Abstract
Prion diseases are transmissible neurodegenerative diseases caused by a conformational isoform of the prion protein (PrP), a host-encoded cell surface sialoglycoprotein. Recent evidence suggests a cytosolic fraction of PrP (cyPrP) functions either as an initiating factor or toxic element of prion disease. When expressed in cultured cells, cyPrP acquires properties of the infectious conformation of PrP (PrPSc), including insolubility, protease resistance, aggregation, and toxicity. Transgenic mice (2D1 and 1D4 lines) that coexpress cyPrP and PrPC exhibit focal cerebellar atrophy, scratching behavior, and gait abnormalities suggestive of prion disease, although they lack protease-resistant PrP. To determine if the coexpression of PrPC is necessary or inhibitory to the phenotype of these mice, we crossed Tg1D4(Prnp+/+) mice with PrP-ablated mice (TgPrnpo/o) to generate Tg1D4(Prnpo/o) mice and followed the development of disease and pathological phenotype. We found no difference in the onset of symptoms or the clinical or pathological phenotype of disease between Tg1D4(Prnp+/+) and Tg1D4(Prnpo/o) mice, suggesting that cyPrP and PrPC function independently in the disease state. Additionally, Tg1D4(Prnpo/o) mice were resistant to challenge with mouse-adapted scrapie (RML), suggesting cyPrP is inaccessible to PrPSc. We conclude that disease phenotype and cellular toxicity associated with the expression of cyPrP are independent of PrPC and the generation of typical prion disease.
Prion diseases are fatal, neurodegenerative disorders possessing a unique transmissibility via the “prion,” a proteinaceous infectious agent. The only known component of the prion is a conformational variant (PrPSc) of host-encoded prion protein (PrPC) (29). PrPSc replicates by templating its conformation onto PrPC, a reaction that requires that only the two isoforms be present (17) but which may be more efficient in the presence of other host factors (8, 11, 12). PrPC is a ubiquitously expressed sialoglycoprotein normally attached to the outer leaflet of the plasma membrane via a glycosylphosphatidyl-inositol (GPI) anchor. Although the primary function of PrPC remains obscure, evidence suggests roles in copper regulation (4, 28), antioxidant activity (3), and intracellular signaling (32). During normal expression, PrPC is translocated into the endoplasmic reticulum (ER) lumen, where it undergoes several posttranslational modifications, including the addition of the GPI anchor, disulfide bond formation, and core glycosylation at two asparagines, before it passes to the Golgi apparatus for further sugar modification and sialation en route to the plasmalemma (7).
Recently, additional forms of PrP have been recognized, including two transmembrane forms (15) and a cytosolic form (25). Cytosolic PrP (cyPrP) has been detected in experimental cell culture systems (21, 31, 35), as well as endogenously in mice by immunohistochemistry (25, 34) and following proteasome inhibition in cultured cells (22, 31). Several lines of evidence suggest that the presence of PrP in the cytoplasm is linked to prion disease. When expressed in the cytosol of Saccharomyces cerevisiae, PrP develops PrPSc-like properties, including insolubility and a relative resistance to proteinase K (PK) (21, 27). It has also been reported that misfolded PrP in the ER is subjected to ER quality control, leading to its retrotranslocation and accumulation within the cytosol, as evidenced under conditions of proteasome inhibition (23), although whether it is poor translocation into the ER rather than active retrotranslocation is currently under debate (13, 31, 34). The accumulation of PrP within the cytosol is proposed to either initiate prion disease or act as a toxic element of disease. Both concepts are supported by the transgenic (Tg) 2D1 and 1D4 mouse lines, which express cyPrP and develop focal cerebellar atrophy and a unique phenotype (23). However, these mice lack spongiform change and PK-resistant PrP in the brain. The latter feature does not exclude this as a prion disease, as there are clear examples of human forms of prion disease that do not develop obvious PK-resistant PrP (e.g., Gerstmann-Sträussler-Scheinker disease) and others that display little to no spongiform change (e.g., fatal insomnia). As such, the role of cyPrP in prion disease requires further study. We asked two specific questions regarding the nature of cyPrP: (i) is the toxicity associated with its expression dependent on the coexpression of PrPC, and (ii) does it participate directly in the generation of PrPSc? Our findings suggest that the toxicity associated with cyPrP expression is independent of PrPC expression, and cyPrP alone is not sufficient to support the propagation of PrPSc in vivo.
MATERIALS AND METHODS
Mouse lines.
The Tg1D4(Prnp+/+) line was kindly provided by Susan Lindquist (Whitehead Institute, Cambridge, MA) and is described in detail elsewhere (23). Briefly, this line was constructed on a C57BL/6 background using the MoPrP.Xho expression vector (ATCC) containing the PrP23-230 construct, which lacks the N-terminal ER entry signal sequence (residues 1 to 22) and the C-terminal GPI anchor consensus sequence (residues 231 to 254). Tg(Prnpo/o) mice were kindly provided by Stanley Prusiner (UCSF, San Francisco, CA) and have been described in detail elsewhere (5). This line develops normally and has been repeatedly shown to be resistant to prion challenge (5, 30). Tg1D4(Prnpo/o) mice were prepared by crossing Tg1D4(Prnp+/+) with Tg(Prnpo/o) mice for more than 10 generations prior to study. To screen for the 1D4 transgene by PCR on genomic DNA isolated from weanling tail clips, the primer pair was 5′-AACCAAGTGTACTACAGGCCA-3′ and 5′-ATTGAAAGAGCTACAGGTGGA-3′, which anneal to an internal coding segment of PrP and the 3′ untranslated region of the MoPrP.Xho vector used to construct the mice. To screen for the Prnpo/o transgene, the primers 5′-TCAGCCTAAATACTGGCAC-3′, 5′-GCCTAGACCACGAGAAATGC-3′, and 5′-GCATCAGCCATGATGGATAC-3′ were used to produce an amplicon of 730 bp from a homozygous knockout mouse or an 880-bp amplicon from a wild-type mouse. Screening for both transgenes was performed to confirm the Tg1D4(Prnpo/o) line. Mice were euthanized by CO2 asphyxiation, and their brains were harvested and prepared for either Western blotting or histological analysis as described below.
