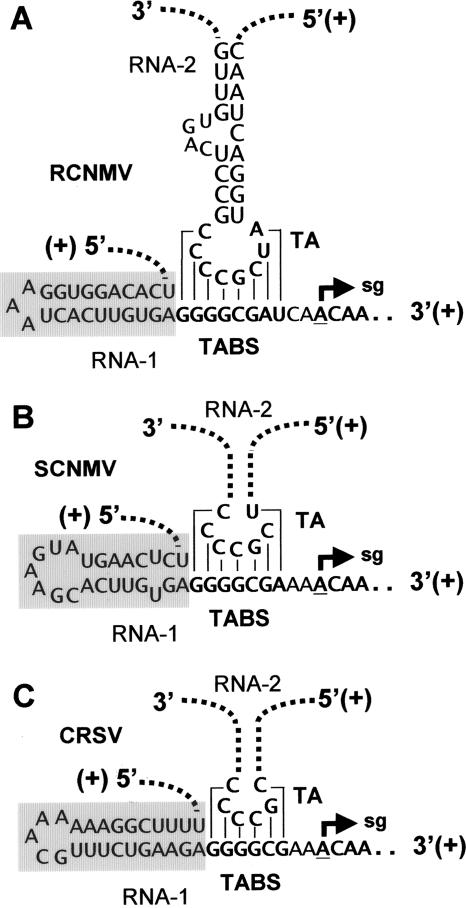
Predicted SL structures located immediately 5′ to the TABS elements in Dianthovirus genomes. The structures correspond to (A) RCNMV, (B) Sweet clover necrotic mosaic virus (SCNMV), and (C) Carnation ringspot virus (CRSV). The TA/TABS interaction for RCNMV occurs between genomic segments RNA1 and RNA2, and similar intermolecular interactions are proposed to occur in SCNMV and CRSV (33). The TA SL for SCNMV and CRSV are not shown and are instead depicted by dotted lines. The proposed SL structures located 5′ to the TABS elements (that are positioned similarly to SL1-sg1 relative to the RS1 element in TBSV) are indicated by gray boxes.
For SL-AS2, the destabilization of its upper stem resulted in relative increases in sg mRNA2 levels, while restoring the stem with a transposed version of the original base pair yielded even higher levels of sg mRNA2 (Fig. 4). This unanticipated result may be related to structural features that are not evident without detailed knowledge of the atomic structures of the different mutants. For example, it is possible that the mutants are able to form unexpected conformations that better facilitate the presentation of AS2. Interestingly, comparative structural analysis of different tombusviruses revealed that, in contrast to those in SL-AS1s, the upper stems in SL-AS2s are not completely conserved in the genera (i.e., the upper stem is not predicted to form in 4 out of 14 species) (46). These variations in nature suggest a greater degree of flexibility for the AS2 context. However, since SL-AS2 structure can influence transcriptional efficiency, the divergence observed could also be related to the optimization of sg mRNA2 levels for the different viral niches.
SL1-sg1 and the DE-A/CE-A interaction: the role of secondary structures 5′ to AS/RS interactions.
Our analysis of SL1-sg1 suggested that it functions in the plus strand and that the stability of its lower stem contributes to the efficient production of sg mRNA1 (Fig. 5). The observation that greater activation occurred when SL1-sg1 was positioned directly adjacent to the AS1/RS1 interaction is consistent with it functioning, at least in part, by stabilizing the AS1/RS1 helix by coaxial stacking (additional functions for SL1-sg1 remain possible). In contrast, for sg mRNA2 transcription, an analogous role could not be established for the similarly positioned DE-A/CE-A interaction (Fig. 6). In line with this latter finding, some tombusviruses have predicted DE-A/CE-A interactions that are not directly adjacent to their corresponding AS2/RS2 helices and thus could not readily act as coaxial stabilizers (47). Therefore, based on these data, the DE-A/CE-A interaction seems to function primarily, if not exclusively, as a long-distance recruiting complex (2).
