Abstract
In previous studies, we have shown that two major respiratory pathogens, influenza virus and parainfluenza virus, produce acute alterations in ion transport upon contacting the apical membrane of the respiratory epithelium. In the present study, we examine the effects on ion transport by the mouse tracheal epithelium of a third major respiratory pathogen, respiratory syncytial virus (RSV). RSV infections are associated with fluid accumulation in the respiratory tract and cause illnesses that range in severity from rhinitis, sinusitis, otitis media, and bronchitis to bronchiolitis and pneumonia. We find that within minutes of RSV contacting the apical membrane; it inhibits amiloride-sensitive Na+ transport by the epithelium. This effect is mediated by protein kinase C and is reproduced by recombinant viral F (fusion) protein. Since this inhibition is not accompanied by any alteration in the epithelial responses to carbachol or to forskolin plus 3-isobutyl-1-methylxanthine (IBMX), it is not due to a nonspecific toxic action of the virus. The inhibition also appears to require Toll-like receptor 4 and the presence of asialogangliosides in the apical membrane. Since the concentration range over which this inhibition is observed (102 to 105 PFU/ml) is comparable to the viral concentrations observed in clinical and experimental RSV infections, it seems likely that direct inhibition by the virus of epithelial Na+ transport may contribute to the fluid accumulation that is observed in RSV infections.
The thickness of the fluid layer covering the respiratory surfaces is determined by the ion transport activity of the respiratory epithelium (5). In particular, it is regulated by the balance between the rate of Na+ absorption through epithelial Na+ channels and the rate of Cl− secretion through cystic fibrosis transmembrane conductance regulator (CFTR) and Ca2+-activated Cl− channels. This is well illustrated by the clearance of the lung fluid at birth, which is due to the activation of the epithelial Na+ channels in the respiratory epithelium (4). Similarly, the dehydration of the respiratory surface which is observed in cystic fibrosis appears to be due to the increased activity of the epithelial Na+ channels consequent on the loss of the normal inhibitory effects of CFTR (2, 29). On the other hand, inactivating mutations of the epithelial Na+ channels, as occur in pseudohypoaldosteronism type I, lead to fluid accumulation in the respiratory tract (19). A number of acquired conditions characterized by increased amounts of respiratory surface fluid have also been found to be associated with abnormal respiratory ion transport. These conditions include high-altitude pulmonary edema (32), hypoxic pulmonary edema (36), and neonatal respiratory distress syndrome (3).
Viral respiratory infections are associated with fluid accumulation in the respiratory tract which can range in severity from rhinitis, sinusitis, otitis media, or bronchitis to pneumonia. Given the role of ion transport by the respiratory epithelium in controlling the amount of respiratory surface fluid, this association suggested to us that respiratory viruses alter epithelial ion transport. When we tested this hypothesis for influenza virus, we found that the virus inhibited epithelial transport of Na+ (20). It did so as a consequence of the hemagglutinin in the viral coat binding to a neuraminidase-sensitive glycoprotein in the apical membrane of the epithelial cells. This, in turn, led to the activation of phospholipase C and protein kinase C. These effects were evident within minutes of the virus contacting the epithelium and did not require infection of the cells (8). Recently, Lan and coworkers extended these findings by showing that influenza virus inhibits the clearance of respiratory surface fluid by a mechanism mediated by phospholipase C, protein kinase C, and Src (26).
Subsequently, we examined the effects of another major respiratory pathogen, parainfluenza virus, on epithelial ion transport. We found that contact of the model parainfluenza virus, Sendai virus, with the epithelium also changes epithelial ion transport so as to promote an increase in respiratory fluid (21). These changes were not, however, limited to the inhibition of epithelial Na+ absorption. The virus also stimulated Cl− secretion through Ca2+-activated Cl− channels. The mechanism by which Sendai virus acted on the epithelium also differed from the mechanism used by influenza virus. Sendai virus triggered the release of ATP from the epithelium, which then acted in an autocrine fashion on apical purinergic receptors to activate phospholipase C, leading to an increase in intracellular Ca2+ and the activation of protein kinase C (21). A very similar mechanism was also recently described for Pseudomonas flagellin-induced effects on airway transport (22).
In the present study, we examine the acute effects of a third major respiratory virus, respiratory syncytial virus (RSV). RSV is the major respiratory pathogen among infants and children less than 5 years of age and causes 20% of childhood admissions to hospitals for respiratory infections (15). It also causes repeated reinfections in both children and adults. Less serious infections lead to nasal congestion, conjunctivitis, and otitis media, while more serious infections include bronchiolitis in children and potentially life-threatening pneumonia in adults (15). Moreover, RSV infection alters the sensitivity of laryngeal chemoreceptors, which may result in prolonged apnea (27). The clinical characteristics of the illness thus suggest that the virus may alter epithelial ion transport. Furthermore, it was recently reported that mice infected with RSV have decreased alveolar fluid clearance and a loss of sensitivity of the alveolar fluid clearance to amiloride, consistent with the virus directly, or indirectly, inhibiting the rate of epithelial Na+ transport (8). We thus examined whether exposure to RSV produces rapid alterations in the rate of ion transport by the respiratory epithelium. We find that it does and that the mechanism involved resembles that used by influenza virus rather than the mechanism used by parainfluenza virus.