Inoculations.
All inoculations were performed via the intracerebral route in 45- to 60-day-old mice, as previously described (24). Inocula consisted of 30 μl of either brain homogenates prepared as a 2.5% (wt/vol) solution in sterile phosphate-buffered saline (PBS) or of cultured cells homogenized in PBS, as described in Results. Brain homogenates included the Rocky Mountain Laboratories (RML) strain of mouse-adapted scrapie and that of symptomatic Tg2D1 mice that express cyPrP and develop disease at 4 to 6 weeks of age (23). For cyPrP generated in cultured cells, N2a cells were transiently transfected with the pCB6+ mammalian expression vector carrying the PrP23-230 cDNA. Transfections were performed using Fugene 6 reagent (Roche Diagnostics) according to the manufacturer's instructions. All mice were monitored for evidence of a phenotype consistent with either typical scrapie (ataxia, scruffy coat, plastic tail, hunched posture, etc.) or that associated with cyPrP-expressing mice (dermatitis, fur loss, decreased spontaneous movement, or ataxia). Symptomatic mice were sacrificed and the brains harvested when death was imminent. Nontransgenic mice inoculated with cyPrP were sacrificed at >800 days to ensure the best chance for disease manifestation of low prion titers. Half of the brain samples were flash-frozen and stored at −80°C until Western blot analysis was performed, while the other half was collected following whole animal perfusion with 4% paraformaldehyde and stored in PBS with 0.1% sodium azide at 4°C for histological analysis.
Proteolysis and immunoblot analysis.
For Western blotting, 10% (wt/vol) brain homogenates were prepared in sterile PBS and diluted 1:2 in 2% N-lauroyl-sarcosine in TNE buffer (10 mM Tris-Cl, 1 mM EDTA, 200 mM NaCl, pH 7.4). To detect proteinase K-resistant PrP, 40 μl of homogenate was incubated with 20 μg/ml PK for 1 h at 37°C and stopped with 2 mM phenylmethylsulfonyl fluoride. For the solubility assay, 10% brain homogenates were prepared in lysis buffer and centrifuged at 4°C for 1 h at 100,000 × g, using a TLA 100 rotor in a Sorvall ultracentrifuge. Equal proportions of supernatant and pellet fractions were subjected to Western blotting. Membranes were probed with D13 antibody (InPro) at 0.1 μg/ml. Molecular weight markers are Precision Plus protein standards (Bio-Rad). Signal was detected on a Bio-Rad ChemiDoc XRS imager, using SuperSignal West Pico chemiluminescent substrate (Pierce). For dot blot analysis, equal volumes of 1% brain homogenates were serially diluted in TNE buffer containing 2% N-lauroyl-sarcosine and passed through nitrocellulose membrane by vacuum before immunoblotting. Quantitation was performed using Quantity One software (Bio-Rad) by plotting density measurements against the dilution factor in the linear range of signal.
Histological analysis.
Mice were asphyxiated with CO2 and perfused via cardiac puncture with 20 ml PBS and then 20 ml 4% paraformaldehyde. Brains were stored in 4% paraformaldehyde for 1 week and then transferred to PBS containing 0.1% sodium azide until paraffin or epon embedding. The blocks were cut with a microtome into 5-μm sections and stained with hematoxylin and eosin or with 1% thioflavin S for detection of amyloid deposits. For the evaluation of astrocytosis, 5-μm paraffin sections were stained with a rabbit antiserum against glial fibrillary acidic protein (DAKO) using an automated tissue stainer (Ventana) following antigen retrieval in a citrate buffer at pH 6 for 40 min at 98°F.
RESULTS
Tg1D4(Prnpo/o) mouse line.