SL1-sg1 is an interesting RNA structure because it is located in the 3′ portion of the p92 ORF. Despite this coding restriction, all sequenced tombusviruses are predicted to contain equivalent SL1-sg1s that are positioned directly adjacent to their AS1/RS1 helices and which, notably, contain co- and monovariations that maintain their stem regions (46). Interestingly, a similarly positioned SL structure is also predicted to be present in RCNMV RNA1, just 5′ to the TABS (which also maps within the 3′ end of the RCNMV RdRp ORF) (Fig. 9A). Comparable SLs with different compositions are also predicted to occur in corresponding positions in two other sequenced dianthoviruses, Sweet clover necrotic mosaic virus and Carnation ringspot virus (Fig. 9B and C). Moreover, the presence of GU base pairs in two of the predicted SLs suggests that they would operate as plus-strand elements (Fig. 9A and C), as proposed for SL1-sg1. Based on these structural parallels, it is possible that the conserved 5′ proximal SLs in dianthoviruses are functionally comparable to SL1-sg1 in TBSV and other tombusviruses.
Spacer 2: a spacer element modulates synthesis of minus and plus strands of sg mRNA2.
The role of the spacer 2 sequence was examined, and it was found to be important for modulating the accumulation of both minus and plus strands of sg mRNA2 (Fig. 7). Although the identity of nucleotides in spacer 2 contributes to this activity, the length of this element was found to be of even greater importance. Previous studies have shown that most sg mRNA2 minus strands (∼80%) terminate with a 3′ end corresponding to the +1 position and do not contain spacer-derived sequences (13). This suggests that the nucleotides complementary to spacer 2 are not normally part of the promoter and that the spacer sequence acts primarily as a plus-strand element that functions in the attenuation process. In this capacity, it would mediate the production of minus strands that contain 3′ termini that are competent for sg mRNA transcription (i.e., contain a functional promoter). However, the perturbation of the spacer could reduce the efficiency of the termination process (leading to reduced amounts of minus strands) (Fig. 7) and/or produce minus strands with altered 3′ ends. The latter possibility could explain the proportionally greater reductions of plus strands observed with the spacer mutants (Fig. 7). That is, a subset of minus-strand templates might contain defective promoters (e.g., containing extra or deleted 3′ residues). Taking this into consideration, the negative effects observed on plus-strand accumulation could be “indirect” consequences of altered structure of the promoter and/or its context that result from aberrant termination. This and other possibilities (such as the spacer trans-activating promoter activity) will be investigated in future studies.
Promoter 2: a promoter region modulates synthesis of both plus and minus strands of sg mRNA2.
The promoters for minus- and plus-strand TBSV genome synthesis have been identified previously at the 3′ termini of the complementary plus and minus strands, respectively (20, 22). In the present study, the internal boundary of the sg mRNA2 promoter element, promoter 2, was delineated by deletion analysis. The data revealed that the sequence from +1 up to and including +6 can mediate sg mRNA2 accumulation at ∼97% efficiency (DP4) (Fig. 8). This finding is in agreement with results from deletion analysis of the Cucumber necrosis virus tombusvirus sg mRNA2 promoter region, which also defined this boarder as +6 (11).
Previous studies showed that the +1 position is a specific determinant of plus-strand sg mRNA synthesis and a key component of the promoter (2, 3). In the present study, some of the substitutions at the +2 and +3 positions (i.e., +2A, +3C, +3A, or +2A+3A) yielded results that were similar to those observed previously for the +1 position (i.e., they exhibited high minus-strand and low plus-strand sg mRNA accumulation), thereby implicating these positions as specific determinants of promoter activity (Fig. 8). However, other substitutions at these same positions (+2C, +2G, +3G or +2G +3G) caused major decreases in both plus and minus levels, pointing to a role in modulating attenuation activity (Fig. 8). Thus, depending on their nucleotide identity, the +2 and +3 positions are able to exert control over both promoter and/or attenuation processes. In contrast, none of the mutations in more downstream regions influenced minus-strand accumulation, suggesting that the attenuation activity does not overlap with positions +4 to +8.