MATERIALS AND METHODS
Viruses.
RSV (strain A2), a kind gift from P. Young (Sir Albert Sakzewski Virus Research Centre, Brisbane, Australia), was propagated in HEp-2 cells (ATCC), which were derived via HeLa contamination and maintained in medium 199 supplemented with 5% fetal calf serum and antibiotics. Semiconfluent (80%) monolayers were infected with RSV at a multiplicity of infection of 1. Supernatants from RSV-infected cultures were collected 4 to 5 days after infection, frozen and thawed twice to disrupt cells, vortexed for 1 min, and clarified by centrifugation at 1,200 × g for 15 min. The virus was titrated prior to use. RSV protein F was purified from virions, a generous gift from D. Spielman (Lederle-Praxis Biologicals, Rochester, NY). RSV protein F preparations may contain a certain fraction of G protein due to interprotein disulfide bonding (1).
Cell culture.
M1 mouse cortical collecting duct cells, provided by C. Korbmacher (Oxford University, United Kingdom), were grown to confluence for 3 days on permeable supports (Transwell-Coll; Costar, Cambridge, MA) in Dulbecco modified Eagle medium-F12 containing 10% fetal calf serum, glutamine (2 mmol/liter), penicillin (100,000 U/liter), streptomycin (100,000 U/liter), and dexamethasone (0.1 μmol/liter).
Ussing chamber experiments.
QS and C57BL mice were killed by cervical dislocation. The trachea was then removed, freed of connective tissue, and divided into small pieces. These pieces were then stored in a chilled solution containing the following (mmol/liter): NaCl (145), KCl (3.8), d-glucose (5), MgCl2 (1), HEPES (5), and Ca2+ gluconate (1.3), pH 7.4. Tissues were mounted in an Ussing chamber with a circular aperture of 0.95-mm2 area. The apical and basolateral surfaces of the epithelium were perfused continuously at a rate of 10 to 20 ml/min (chamber volume, 2 ml) at 37°C. The bath solution contained the following (mmol/liter): NaCl (145), KH2PO4 (0.4), K2HPO4 (1.6), d-glucose (5), MgCl2 (1), and Ca2+ gluconate (1.3), pH 7.4. All experiments were carried out under open-circuit conditions. The transepithelial potential difference (Vte) was recorded relative to the luminal side of the epithelium, and the current was regarded as positive when conventional current flowed from the apical to the serosal side of the epithelium. The transepithelial resistance (Rte) was determined by applying short (1-s) current pulses (ΔI = 0.5 μA) across the mucosa. Voltage deflections recorded in the absence of the mucosa were subtracted from those obtained in its presence, and Rte was calculated according to Ohm's law (Rte = ΔVte/ΔI). Tissues were accepted only if the Rte exceeded that obtained for an empty chamber by at least a factor of 3. Under these conditions, the recordings were stable for 3 to 4 h.
Compounds and statistics.
All compounds used were of the highest available grade of purity. 3-Isobutyl-1-methylxanthine (IBMX), forskolin, amiloride, bisindolylmaleimide 1 (BIM1), carbachol, apyrase, and suramin were obtained from Sigma (Deisenhofen, Germany). 1-Phenyl-2-hexadecanoylamino-3-morpholino-1-propanol (PPMP) was obtained from Adelab Scientific (Norwood, South Australia), and neuraminidase was obtained from Boehringer (Mannheim, Germany). Results are presented as means ± standard errors of the means (SEM) (n = the number of preparations tested). Statistical significance was assessed using paired or unpaired Student's t tests at a P value of <0.05.
RESULTS
Baseline electrical properties of the mouse tracheal epithelium.
Under open-circuit conditions, Vte was 9.1 ± 0.5 mV (n = 32) and Rte was 67.9 ± 5.1 Ωcm2 (n = 32), resulting in an equivalent short-circuit current (IscEq) of 137.3 ± 11.5 μA/cm−2 (n = 32). The addition of the inhibitor of epithelial Na+ channels, amiloride (10 μmol/liter), to the solution bathing the apical membrane of the epithelium reduced the equivalent short-circuit current to 24.3 ± 1.9 μA/cm−2 (n = 32). This indicates that electrogenic ion transport by the tracheal epithelium was dominated by the transport of Na+ through epithelial Na+ channels, consistent with other studies of this tissue (23).
Effects of RSV on the mouse tracheal epithelium.