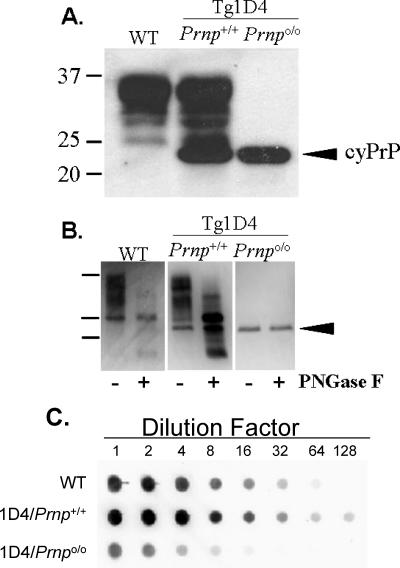
The normal cellular isoform of prion protein (PrPC) can be distinguished from cyPrP by its relative migration rate (Mr) and profile on Western blotting. PrPC appears as three major bands ranging from ∼25 to 35 kDa, representing unglycosylated and variably glycosylated PrP, whereas cyPrP, which lacks the N-terminal signal peptide and C-terminal GPI anchor signal sequence, runs as a single fraction with an molecular size of ∼23 kDa. Compared with normal mice, Tg1D4(Prnp+/+) mice display an additional immunoreactive band with an molecular size of ∼23 kDa, and this same band is detected in isolation in Tg1D4(Prnpo/o) mice, confirming both homozygous knockout of PrPC and expression of cyPrP (Fig. 1A). In addition, the relative intensity of the cyPrP band was comparable between Tg1D4(Prnp+/+) and Tg1D4(Prnpo/o) brain homogenates, suggesting that the transgene array remained intact during cross-breeding. Treatment with peptide N-glycosidase F (PNGase F), to remove asparagine-linked carbohydrates, confirmed that cyPrP is not glycosylated (Fig. 1B). This treatment also allowed us to make a rough densitometric comparison of the relative levels of cyPrP and total PrPC in the same brain, which was calculated as 20%. In addition, as a more sensitive assessment of relative concentration, we compared the PrP signals of serially diluted brain homogenates prepared from non-Tg, Tg1D4(Prnp+/+), and Tg1D4(Prnpo/o) mice, which confirmed that cyPrP levels are roughly 20% of normal levels of PrPC in non-Tg mice (Fig. 1C). Notably, this comparison shows that the PrP levels detected in Tg1D4(Prnpo/o) mice reflect the difference between Tg1D4(Prnp+/+) and non-Tg mice.
FIG. 1.
PrP characteristics in 1D4 mouse lines. A. Patterns of expression of PrPC and cyPrP from non-Tg (wild-type [WT]), Tg1D4(Prnp+/+), and Tg1D4(Prnpo/o) mice, detected by mouse-specific anti-PrP D13 antibody. Each lane represents ∼10 μg of total protein prepared from the brain of a representative mouse from each line. PrPC ranges from 25 to 35 kDa (lanes 1 and 2), representing the typical pattern of unglycosylated and glycosylated fractions, whereas cyPrP, because of the absence of the GPI anchor, migrates slightly faster, as a single ∼23-kDa band (lanes 2 and 3). This blot was overexposed to emphasize the banding patterns and may overestimate the level of cyPrP present. B. PrP from each mouse line was treated with PNGase F to confirm that cyPrP is not glycosylated. This treatment also allows a crude comparison of the relative levels of cyPrP and PrPC within the same brain of a Tg1D4(Prnp+/+) mouse. Densitometry estimated cyPrP to represent at ∼20 to 25% of PrPC. C. Serial dilutions of 1% brain homogenates prepared in lysis buffer from each mouse line were performed to more accurately assess the relative levels of PrPC and cyPrP. The image was processed on a Bio-Rad XRS document imager, and Quantity One (Bio-Rad) software was used to calculate the relative density against the dilution factor. This confirmed the expression level of cyPrP to be ∼20% that of wild-type mice. The higher total level of PrP in Tg1D4(Prnp+/+) mice reflects the combination of cyPrP and endogenous PrPC.
Phenotypes of Tg1D4 mouse lines.
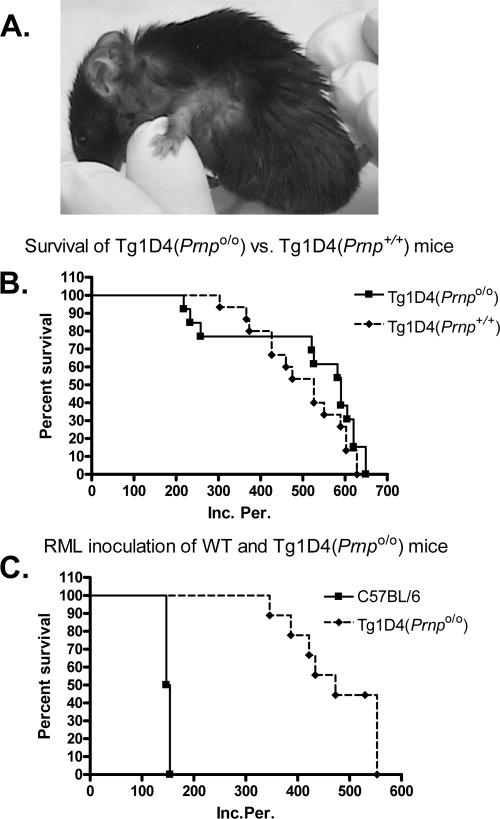
The most prominent and early feature we observed in Tg1D4(Prnp+/+) mice was scratching behavior (pruritus), particularly around the neck and forelimbs (Fig. 2A), which began anywhere from 233 to 649 days of age (average 512 ± 45 days [mean ± standard error]; n = 13). The scratching was so intense in some mice that inflammation and infection developed in many, making it difficult to maintain them for extended periods. Thinning of the coat generally followed. Overt ataxia was not routinely observed. We considered this a possible result of culling the animals prior to the end of their natural life span, however; many survived well past 500 days, far beyond the time at which obvious ataxia is reported to develop in this line (23). In the initial report on Tg1D4(Prnp+/+) mice, ataxia was observed in ∼15% of mice. We observed clinically apparent ataxia in 1 of 13 mice (7.7%) but in 1 of 8 (12%) that survived beyond 500 days, which compares roughly with the described phenotype.
FIG. 2.