For attenuation activity, the nucleotides at +2 and +3 could assist in the RdRp stalling and/or dissociation process. However, the situation is complicated by the fact that these residues are copied; thus, it is conceivable that the nucleotides in either strand or in both strands are involved. The latter possibility would be relevant if the stability between the template nascent-strand duplex in the elongating RdRp complex contributes to termination. Indeed, efficient minus-strand synthesis was facilitated by the wt U residues in positions +2 and +3 or their substitution with one or two A residues (Fig. 8). The weaker AU or UA base pairs that would form in the template nascent-strand duplex could facilitate RdRp nascent-strand stalling and/or dissociation from the template. However, a C at +3 also yielded high levels of minus strands, indicating a possible exception to this putative mechanism or a more complicated scheme involving nucleotide identity. The mechanism involving a weak template nascent-strand duplex is appealing because the AS2/RS2 interaction and spacer element, both of which also affect minus-strand accumulation (13) (Fig. 7), can be functionally incorporated into the process. In this putative scenario, the AS2/RS2 interaction would cause the actively copying polymerase to stall, while the spacer element would ensure the positioning of +2 and +3 within the template nascent-strand duplex. Thus, if applicable, the coordinated function of three different elements would act to control the attenuation process that leads to the generation of sg mRNA2 minus strands.
The coupled dual activity of this region in mediating both plus and minus strand accumulation made the assessment of promoter function challenging. The results suggest that the core promoter may be as small as +1 to +4; however, other more distal sequences are also involved. For instance, the U-to-G substitutions in the +4-to-+8 region preferentially inhibited plus-strand accumulation, supporting the importance of this more distal region for promoter activity. In general, the +2-to-+8 region is U rich (i.e., at five of seven positions) and the naturally occurring U residues are preferred at +2, +3, and in the +4-to-+8 region (Fig. 8). Consistent with the importance of U residues for promoter function, in vitro analysis of the similarly structured promoter for plus-strand genome synthesis revealed that substituting the +2-to-+8 region with either A-rich or GC-rich sequences caused the inhibition of RdRp activity (22). The mechanism by which particular residues facilitate the activity of TBSV transcriptional promoters is unclear, but possibilities include (i) interacting specifically with the RdRp and/or accessory proteins (12, 32, 34), (ii) mediating the transition of the RdRp from initiation to elongation (9), and/or (iii) facilitating access to the promoter by being unstructured or forming a weak duplex with its complementary strand (23). What is intriguing is that such promoter activities would have to be carefully coordinated with the other role of this region in the production of minus-strand templates (i.e., the attenuation process). Consequently, a careful balancing act would be required to satisfy the dual structure/function activities of these highly integrated RNA elements.
Functional integration of newly defined RNA elements into a PT model.
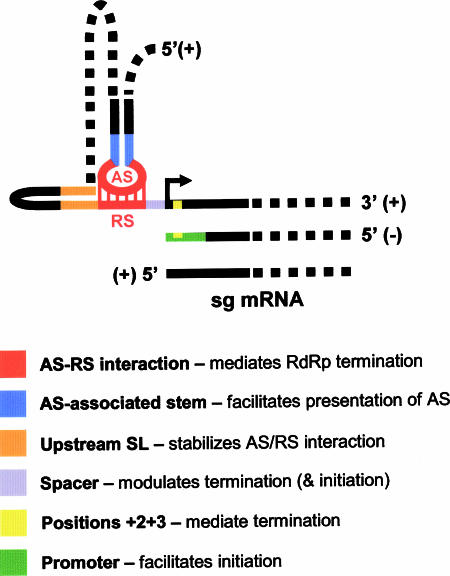
This study has revealed that sg mRNA transcription in TBSV is regulated by a multicomponent RNA-based control system. The diverse RNA elements that have been characterized can be incorporated into a general PT model for sg mRNA transcription (Fig. 10). Below, functions for the different RNA elements are proposed. (i) The SLs that encompass the AS elements act to facilitate the presentation of their AS sequences in single-stranded form and promote the efficient formation of AS/RS interactions. (ii) Local SLs positioned immediately 5′ to RSs (e.g., SL1-sg1) function (at least partly) via coaxial stacking to stabilize corresponding AS/RS interactions. (iii) Spacer elements modulate both the attenuation process that leads to minus-strand production and the promoter activity that drives sg mRNA transcription (though the latter may result from an indirect effect related to minus-strand template structure). (iv) Positions +2 and +3 (and/or their complements in the plus strand) influence the efficiency of minus-strand production, potentially by facilitating RdRp stalling/dissociation. (v) Lastly, promoter elements function to mediate the initiation of the RdRp and direct efficient plus-strand synthesis. The mechanistic scenarios presented above show that the newly defined RNA elements can be readily integrated into a PT model (Fig. 10). Accordingly, this multicomponent RNA-based control system could serve as a useful prototype for other viruses thought to utilize a premature termination mechanism for sg mRNA transcription (7, 14, 27, 35, 39, 41, 48).