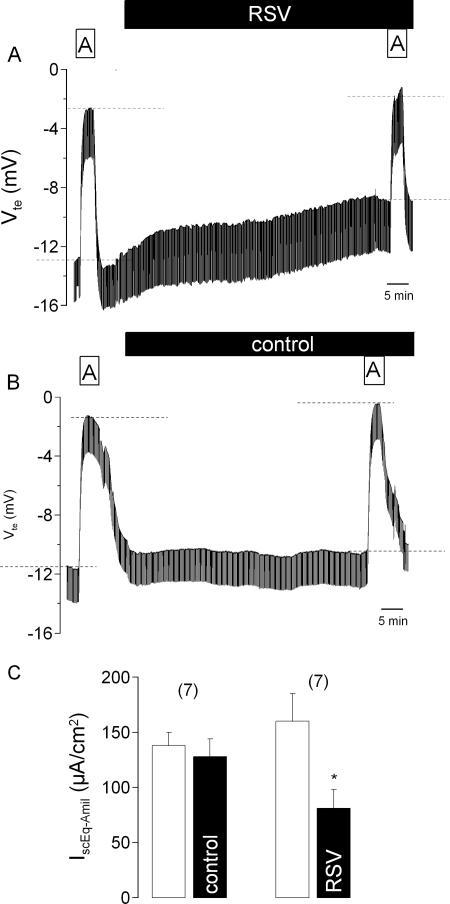
We found that exposure of the apical membrane of the mouse tracheal epithelium to 2 × 104 PFU/ml of RSV for 1 h reduced Vte from 13.1 ± 1.5 mV (n = 7) to 8.1 ± 1.1 mV (n = 7) (Fig. 1A) and increased Rte from 74.1 ± 7.1 Ωcm2 (n = 7) to 87.8 ± 8.6 Ωcm2 (n = 7). It should be noted that RSV did not cause an immediate transient increase in Vte coupled with a reduction in Rte (Fig. 1A) of the type that we have previously found to accompany the exposure of the tracheal epithelium to Sendai virus (21). Incubation for 1 h in the absence of RSV did not significantly change the electrical properties of the epithelium (Fig. 1B). Vte prior to incubation in the control medium, 13.8 mV ± 1.9 mV (n = 7), did not differ significantly from that observed after 1 h of incubation, 12.1 ± 1.7 mV (n = 7). Similarly, there was also no change in Rte during control incubation. At time zero, Rte was 78.8 ± 7.1 Ωcm2 (n = 7), which was not significantly different from Vte observed after 1 h of incubation in control medium, 81.8 ± 8.6 Ωcm2 (n = 7). Moreover, incubation of the tissues with supernatants isolated from noninfected HEp-2 cells had no effect on amiloride-sensitive transport (20.2 ± 3.0 μA/cm2 versus 21.7 ± 1.9 μA/cm2; n = 6).
FIG. 1.
Effect of RSV on amiloride (10 μmol/liter)-sensitive Na+ absorption in the mouse trachea. Original Ussing chamber recordings of Vte before and during apical exposure for 1 h to 2 × 104 PFU/ml RSV (A) or control buffer (B). Dashed lines in panels A and B indicate the effects of amiloride. Panel C summarizes the measurements of the amiloride-sensitive Na+ current (IscEq-Amil) obtained under control conditions and after incubation with either control buffer solution or RSV. The data are presented as means ± SEM (with the number of experiments in parentheses). The asterisk indicates a statistically significant difference compared to the control.
As is evident from Fig. 1A and B, exposure to RSV for 1 h reduced the magnitude of the response of Vte to amiloride, suggesting that RSV had inhibited the amiloride-sensitive Na+ transport. This is confirmed in Fig. 1C, which shows that exposure to RSV reduced the amiloride-sensitive component of the short-circuit current by approximately 50%. Incubation for 1 h without RSV, however, had no effect (Fig. 1C). We further found that the inhibition of the amiloride-sensitive short-circuit current produced by apical exposure to RSV for 1 h was dependent on the concentration of the virus over the range of 100 to 100,000 PFU/ml (Fig. 2).
FIG. 2.
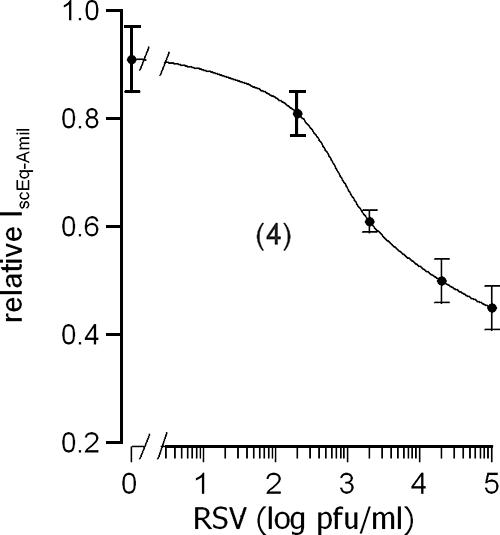
Concentration-response curve for the inhibitory effect of application for 1 h to the apical membrane of RSV on the amiloride-sensitive short-circuit current (IscEq-Amil) in the mouse trachea. In this figure, the IscEq-Amil measured 1 h after exposure to RSV has been normalized to the IscEq-Amil measured prior to exposure to give the relative IscEq-Amil. The data are presented as means ± SEM (with the number of experiments in parentheses).