Tg1D4(Prnpo/o) mice develop spontaneous disease but not scrapie. A. The earliest symptom in cyPrP-expressing mice was scratching behavior (pruritis). Note the loss of fur around the neck and forelimb as a result of persistent scratching. B. Fifteen Tg1D4(Prnpo/o) mice and 13 Tg1D4(Prnp+/+) mice were monitored for signs of spontaneous disease, and results were plotted as Kaplan-Meier survival curves. Survival was not significantly different from that of Tg1D4(Prnp+/+) mice (χ2 = 1.47; P = 0.24). C. Six non-Tg and nine Tg1D4(Prnpo/o) mice were monitored for signs of scrapie and 1D4 phenotype following intracerebral inoculation of 2.5% RML isolate of mouse-passaged scrapie, and results were plotted as survival curves. None of the Tg1D4(Prnpo/o) mice developed signs of scrapie, but they did develop signs typical of the 1D4 phenotype.
Tg1D4(Prnpo/o) mice were phenotypically indistinguishable from the parental Tg1D4(Prnp+/+) mouse line. They developed pruritus with a similar variability of onset that was not significantly different from that of the Tg1D4(Prnp+/+) mice (498 ± 27 days versus 512 ± 14 days) (Fig. 2B and Table 1). The presence of gait difficulty was also similar in frequency and late in onset in these mice. However, 10 of 13 animals set up for long-term observation survived beyond 500 days, and only 2 of these animals displayed reduced spontaneous mobility and associated ataxia. In addition to the clinical phenotype, the histologic picture at time of death was similar between Tg1D4(Prnp+/+) and Tg1D4(Prnpo/o) mice; the degree of focal cerebellar granule cell layer atrophy, the principle pathological feature of cyPrP expression, was similar in the two lines, as was astrogliosis, as defined by glial fibrillary acidic protein (GFAP) staining (see Fig. 4). No other pathological features were evident. These results suggest cyPrP toxicity is independent of PrPC expression.
TABLE 1.
Time to disease for all experiments
| Mouse line | Inoculum | Ns/Nta | Phenotype | Incubation period (days) ± SE |
|---|---|---|---|---|
| Tg1D4(Prnpo/o) | 13/13 | Scratching, some ataxia | 512 ± 14 | |
| Tg1D4(Prnp+/+) | 15/15 | Scratching, some ataxia | 498 ± 27 | |
| Non-Tg | RML | 6/6 | Severe ataxia, hunched | 151 ± 1.6 |
| Tg1D4(Prnpo/o) | RML | 0/9 | Scratching, some ataxia | Not sickd |
| Non-Tg | BHb (Tg2D1) | 0/13 | Normal | Not sick, >800 |
| Non-Tg | cyPrP (N2a) | 0/6 | Normal | Not sick, >800 |
| Non-Tg | cyPrP-PI (N2a) | 0/6 | Normal | Not sick, >800 |
| Non-Tg | Mock (N2a) | 0/5 | Normal | Not sick, >800 |
| Non-Tg | RMLc | 5/5 | Severe ataxia, hunched | 167 ± 1.0 |
Ns/Nt, number sick/number tested.
BH, brain homogenate.
RML inoculum prepared separately.
No symptoms of typical mouse scrapie.
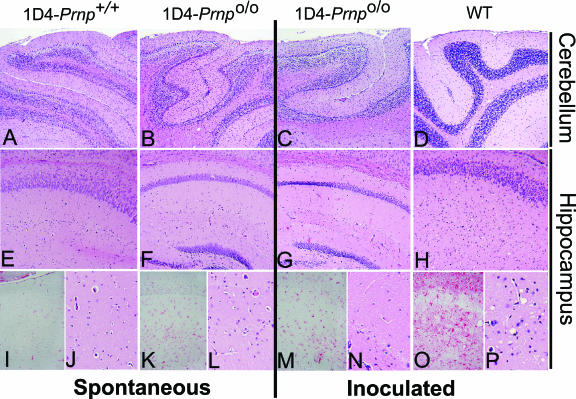
FIG. 4.
Pathological features of normal and Tg mice. Representative histological sections from the cerebellum (A to D) and hippocampus (E to P) of each experimental group, at low (A to H) and high (I to P) (×4) magnification, stained with hematoxylin and eosin (all except images in panels I, K, M, and O) to assess spongiform change or with anti-GFAP (I, K, M, and O) to assess gliosis within the hippocampus. Cerebellar granule cell layer atrophy was associated with cyPrP expression in both Tg1D4(Prnp+/+) and Tg1D4(Prnpo/o) mice, compared with age-matched wild-type (WT) mice. No vacuolation was evident in these mice at any level of magnification. When challenged with RML, Tg1D4(Prnpo/o) mice >500 days old did not show signs of vacuolation or gliosis, compared with the extensive vacuolation and gliosis present in 150-day-old WT mice that were symptomatic with clinical (ataxia, rough coat, hunched posture, and plastic tail) and biochemical (PK-resistant PrP) evidence of scrapie.
cyPrP as a substrate for prion conversion.