FIG. 10.
A refined PT model for sg mRNA transcription in TBSV. The model presented (not to scale) is generic and incorporates RNA elements involved in sg mRNA1 and/or sg mRNA2 transcription. A list of proposed functions for the color-coded RNA elements in the schema is provided. See the text for details.
Supplementary Material
Acknowledgments
We thank members of our laboratory for reviewing the manuscript.
This work was supported by the NSERC, PREA, and CRC.
Footnotes
Published ahead of print on 13 December 2006.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Buck, K. W. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47:159-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi, I. R., M. Ostrovsky, G. Zhang, and K. A. White. 2001. Regulatory activity of distal and core RNA elements in tombusvirus subgenomic mRNA2 transcription. J. Biol. Chem. 276:41761-41768. [DOI] [PubMed] [Google Scholar]
- 3.Choi, I. R., and K. A. White. 2002. An RNA activator of subgenomic mRNA1 transcription in tomato bushy stunt virus. J. Biol. Chem. 277:3760-3766. [DOI] [PubMed] [Google Scholar]
- 4.Fabian, M. R., H. Na, D. Ray, and K. A. White. 2003. 3′-terminal RNA secondary structures are important for accumulation of tomato bushy stunt virus DI RNAs. Virology 313:567-580. [DOI] [PubMed] [Google Scholar]
- 5.Fabian, M. R., and K. A. White. 2004. 5′-3′ RNA-RNA interaction facilitates cap- and poly(A) tail-independent translation of tomato bushy stunt virus mRNA: a potential common mechanism for Tombusviridae. J. Biol. Chem. 279:28862-28872. [DOI] [PubMed] [Google Scholar]
- 6.Fabian, M. R., and K. A. White. 2006. Analysis of a 3′-translation enhancer in a tombusvirus: a dynamic model for RNA-RNA interactions of mRNA termini. RNA 12:1304-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowda, S., T. Satyanarayana, M. A. Ayllon, M. R. Albiach-Marti, M. Mawassi, S. Rabindran, S. M. Garnsey, and W. O. Dawson. 2001. Characterization of the cis-acting elements controlling subgenomic mRNAs of Citrus tristeza virus: production of positive- and negative-stranded 3′-terminal and positive-stranded 5′-terminal RNAs. Virology 286:134-151. [DOI] [PubMed] [Google Scholar]
- 8.Hearne, P. Q., D. A. Knorr, B. I. Hillman, and T. J. Morris. 1990. The complete genome structure and synthesis of infectious RNA from clones of tomato bushy stunt virus. Virology 177:141-151. [DOI] [PubMed] [Google Scholar]
- 9.Hema, M., and C. C. Kao. 2004. Template sequence near the initiation nucleotide can modulate brome mosaic virus RNA accumulation in plant protoplasts. J. Virol. 78:1169-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain, S., J. Pan, Y. Chen, Y. Yang, J. Xu, Y. Peng, Y. Wu, Z. Li, Y. Zhu, P. Tien, and D. Guo. 2005. Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J. Virol. 79:5288-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston, J. C., and D. M. Rochon. 1995. Deletion analysis of the promoter for the cucumber necrosis virus 0.9-kb subgenomic RNA. Virology 214:100-109. [DOI] [PubMed] [Google Scholar]
- 12.Kim, C. H., C. C. Kao, and I. Tinoco, Jr. 2000. RNA motifs that determine specificity between a viral replicase and its promoter. Nat. Struct. Biol. 7:415-423. [DOI] [PubMed] [Google Scholar]
- 13.Lin, H.-X., and K. A. White. 2004. A complex network of RNA-RNA interactions controls subgenomic mRNA transcription in a tombusvirus. EMBO J. 23:3365-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindenbach, B. D., J. Y. Sgro, and P. Ahlquist. 2002. Long-distance base pairing in flock house virus RNA1 regulates subgenomic RNA3 synthesis and RNA2 replication. J. Virol. 76:3905-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 16.Miller, W. A., T. W. Dreher, and T. C. Hall. 1985. Synthesis of brome mosaic virus subgenomic RNA in vitro by internal initiation on (−)-sense genome RNA. Nature 313:68-70. [DOI] [PubMed] [Google Scholar]