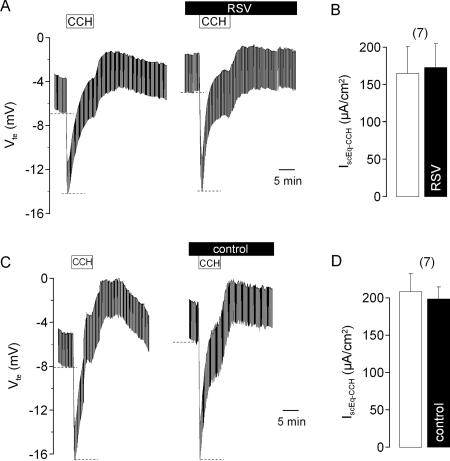
We then investigated whether exposure to RSV affected any other transport properties of the epithelium in the absence of amiloride. We first examined the effects of RSV on the Ca2+-activated secretory pathway (Fig. 3). We found that the serosal addition of 100 μmol/liter carbachol produced an increase in IscEq of 173 ± 32 μA/cm2 (n = 7) in tissues exposed apically for 1 h to RSV. This response did not differ significantly from their response to serosal carbachol prior to RSV exposure, 165 ± 36 μA/cm2 (n = 7). In additional control experiments, we confirmed that the increase in the short-circuit current evoked by carbachol in tissues incubated in control medium for 1 h, 208 ± 24 μA/cm2 (n = 7), did not differ significantly from the response observed prior to incubation, 199 ± 16 μA/cm2 (n = 7). Hence, exposure to RSV did not affect the Ca2+-activated secretory pathway.
FIG. 3.
Effect of RSV on carbachol (CCH) (100 μmol/liter)-induced ion transport in the mouse trachea. Original Ussing chamber recordings of Vte and the CCH responses prior to and following apical exposure for 1 h to RSV (1 × 104 PFU/ml) (A) or to control buffer (C). Panel B summarizes the effects of 1 h of exposure to RSV on the CCH-induced change in the short-circuit current (IscEq-CCH). Dashed lines in panels A and C indicate the effects of CCH. Panel D summarizes the effects of 1 h of exposure to control buffer on the CCH-induced change in IscEq-CCH. The data are presented as means ± SEM (with the number of experiments in parentheses).
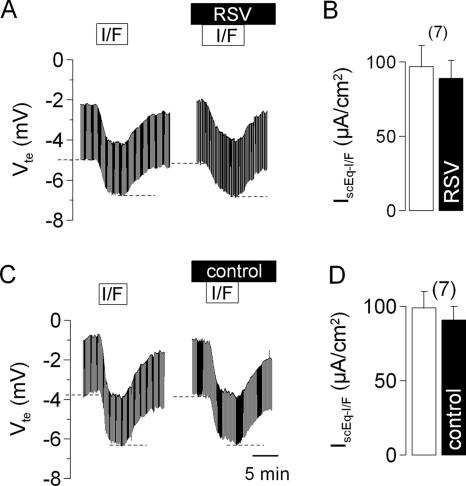
We also examined whether RSV affected the cyclic AMP-activated secretory pathway (Fig. 4). We found that in tissues exposed to RSV for 1 h, the serosal addition of a combination of 2 μmol/liter forskolin and 10 μmol/liter IBMX produced an increase in the short-circuit current, 89 ± 12 μA/cm2 (n = 7), which was not significantly different from the response observed prior to RSV exposure, 97 ± 14 μA/cm2 (n = 7). We further found that the short-circuit current response to forskolin plus IBMX, when added to tissues incubated in control medium for 1 h (91 ± 9 μA/cm2; n = 7), was not different to their response prior to incubation (99 ± 11 μA/cm2; n = 7). Hence, exposure to RSV does not affect the cyclic AMP-activated secretory pathway. Considering this finding with our finding that RSV does not affect the response to carbachol (see above), we conclude that the inhibition of amiloride-sensitive Na+ absorption by RSV is selective and is not due to a nonspecific toxic effect on the tracheal epithelium.
FIG. 4.
Effect of RSV on IBMX (100 μmol/liter) and forskolin (2 μmol/liter)-induced ion transport in the mouse trachea. Original Ussing chamber recordings of Vte and the responses to IBMX plus forskolin (I/F) prior to and following apical exposure for 1 h to RSV (2 × 104 PFU/ml) (A) or control buffer (C). Panel B summarizes the effects of 1 h of exposure to RSV on the IBMX-plus-forskolin-induced change in the short-circuit current (IscEq-I/F). Dashed lines in panels A and C indicate the effects of IBMX and forskolin. Panel D summarizes the effects of 1 h of exposure to control buffer on the IBMX-plus-forskolin-induced change in IscEq-I/F. The data are presented as means ± SEM (with the number of experiments in parentheses).
Effects of the RSV F protein.