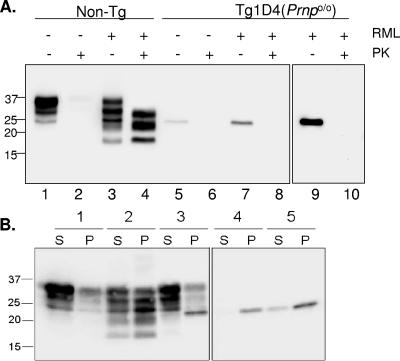
To address whether cyPrP is involved in the propagation of prions, we challenged Tg1D4(Prnpo/o) mice with an intracerebral inoculation of the RML isolate of scrapie. Whereas all six non-Tg control mice inoculated with RML developed stereotypical signs of scrapie at 151 ± 1.6 days, none of the Tg1D4(Prnpo/o) mice developed such symptoms, despite survival of some beyond 500 days of incubation (Fig. 2C and Table 1). Animals developed scratching, loss of coat and, in some, reduced mobility that was indistinguishable from uninoculated Tg1D4(Prnpo/o) mice but clearly distinct from the typical scrapie phenotype. A consistent feature of scrapie in mice inoculated with RML is the accumulation of PK-resistant PrPSc within the brain (6). We sought to determine if PK-sensitive cyPrP is converted to PK-resistant cyPrPSc in vivo when challenged with prions, despite no evidence of disease in RML-inoculated Tg1D4(Prnpo/o) mice. Brain homogenates prepared from prion-inoculated non-Tg and Tg1D4(Prnpo/o) mice were digested with 20 μg/ml PK and assayed by Western blotting. As expected, non-Tg mice inoculated with RML displayed high levels of PK-resistant PrPSc (Fig. 3A, lanes 1 to 4). Additionally, when compared to uninoculated mice, proteolytic fragments with a lower Mr are evident, reflecting the accumulation of PrPSc (1). In contrast, no PK-resistant PrP was detected in Tg1D4(Prnpo/o) mice inoculated with RML (Fig. 3A, lanes 5 to 8). As a further check for cyPrPSc, we determined if the solubility characteristics of cyPrP obtained from Tg1D4(Prnpo/o) mice following RML inoculation differed from uninoculated mice. Figure 3B demonstrates that while the major fraction of PrP in non-Tg mice is soluble, PrP from scrapie-infected non-Tg mice is predominantly insoluble. In both Tg1D4(Prnp+/+) and Tg1D4(Prnpo/o) mice, the cyPrP band at ∼23 kDa partitioned almost entirely to the insoluble fraction, and this fraction was not altered in mice inoculated with RML. Thus, cyPrP is not a substrate for conversion to PrPSc when challenged with RML prions in vivo.
FIG. 3.
cyPrP does not produce PK-resistant PrP. A. Western blot of brain homogenates from non-Tg and Tg1D4(Prnpo/o) mice inoculated with RML or vehicle and submitted to PK digestion. Non-Tg mice (lanes 1 to 4) were symptomatic with typical scrapie-like symptoms and died at ∼150 days, whereas the Tg1D4(Prnpo/o) mice (lanes 5 to 10) were killed at ∼500 days, following symptoms typical of the cyPrP phenotype. Brain homogenates were digested with 20 μg/ml PK, as described in the text. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed with a 14% acrylamide gel, and membranes were probed with the D13 antibody. PK-resistant PrP was evident in non-Tg homogenates, but not in Tg1D4(Prnpo/o) homogenates, even when the blot was significantly overexposed (last panel). B. Solubility characteristics of PrP from brains of mice expressing cyPrP. Supernatant (S) and pellet (P) fractions from brain homogenates of non-Tg (samples 1 and 2) and Tg1D4(Prnpo/o) (samples 4 and 5) mice in control (samples 1 and 4) and RML-inoculated (samples 2 and 5) mice were compared. The solubility profile of PrP in Tg1D4(Prnp+/+) mice is presented in sample 3. Note that cyPrP partitions nearly completely to the insoluble fraction, whether expressed in the presence or absence of PrPC. Samples 4 and 5 were run on the same gel but separated from the remainder of the samples.
Pathological features of cyPrP and prion infection.
Brain sections from several mice within each group were assessed for pathological features of prion disease or alteration of underlying cyPrP-related pathology. Tissues were examined for vacuolation (spongiform change), gliosis, and amyloid deposition, using hematoxylin and eosin staining, immunohistochemistry with anti-GFAP antibody, and thioflavin S staining, respectively. Representative sections of the forebrain, at the level of the hippocampus and cerebellum, are presented in Fig. 4. As described previously by Ma et al. (23), atrophy of the granule cell layer of the cerebellum is the primary pathological feature of Tg1D4(Prnp+/+) mice, and we consistently observed this in all adult Tg1D4(Prnp+/+) mice. We found no obvious differences in the location and degree of pathology in Tg1D4(Prnpo/o) compared with Tg1D4(Prnp+/+) mice (Fig. 4). In addition, there was a comparable level of gliosis evident in the cerebellum (not shown), yet a minimal level in the forebrain, between the two lines. There was no evidence of spongiform change in either line, even at more than 500 days of age, when the cyPrP phenotype was evident. Mice that displayed a reduction in mobility were included in the histologic assessment and found to have the same pathological profile as those that showed only the scratching behavior.
In non-Tg mice (Fig. 4) inoculated with RML, profound spongiform change and gliosis was evident throughout the brain at the time of sacrifice. In stark contrast, after more than 500 days of observation following RML inoculation, Tg1D4(Prnpo/o) mice did not exhibit spongiform change. In addition, none of the mice developed amyloid deposits, as defined by thioflavin S staining (not shown).
cyPrP and transmissibility.