- 17.Miller, W. A., and G. Koev. 2000. Synthesis of subgenomic RNAs by positive-strand RNA viruses. Virology 273:1-8. [DOI] [PubMed] [Google Scholar]
- 18.Na, H., M. R. Fabian, and K. A. White. 2006. Conformational organization of the 3′ untranslated region in the tomato bushy stunt virus genome. RNA 12:2199-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Na, H., and K. A. White. 2006. Structure and prevalence of replication silencer-3′ terminus RNA interactions in Tombusviridae. Virology 345:305-316. [DOI] [PubMed] [Google Scholar]
- 20.Nagy, P. D., and J. Pogany. 2000. Partial purification and characterization of Cucumber necrosis virus and Tomato bushy stunt virus RNA-dependent RNA polymerases: similarities and differences in template usage between tombusvirus and carmovirus RNA-dependent RNA polymerases. Virology 276:279-288. [DOI] [PubMed] [Google Scholar]
- 21.Oster, S. K., B. Wu, and K. A. White. 1998. Uncoupled expression of p33 and p92 permits amplification of tomato bushy stunt virus RNAs. J. Virol. 72:5845-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panavas, T., J. Pogany, and P. D. Nagy. 2002. Analysis of minimal promoter sequences for plus-strand synthesis by the Cucumber necrosis virus RNA-dependent RNA polymerase. Virology 296:263-274. [DOI] [PubMed] [Google Scholar]
- 23.Panavas, T., J. Stork, and P. D. Nagy. 2006. Use of double-stranded templates by the tombusvirus replicase in vitro: implications for the mechanism of plus-strand initiation. Virology 352:110-120. [DOI] [PubMed] [Google Scholar]
- 24.Pasternak, A. O., W. J. Spaan, and E. J. Snijder. 2006. Nidovirus transcription: how to make sense? J. Gen. Virol. 87:1403-1421. [DOI] [PubMed] [Google Scholar]
- 25.Pasternak, A. O., E. van den Born, W. J. Spaan, and E. J. Snijder. 2001. Sequence requirements for RNA strand transfer during nidovirus discontinuous subgenomic RNA synthesis. EMBO J. 20:7220-7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogany, J., M. R. Fabian, K. A. White, and P. D. Nagy. 2003. A replication silencer element in a plus-strand RNA virus. EMBO J. 22:5602-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price, B. D., M. Roeder, and P. Ahlquist. 2000. DNA-directed expression of functional flock house virus RNA1 derivatives in Saccharomyces cerevisiae, heterologous gene expression, and selective effects on subgenomic mRNA synthesis. J. Virol. 74:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu, W., J. W. Park, and H. B. Scholthof. 2002. Tombusvirus P19-mediated suppression of virus-induced gene silencing is controlled by genetic and dosage features that influence pathogenicity. Mol. Plant-Microbe Interact. 15:269-280. [DOI] [PubMed] [Google Scholar]
- 29.Qu, F., and T. J. Morris. 2002. Efficient infection of Nicotiana benthamiana by Tomato bushy stunt virus is facilitated by the coat protein and maintained by p19 through suppression of gene silencing. Mol. Plant-Microbe Interact. 15:193-202. [DOI] [PubMed] [Google Scholar]
- 30.Sawicki, S. G., and D. L. Sawicki. 1998. A new model for coronavirus transcription. Adv. Exp. Med. Biol. 440:215-219. [DOI] [PubMed] [Google Scholar]
- 31.Scholthof, H. B., K. B. Scholthof, M. Kikkert, and A. O. Jackson. 1995. Tomato bushy stunt virus spread is regulated by two nested genes that function in cell-to-cell movement and host-dependent systemic invasion. Virology 213:425-438. [DOI] [PubMed] [Google Scholar]
- 32.Siegel, R. W., L. Bellon, L. Beigelman, and C. C. Kao. 1998. Moieties in an RNA promoter specifically recognized by a viral RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. USA 95:11613-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sit, T. L., A. A. Vaewhongs, and S. A. Lommel. 1998. RNA-mediated trans-activation of transcription from a viral RNA. Science 281:829-832. [DOI] [PubMed] [Google Scholar]