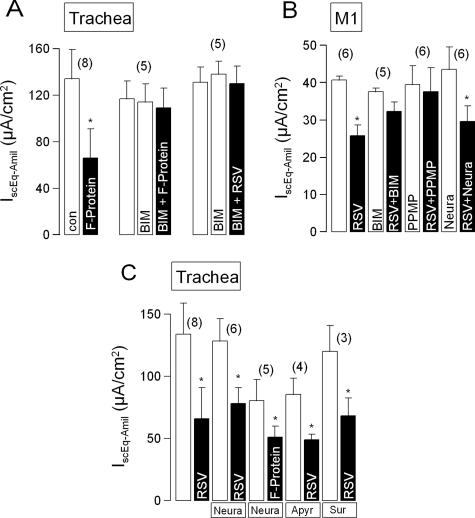
The RSV F, or fusion, protein mediates the fusion of viral particles with the membrane of uninfected cells and has been shown to trigger many of the cellular responses to infection with RSV (13). Thus, we investigated whether the effect of intact RSV on the amiloride-sensitive Na+ current could be reproduced by the F protein. As shown in Fig. 5A, we found that the amiloride-sensitive short-circuit current is significantly reduced by exposure of the apical membrane for 1 h to 2.5 μg/ml of recombinant F protein. Since the actions of influenza virus are mediated by protein kinase C (20), we then investigated whether the actions of the F protein could be blocked by the potent inhibitor of protein kinase C, BIM-I (100 nmol/liter). We found that they were. We further found that incubation in BIM-I prevented the inhibitory effect of the intact virus (Fig. 5A).
FIG. 5.
(A) Summary of the effects on the amiloride-sensitive short-circuit current (IscEq-Amil) across the mouse tracheal epithelium of apical application for 1 h of the RSV F protein (2.5 μg/ml), the RSV F protein (2.5 μg/ml) in the presence of the protein kinase C inhibitor BIM-I (100 nmol/liter), and intact RSV (105 PFU/ml) in the presence of BIM-I (100 nmol/liter). (B) Summary of the effects on IscEq-Amil across cultured mouse collecting duct (M1) cell monolayers of application for 1 h of RSV (2 × 105 PFU/ml) and RSV (2 × 105 PFU/ml) in the presence of BIM1 (100 nmol/liter). Also shown are the effects on the inhibition of IscEq by RSV (2 × 105 PFU/ml) of preincubation of the monolayers in PPMP (40 μmol/liter) for 24 h or preincubation in neuraminidase (0.5 U/ml) for 30 min. (C) Summary of the effects on IscEq-Amil across the mouse tracheal epithelium of 1 h of apical exposure to RSV (2 × 105 PFU/ml) or the F protein (2.5 μg/ml) following pretreatment of the apical surface of the epithelium with neuraminidase (Neura) (0.5 U/ml) for 30 min or when apyrase (Apyr) (2 U/ml) or suramin (Sur) (100 μmol/liter) have been included in the apical bathing solution. The data are presented as means ± SEM (with the number of experiments in parentheses). The asterisks indicate statistically significant differences compared to the corresponding controls. con, control.
RSV inhibits amiloride-sensitive Na+ absorption in cultured epithelia.
We then examined whether substromal cells are required for the inhibitory effects of RSV on amiloride-sensitive Na+ absorption. For this purpose, we used cultured monolayers of the M1 mouse collecting duct cell line, which we have previously shown to have the same responses to influenza virus and parainfluenza virus as does the tracheal epithelium (20,21). As is shown in Fig. 5B, we found that amiloride-sensitive Na+ absorption in M1 cell monolayers is significantly inhibited by RSV. Furthermore, this inhibition can be prevented by treating the monolayer with 100 nmol/liter BIM1, in agreement with our findings for the mouse tracheal epithelium (see above).
Nature of the apical receptor by which RSV alters epithelial ion transport.
When UV light-inactivated RSV was applied to mouse airways, 65% (n = 5) of the amiloride-sensitive transport was inhibited, further suggesting that RSV inhibits transport through binding to the cell membrane. We investigated whether the actions of RSV on cultured M1 cells are mediated by a glycoprotein or a glycoplipid. Our previous studies have shown that influenza virus acts via a neuraminidase-sensitive glycoprotein in the apical membrane of the epithelial cells (20), whereas the action of parainfluenza virus requires an apical membrane glycolipid, the synthesis of which can be inhibited by incubation of the cells in PPMP, an inhibitor of the enzyme glucosylceramide synthase (12, 21). In the present experiments, we found that preincubation of the apical surface of M1 cell monolayers in neuraminidase (0.5 U/ml) for 30 min had no impact on the inhibition of the amiloride-sensitive current produced by the subsequent addition of RSV (Fig. 5B). Similarly, preincubation of the apical surface of the tracheal epithelium with neuraminidase (1 U/ml) for 30 min did not prevent the reduction of the amiloride-sensitive current produced by intact RSV or recombinant F protein (Fig. 5C). Preincubation of M1 cell monolayers for 24 h in 40 μmol/liter PPMP did, however, abolish the inhibitory action of RSV F protein on amiloride-sensitive Na+ absorption (Fig. 5B).
Potential role for Toll-like receptor type 4.