Although cyPrP does not appear to be converted to PK-resistant PrP, it is insoluble, and it is well known that prion transmission can occur in the absence of detectable PK-resistant PrP (18). We, therefore, attempted to transmit disease using cyPrP generated from two sources: cultured cells and whole animals. Mouse neuroblastoma (N2a) cells were transiently transfected to express PrP23-231, which lacks the ER entry signal sequence and GPI anchor signal (i.e., cyPrP), in the absence and presence of 5 μM of the proteasome inhibitor MG132 for 24 h. At the end of the incubation period, 1 × 108 cells were detergent lysed and centrifuged at 16,000 × g to pellet insoluble cyPrP. The pellet was resuspended in sterile PBS, and 30 μl was inoculated intracerebrally as a crude extract into non-Tg (C57BL/6) mice. A third inoculum was prepared as a 10% (wt/vol) brain homogenate in sterile PBS from a 7-week-old symptomatic Tg2D1 mouse. This line is a derivative of the Tg1D4(Prnp+/+) line that expresses 1.2 times more cyPrP and develops a disease phenotype by 4 weeks of age (23). Thirteen non-Tg mice were inoculated with Tg2D1 brain homogenate, and six mice each were inoculated with N2a-derived cyPrP expressed in the absence or presence of the proteasome inhibitor. After more than 800 days of observation, none of the mice developed symptoms of prion disease (Table 1), nor was PK-resistant PrP detected in brain homogenate of sacrificed animals (not shown). To ensure that mice challenged with cyPrP inocula did not carry PrPSc at levels that were undetectable by Western blotting, we repassaged brain from one of the mice inoculated with Tg2D1 brain homogenate into six additional non-Tg mice. After ∼475 days, the mice were still asymptomatic.
DISCUSSION
Much contrasting evidence has been generated regarding the possible role of cyPrP in prion disease. Different reports have conferred upon it dissimilar properties of protection or toxicity, and these properties seem to depend on cell type and expression level. For instance, Tg1D4 and Tg2D1 mice that express cyPrP on a wild-type PrP background display thinning of the cerebellar granule cell layer in association with signs of ataxia and pruritus (23), and expression of PrP into the cytosol of mouse neuroblastoma (N2a) cells led to apoptotic cell death (20). In addition, cyPrP recovered from cultured mammalian cells or yeast attains some properties of PrPSc, namely, aggregation, insolubility, and protease resistance (20, 27). Conversely, cyPrP accumulation by proteasome inhibition or injection of cDNA into the cytosol protected human primary neurons against Bax-mediated cell death (31), and PrP carrying disease-linked familial mutations was also nontoxic to N2a cells following their accumulation in the cytosol (13). Thus, given the diversity of reported effects of cyPrP, we sought to assess the role of cyPrP in prion disease in vivo.
cyPrP toxicity is independent of PrPC.
Tg1D4(Prnpo/o) mice, lacking background expression of PrPC, displayed spontaneous disease with similar characteristics as Tg1D4(Prnp+/+) mice. Since disease features were neither mitigated nor worsened by eliminating endogenous PrPC from Tg1D4(Prnp+/+) mice, the toxicity of cyPrP appears to occur by a mechanism that does not require the participation of PrPC. This differs from other prion-associated toxic agents requiring PrPC expression for toxicity, such as the toxic PrP106-126 peptide (2). Moreover, in contrast to the toxicity associated with the PrP partial homologue doppel, which is neurotoxic only in the absence of PrP (26), PrPC expression does not rescue cyPrP toxicity.
cyPrP is not a substrate for prion propagation.
Mice that express cyPrP in the absence of PrPC were not susceptible to prion challenge. It is well known that the level of PrPC is inversely correlated with the incubation period, which raises the possibility that the low level of cyPrP could result in a prolongation of the incubation period beyond the typical period of ∼150 days. However, we found no evidence of scrapie-like clinical features in mice observed beyond 500 days, more than three times the normal incubation period. To ensure that we did not miss subclinical disease, the brains from all mice were examined histologically for evidence of spongiform change and/or plaque deposition, but none was evident. This resistance to prions, despite the presence of PrP within the brain, provides important clues to the nature of prion replication. First, cyPrP is insufficient to propagate prions in vivo. This may result from two possibilities: (i) PrPSc and cyPrP fractions reside in separate cellular compartments that do not meet, or (ii) cyPrP is unable to attain the PrPSc conformation. Several reports suggest that cyPrP, which is unglycosylated and does not contain the C-terminal disulfide bond, is capable of being converted to PrPSc (20, 27, 33). For instance, recombinant truncated PrP (PrP89-230) can attain infectivity when bioassayed in a mouse that overexpresses PrP89-230 containing the GPI anchor (19), and mice that express extracellular PrP lacking the GPI anchor, which is primarily unglycosylated, can replicate PrPSc in vivo only in the presence of PrPC coexpression (10). Thus, the resistance of Tg1D4(Prnpo/o) mice to prions is likely due to the absence of cell surface expression of PrPC.
Early studies supported anchoring of PrPC to the plasmalemma as a necessary feature for susceptibility to prion infection (9). More recent work shows PrPSc remains extracellular during replication, either on the cell surface or within intracellular luminal compartments of the endocytic pathway (1, 10). For PrPSc to convert cyPrP, the inoculum would have to gain entry into the cytosol, but once converted and released from the apoptotic cell, entry to a neighboring cell might not be possible in the absence of surface-bound PrPC, thus preventing propagation of prions throughout the brain. This physical separation and independence of cyPrP and PrPC is further supported by the absence of a phenotypic difference between Tg1D4(Prnp+/+) and Tg1D4(Prnpo/o) mice.