- 34.Sivakumaran, K., S. K. Choi, M. Hema, and C. C. Kao. 2004. Requirements for brome mosaic virus subgenomic RNA synthesis in vivo and replicase-core promoter interactions in vitro. J. Virol. 78:6091-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smits, S. L., A. L. W. van Vliet, K. Segeren, H. el Azzouzi, M. van Essen, and R. J. de Groot. 2005. Torovirus non-discontinuous transcription: mutational analysis of a subgenomic mRNA promoter. J. Virol. 79:8275-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sola, I., J. L. Moreno, S. Zuniga, S. Alonso, and L. Enjuanes. 2005. Role of nucleotides immediately flanking the transcription-regulating sequence core in coronavirus subgenomic mRNA synthesis. J. Virol. 79:2506-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatsuta, M., H. Mizumoto, M. Kaido, K. Mise, and T. Okuno. 2005. The Red clover necrotic mosaic virus RNA2 trans-activator is also a cis-acting RNA2 replication element. J. Virol. 79:978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Marle, G., J. C. Dobbe, A. P. Gultyaev, W. Luytjes, W. J. Spaan, and E. J. Snijder. 1999. Arterivirus discontinuous mRNA transcription is guided by base pairing between sense and antisense transcription-regulating sequences. Proc. Natl. Acad. Sci. USA 96:12056-12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Vliet, A. L., S. L. Smits, P. J. Rottier, and R. J. de Groot. 2002. Discontinuous and non-discontinuous subgenomic RNA transcription in a nidovirus. EMBO J. 21:6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White, K. A. 1996. Formation and evolution of tombusvirus defective interfering RNAs. Semin. Virol. 7:409-416. [Google Scholar]
- 41.White, K. A. 2002. The premature termination model: a possible third mechanism for subgenomic mRNA transcription in (+)-strand RNA viruses. Virology 304:147-154. [DOI] [PubMed] [Google Scholar]
- 42.White, K. A., and T. J. Morris. 1994. Nonhomologous RNA recombination in tombusviruses: generation and evolution of defective interfering RNAs by stepwise deletions. J. Virol. 68:14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, K. A., and P. D. Nagy. 2004. Advances in the molecular biology of tombusviruses: gene expression, genome replication and recombination. Prog. Nucleic Acids Res. Mol. Biol. 78:188-226. [DOI] [PubMed] [Google Scholar]
- 44.Wu, B., and K. A. White. 1999. A primary determinant of cap-independent translation is located in the 3′-proximal region of the tomato bushy stunt virus genome. J. Virol. 73:8982-8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyatt, J. R., J. D. Puglisi, and I. Tinoco, Jr. 1989. RNA folding: pseudoknots, loops and bulges. Bioessays 11:100-106. [DOI] [PubMed] [Google Scholar]
- 46.Xu, W. 2006. Role of RNA secondary structures in tombusvirus subgenomic mRNA transcription. M.S. thesis. York University, Toronto, Canada.
- 47.Zhang, G., V. Slowinski, and K. A. White. 1999. Subgenomic mRNA regulation by a distal RNA element in a (+)-strand RNA virus. RNA 5:550-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong, W., and R. R. Rueckert. 1993. Flock house virus: down-regulation of subgenomic RNA3 synthesis does not involve coat protein and is targeted to synthesis of its positive strand. J. Virol. 67:2716-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuker, M., D. H. Mathews, and D. H. Turner. 1999. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide, p. 11-43. In J. Barciszewski and B. F. C. Clark (ed.), RNA biochemistry and biotechnology. NATO ASI Series. Kluwer Academic Publishers, Berlin, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.