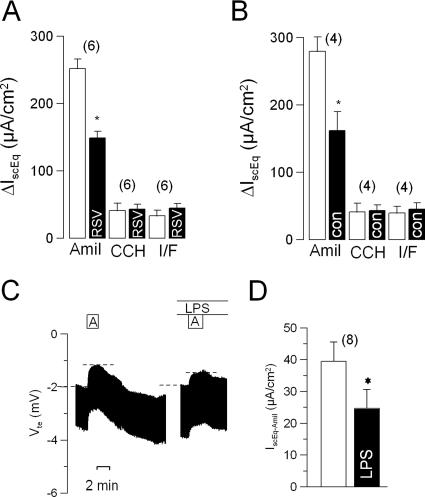
Some effects of RSV on cellular behavior have been reported to be mediated by Toll-like receptor 4 (14, 16); hence, we examined the effects of RSV on the tracheal epithelium of C3H/HeJ mice, a mouse strain in which Toll-like receptor 4 has been rendered unable to activate intracellular signaling pathways by a natural mutation in its TIR domain (31). When measured under baseline conditions, the transport properties of the tracheal epithelium of C3H/HeJ mice were quite different from those of the C57BL and QS strains. In particular, the short-circuit current activated by the serosal addition of 100 μmol/liter carbachol, 41 ± 13 μA/cm2 (n = 4) (Fig. 6), was approximately 25% of that observed in the C57BL and QS strains, consistent with the reported reduction in bronchoconstriction provoked by methacholine and acetylcholine in the C3H/HeJ strain (9). Similarly, the addition of 2 μmol/liter forskolin plus 10 μmol/liter IBMX to the serosal solution induced an increase in the short-circuit current of only 39 ± 10 μA/cm2 (n = 4), which was roughly 50% of the response in the C57BL and QS strains. In contrast, the amiloride-sensitive short-circuit current, 280 ± 21 μA/cm2 (n = 4), was approximately twice that observed in the C57BL and QS strains but then declined to 162 ± 28 μA/cm2 during 1 h of perfusion under control conditions, while the responses to carbachol and to forskolin plus IBMX remained unchanged (Fig. 6B). Incubation of the tracheal epithelium from C3H/HeJ mice with RSV for 1 h was accompanied by a decline in the amiloride-sensitive short-circuit current from 280 ± 21 μA/cm2 (n = 8) at time zero to 148 ± 9 μA/cm2 after 1 h. The responses to carbachol and to forskolin plus IBMX did not change significantly during incubation with RSV. The decline in the amiloride-sensitive short-circuit current observed during 1 h of incubation with RSV, 42%, did not differ from the decline we observed under control conditions, 43%. Hence, it appears that RSV may require functional type 4 Toll-like receptors to exert its effects on epithelial ion transport. This is further supported by the finding that lipopolysaccharides (10 μg/ml) also inhibited amiloride-sensitive transport (Fig. 6C and D).
FIG. 6.
Summaries of the effects of RSV (A) or control (con) (B) incubation on short-circuit current responses (ΔIscEq) to amiloride (Amil), carbachol (CCH), and forskolin plus IBMX (I/F). Dashed lines in panel C indicate the effects of amiloride. The effects of lipopolysaccharide (LPS) (10 μM) on transepithelial voltage and amiloride-sensitive transport (C) and amiloride-sensitive IscEq (D) are shown. The data are presented as means ± SEM (with the number of experiments in parentheses). The asterisks indicate statistically significant differences compared to the corresponding controls.
Are the effects of RSV mediated by the autocrine action of UTP?
It was recently reported that RSV infection in mice leads to a decrease in the amiloride-sensitive component of alveolar fluid clearance, apparently by a mechanism involving the activation of suramin-sensitive purinergic receptors by UTP (8). Furthermore, our own studies on parainfluenza virus indicated that this virus inhibits amiloride-sensitive Na+ absorption by the autocrine action of ATP on apical purinergic receptors (21). Hence, we examined whether the effects of RSV on the tracheal epithelium are mediated by the activation of purinergic receptors or the release of UTP or ATP. We found that the inclusion of 2 U/ml apyrase, an enzyme which degrades both ATP and UTP, in the solution bathing the apical membrane did not prevent the RSV F protein from reducing the amiloride-sensitive short-circuit current from 85.4 ± 13.1 μA/cm2 (n = 4) to 49.0 ± 4.4 μA/cm2 (n = 4). Furthermore, the inclusion of 100 μmol/liter suramin, a blocker of P2Y receptors, in the solution bathing the apical membrane did not prevent the RSV F protein from reducing the amiloride-sensitive short-circuit current from 120.4 ± 21.1 μA/cm2 (n = 3) to 68.1 ± 14.5 μA/cm2 (n = 3). Hence, the actions of the RSV F protein are not mediated by the action of ATP or UTP on apical purinergic receptors.