It is interesting to contrast the PrP(GPI−)(Prnpo/o) and 1D4(Prnpo/o) transgenic mice, as the findings provided from these two lines complement each other. PrP(GPI−) includes the ER signal sequence that directs PrP into the ER during translation. However, in the absence of the GPI anchor, which links PrPC to the outer leaflet of the plasmalemma, PrP(GPI−) is secreted to the extracellular space, where it may self-associate in the form of PrP amyloid or, following the exogenous administration of PrPSc, be converted to PK-resistant PrP. Importantly, when expressed in the absence of PrPC, PrP(GPI−) is not toxic to neurons and does not produce a clinical phenotype, suggesting that extracellular deposits of misfolded PrP are not, on their own, damaging to neurons. However, when expressed in the presence of PrPC, PrP(GPI−) acquires toxicity, presumably by interacting with PrPC and entering the endocytic pathway, where intracellular PrPSc accumulates and inflicts neuronal damage. In Tg1D4(Prnpo/o) mice, because cyPrP also lacks the signal sequence for ER entry in addition to that for GPI anchor attachment, it is excluded from the secretory pathway and therefore may accumulate intracellularly and induce toxicity, independent of PrPC expression. But because of its intracellular location, it appears to be protected from exogenously administered PrPSc, which remains largely extracellular in the absence of cell surface PrPC.
cyPrP is not transmissible.
The finding that Tg1D4(Prnp+/+) mice develop isolated cerebellar pathology, rather than widespread brain disease, suggests a lack of cell-to-cell transmission of cyPrP and a separation of toxicity from transmissibility. Our inability to induce prion disease in mice by inoculation of cyPrP agrees with this assumption. After an extended period of observation beyond 800 days, we saw no clinical, pathological, or biochemical evidence of typical prion disease in non-Tg mice inoculated with cyPrP derived from symptomatic cyPrP-expressing mice or with lysates prepared from mammalian cells overexpressing cyPrP. While these results suggest cyPrP is not transmissible, a low level of infectivity that may require an incubation period longer than the normal life span of mice cannot be ruled out (16). To test for this, we repassaged brain from an inoculated mouse that survived 800 days to a group of six healthy mice, none of which developed disease after nearly 500 days. Additional attempts to use cyPrP to stimulate prion propagation in mice that overexpress PrPC to high levels, such as the tga20 mice (14), or mice that express PrP89-230 at 16 times the normal level and were susceptible to recombinant PrPSc (19) might be necessary to uncover an extremely low level of infectivity.
In summary, we conclude that cyPrP-related toxicity is independent of PrPC expression, and cyPrP does not contribute to PrPSc propagation in vivo. While this work does not rule out a role for cyPrP in the initiation of prion disease, it raises further questions regarding how cyPrP might participate in prion generation or development. Additional studies to assess a possible correlation of disease state with levels of cyPrP would be helpful, as would the induction of true transmissible prion disease in 1D4 mice, perhaps by further taxing the proteasome in vivo.
Acknowledgments
This work was supported by NIH grants RO1 NS046037 and R01 NS051480-01 and the Brain Research Foundation.
Footnotes
Published ahead of print on 20 December 2006.
REFERENCES
- 1.Borchelt, D. R., A. Taraboulos, and S. B. Prusiner. 1992. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J. Biol. Chem. 267:16188-16199. [PubMed] [Google Scholar]
- 2.Brown, D. R., J. Herms, and H. A. Kretzschmar. 1994. Mouse cortical cells lacking cellular PrP survive in culture with a neurotoxic PrP fragment. Neuroreport 5:2057-2060. [DOI] [PubMed] [Google Scholar]
- 3.Brown, D. R., W. J. Schulz-Schaeffer, B. Schmidt, and H. A. Kretzschmar. 1997. Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp. Neurol. 146:104-112. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P., and R. Bradley. 1998. 1755 and all that: a historical primer of transmissible spongiform encephalopathy. BMJ 317:1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 6.Bueler, H., A. Raeber, A. Sailer, M. Fischer, A. Aguzzi, and C. Weissmann. 1994. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol. Med. 1:19-30. [PMC free article] [PubMed] [Google Scholar]
- 7.Campana, V., D. Sarnataro, and C. Zurzolo. 2005. The highways and byways of prion protein trafficking. Trends Cell Biol. 15:102-111. [DOI] [PubMed] [Google Scholar]
- 8.Castilla, J., P. Saa, C. Hetz, and C. Soto. 2005. In vitro generation of infectious scrapie prions. Cell 121:195-206. [DOI] [PubMed] [Google Scholar]
- 9.Caughey, B., and G. J. Raymond. 1991. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 266:18217-18223. [PubMed] [Google Scholar]
- 10.Chesebro, B., M. Trifilo, R. Race, K. Meade-White, C. Teng, R. LaCasse, L. Raymond, C. Favara, G. Baron, S. Priola, B. Caughey, E. Masliah, and M. Oldstone. 2005. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308:1435-1439. [DOI] [PubMed] [Google Scholar]
- 11.DebBurman, S. K., G. J. Raymond, B. Caughey, and S. Lindquist. 1997. Chaperone-supervised conversion of prion protein to its protease-resistant form. Proc. Natl. Acad. Sci. USA 94:13938-13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deleault, N. R., R. W. Lucassen, and S. Supattapone. 2003. RNA molecules stimulate prion protein conversion. Nature 425:717-720. [DOI] [PubMed] [Google Scholar]
- 13.Fioriti, L., S. Dossena, L. R. Stewart, R. S. Stewart, D. A. Harris, G. Forloni, and R. Chiesa. 2005. Cytosolic prion protein (PrP) is not toxic in N2a cells and primary neurons expressing pathogenic PrP mutations. J. Biol. Chem. 280:11320-11328. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 15.Hegde, R. S., J. A. Mastrianni, M. R. Scott, K. A. DeFea, P. Tremblay, M. Torchia, S. J. DeArmond, S. B. Prusiner, and V. R. Lingappa. 1998. A transmembrane form of the prion protein in neurodegenerative disease. Science 279:827-834. [DOI] [PubMed] [Google Scholar]
- 16.Hill, A. F., and J. Collinge. 2003. Subclinical prion infection in humans and animals. Br. Med. Bull. 66:161-170. [DOI] [PubMed] [Google Scholar]
- 17.Kocisko, D. A., J. H. Come, S. A. Priola, B. Chesebro, G. J. Raymond, P. T. Lansbury, and B. Caughey. 1994. Cell-free formation of protease-resistant prion protein. Nature 370:471-474. [DOI] [PubMed] [Google Scholar]
- 18.Lasmezas, C. I., J. P. Deslys, O. Robain, A. Jaegly, V. Beringue, J. M. Peyrin, J. G. Fournier, J. J. Hauw, J. Rossier, and D. Dormont. 1997. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275:402-405. [DOI] [PubMed] [Google Scholar]
- 19.Legname, G., I. V. Baskakov, H. O. Nguyen, D. Riesner, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 2004. Synthetic mammalian prions. Science 305:673-676. [DOI] [PubMed] [Google Scholar]
- 20.Ma, J., and S. Lindquist. 2002. Conversion of PrP to a self-perpetuating PrPSc-like conformation in the cytosol. Science 298:1785-1788. [DOI] [PubMed] [Google Scholar]
- 21.Ma, J., and S. Lindquist. 1999. De novo generation of a PrPSc-like conformation in living cells. Nat. Cell Biol. 1:358-361. [DOI] [PubMed] [Google Scholar]
- 22.Ma, J., and S. Lindquist. 2001. Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. Proc. Natl. Acad. Sci. USA 98:14955-14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, J., R. Wollmann, and S. Lindquist. 2002. Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science 298:1781-1785. [DOI] [PubMed] [Google Scholar]
- 24.Mastrianni, J. A., S. Capellari, G. C. Telling, D. Han, P. Bosque, S. B. Prusiner, and S. J. DeArmond. 2001. Inherited prion disease caused by the V210I mutation: transmission to transgenic mice. Neurology 57:2198-2205. [DOI] [PubMed] [Google Scholar]
- 25.Mironov, A., Jr., D. Latawiec, H. Wille, E. Bouzamondo-Bernstein, G. Legname, R. A. Williamson, D. Burton, S. J. DeArmond, S. B. Prusiner, and P. J. Peters. 2003. Cytosolic prion protein in neurons. J. Neurosci. 23:7183-7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore, R. C., P. Mastrangelo, E. Bouzamondo, C. Heinrich, G. Legname, S. B. Prusiner, L. Hood, D. Westaway, S. J. DeArmond, and P. Tremblay. 2001. Doppel-induced cerebellar degeneration in transgenic mice. Proc. Natl. Acad. Sci. USA 98:15288-15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norstrom, E. M., and J. A. Mastrianni. 2005. The AGAAAAGA palindrome in PrP is required to generate a productive PrPSc-PrPC complex that leads to prion propagation. J. Biol. Chem. 280:27236-27243. [DOI] [PubMed] [Google Scholar]
- 28.Pauly, P. C., and D. A. Harris. 1998. Copper stimulates endocytosis of the prion protein. J. Biol. Chem. 273:33107-33110. [DOI] [PubMed] [Google Scholar]
- 29.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prusiner, S. B., D. Groth, A. Serban, R. Koehler, D. Foster, M. Torchia, D. Burton, S. L. Yang, and S. J. DeArmond. 1993. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc. Natl. Acad. Sci. USA 90:10608-10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roucou, X., Q. Guo, Y. Zhang, C. G. Goodyer, and A. C. LeBlanc. 2003. Cytosolic prion protein is not toxic and protects against Bax-mediated cell death in human primary neurons. J. Biol. Chem. 278:40877-40881. [DOI] [PubMed] [Google Scholar]
- 32.Schneider, B., V. Mutel, M. Pietri, M. Ermonval, S. Mouillet-Richard, and O. Kellermann. 2003. NADPH oxidase and extracellular regulated kinases 1/2 are targets of prion protein signaling in neuronal and nonneuronal cells. Proc. Natl. Acad. Sci. USA 100:13326-13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taraboulos, A., D. Serban, and S. B. Prusiner. 1990. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110:2117-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, X., F. Wang, M. S. Sy, and J. Ma. 2005. Calpain and other cytosolic proteases can contribute to the degradation of retro-translocated prion protein in the cytosol. J. Biol. Chem. 280:317-325. [DOI] [PubMed] [Google Scholar]
- 35.Yedidia, Y., L. Horonchik, S. Tzaban, A. Yanai, and A. Taraboulos. 2001. Proteasomes and ubiquitin are involved in the turnover of the wild-type prion protein. EMBO J. 20:5383-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]