DISCUSSION
We have found that exposure of the apical membrane of the tracheal epithelium to RSV produces a rapid inhibition of amiloride-sensitive Na+ absorption. This inhibition is mediated by protein kinase C, can be reproduced by the F protein in the viral coat, and appears to be dependent on the presence of asialogangliosides in the apical membrane. Given that the responses of the epithelium to stimulation with carbachol or with forskolin plus IBMX are not affected by RSV, the effect of the virus on epithelial Na+ transport cannot be regarded as the consequence of a “nonspecific” toxic effect of the pathogen on the epithelium.
The inhibition of amiloride-sensitive Na+ transport produced by RSV appears to require functional type 4 Toll-like receptors. This is consistent with the evidence that these receptors mediate many cellular responses to RSV (6, 14, 24). A role for type 4 Toll-like receptors in mediating the effects of RSV on epithelial ion transport is also supported by the ability of these receptors to respond to the RSV F protein (24) and by the known role of asialogangliosides in Toll-like receptor signaling (33). Whatever the exact nature of the receptor is by which RSV exerts these effects, it must be intrinsic to the epithelial cells and not mediated by the immune system, as we found that RSV could inhibit amiloride-sensitive Na+ transport across monolayers of the M1 collecting duct cell line, which are free of immune cells. The inhibitory effects of RSV on ion channels could also be related to the changes in sensitivity of laryngeal chemoreceptors, which may even lead to fatal apnea. In this respect, the results may be relevant to the pathogenesis of the sudden infant death syndrome associated with RSV infection (27).
The concentration range over which the inhibition is observed, 102 to 105 PFU/ml, is comparable to the levels observed during RSV infections both in animal models and in humans. For example, Davis and coworkers found that RSV levels in lung tissue following nasal inoculation of mice reached 104 PFU/g (8). Similarly, levels ranging from 104 to 105 PFU/g lung tissue have been reported in guinea pigs (7) and cotton rats (18), while concentrations of RSV in nasal and tracheal secretions in children with RSV infections average 105 PFU/ml and can be as high as 107 PFU/ml (30). Furthermore, the available information indicates that the concentrations of the F protein in respiratory secretions are substantially greater than would be expected from the concentration of viable RSV (17).
While our finding that RSV inhibits amiloride-sensitive Na+ transport in isolated epithelia agrees with the recently published report that the amiloride-sensitive component of lung fluid absorption is abolished in mice infected with RSV (8), there are differences between the findings of the two studies. In particular, Davis and coworkers found that the inhibitory effect of RSV infection on the amiloride-sensitive component of airway fluid clearance could be overcome by the instillation into the lungs of agents, such as suramin, which block apical P2Y receptors (8). They further found that instillation of the enzyme apyrase, which degrades both UTP and ATP, blocked the inhibitory effect of RSV infection whereas instillation of the enzyme hexokinase, which degrades ATP but not UTP, did not (8). That Davis et al. identified a role for the autocrine action of UTP in the effects of RSV, which we have not observed, is likely to be due to the marked differences between the two experimental situations; however, we have no mechanistic explanation for it. It is noteworthy that the mechanism by which RSV produces acute alterations in the rate of epithelial Na+ absorption differs from the mechanisms used by influenza virus and parainfluenza virus. As described in the introduction, influenza virus inhibits amiloride-sensitive Na+ transport as a consequence of the hemagglutinin in the viral coat binding a neuraminidase-sensitive glycoprotein in the apical membrane of the epithelium, leading to the activation of phospholipase C and protein kinase C and the inhibition of Na+ absorption (20). Sendai virus, a parainfluenza virus, on the other hand, affects epithelial transport as a consequence of triggering ATP release from the epithelial cells by a glycolipid-dependent mechanism. The ATP then stimulates apical purinergic receptors, leading to the activation of the Ca2+-activated Cl− current and the inhibition of the amiloride-sensitive Na+ current (21). RSV, in inhibiting amiloride-sensitive Na+ but leaving Cl− secretion unaffected and in using a glycolipid-dependent signaling pathway, thus appears to be using elements of both the mechanism used by influenza virus and the mechanism used by parainfluenza virus.
RSV thus joins the growing list of respiratory pathogens that have been reported to directly affect epithelial ion transport. In addition to the respiratory viruses mentioned in the previous paragraph, bacterial pathogens have been found to alter ion transport by the respiratory epithelium so as to promote fluid accumulation in the respiratory tract. Pseudomonas aeruginosa, for example, has been reported to acutely inhibit Na+ absorption by respiratory epithelia in a variety of species (10, 11, 22, 34). This inhibition has variously been reported to require adhesion of the bacterium to the epithelium (10) or to involve products secreted by the bacterium into the extracellular medium, such as rhamnolipids (11) and heat-stable hemolysin (34). Lipopolysaccharide from Klebsiella pneumoniae has been reported to inhibit Na+ absorption by the canine tracheal epithelium (35), while Mycobacterium tuberculosis (37) and Mycoplasma pulmonis (25) have been reported to inhibit Na+ transport across cultured respiratory epithelia. In a previous paper on this subject (21), we suggested, on the basis of the mechanistic similarities between the epithelial responses to parainfluenza virus, to Pseudomonas aeruginosa, and to mechanical trauma, that these responses were all examples of a stereotypic epithelial defense mechanism that operates to sweep noxious agents away from the epithelial surface. The marked differences among the mechanisms by which influenza virus, parainfluenza virus, and RSV alter epithelial electrolyte transport are not really compatible, however, with this proposal. Rather, it would appear that these viruses have independently developed mechanisms for regulating epithelial ion transport so as to increase fluid accumulation in the respiratory tract, presumably in order to foster their spread throughout the host and from host to host. Finally, the actions of respiratory viruses on epithelial transport are an interesting example of the capacity of viruses to harness host systems to their own benefit, as has previously been observed in the dependency of viral replication on the activity of the host cells' own signaling systems (28).
Acknowledgments
This project was supported by the National Health and Medical Research Council of Australia, the Australian Research Council, Cystic Fibrosis Australia, the Else-Kröner-Fresenius-Stiftung, and Deutsche Forschungsgemeinschaft SFB699 projects A6 and A7. D.I.C. and K.K. were fellows of the Medical Foundation of the University of Sydney.
We thank Brian Oliver (Department of Pharmacology, University of Sydney) for supplying us with HEp-2 cells and RSV and J. Ousingsawat for excellent technical assistance.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Arumugham, R. G., S. W. Hildreth, and P. R. Paradiso. 1989. Interprotein disulfide bonding between F and G glycoproteins of human respiratory syncytial virus. Arch. Virol. 105:65-79. [DOI] [PubMed] [Google Scholar]
- 2.Bachhuber, T., J. König, T. Voelcker, B. Mürle, R. Schreiber, and K. Kunzelmann. 2005. Chloride interference with the epithelial Na+ channel ENaC. J. Biol. Chem. 280:31587-31594. [DOI] [PubMed] [Google Scholar]
- 3.Barker, P. M., C. W. Gowen, E. E. Lawson, and M. R. Knowles. 1997. Decreased sodium ion absorption across nasal epithelium of very premature infants with respiratory distress syndrome. J. Pediatr. 130:373-377. [DOI] [PubMed] [Google Scholar]
- 4.Barker, P. M., and R. E. Olver. 2002. Invited review: clearance of lung liquid during the perinatal period. J. Appl. Physiol. 93:1542-1548. [DOI] [PubMed] [Google Scholar]
- 5.Boucher, R. C. 2003. Regulation of airway surface liquid volume by human airway epithelia. Pfluegers Arch. 445:495-498. [DOI] [PubMed] [Google Scholar]
- 6.Bowie, A. G., and I. R. Haga. 2005. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42:859-867. [DOI] [PubMed] [Google Scholar]
- 7.Dakhama, A., T. Z. Vitalis, and R. G. Hegele. 1997. Persistence of respiratory syncytial virus (RSV) infection and development of RSV-specific IgG1 response in a guinea-pig model of acute bronchiolitis. Eur. Respir. J. 10:20-26. [DOI] [PubMed] [Google Scholar]
- 8.Davis, I. C., W. M. Sullender, J. M. Hickman-Davis, J. R. Lindsey, and S. Matalon. 2004. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L112-L120. [DOI] [PubMed] [Google Scholar]
- 9.Duguet, A., K. Biyah, E. Minshall, R. Gomes, C. G. Wang, M. Taoudi-Benchekroun, J. H. Bates, and D. H. Eidelman. 2000. Bronchial responsiveness among inbred mouse strains. Role of airway smooth-muscle shortening velocity. Am. J. Respir. Crit. Care Med. 161:839-848. [DOI] [PubMed] [Google Scholar]
- 10.Evans, D. J., P. S. Matsumoto, J. H. Widdicombe, C. Li-Yun, A. A. Maminishkis, and S. S. Miller. 1998. Pseudomonas aeruginosa induces changes in fluid transport across airway surface epithelia. Am. J. Physiol. 275:C1284-C1290. [DOI] [PubMed] [Google Scholar]
- 11.Graham, A., D. M. Steel, R. Wilson, P. J. Cole, E. W. Alton, and D. M. Geddes. 1993. Effects of purified Pseudomonas rhamnolipids on bioelectric properties of sheep tracheal epithelium. Exp. Lung Res. 19:77-89. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero, C. A., S. Zarate, G. Corkidi, S. Lopez, and C. F. Arias. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacking, D., and J. Hull. 2002. Respiratory syncytial virus—viral biology and the host response. J. Infect. 45:18-24. [DOI] [PubMed] [Google Scholar]
- 14.Haeberle, H. A., R. Takizawa, A. Casola, A. R. Brasier, H. J. Dieterich, N. Van Rooijen, Z. Gatalica, and R. P. Garofalo. 2002. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and Toll-like receptor 4-dependent pathways. J. Infect. Dis. 186:1199-1206. [DOI] [PubMed] [Google Scholar]
- 15.Hashem, M., and C. B. Hall. 2003. Respiratory syncytial virus in healthy adults: the cost of a cold. J. Clin. Virol. 27:14-21. [DOI] [PubMed] [Google Scholar]
- 16.Haynes, L. M., D. D. Moore, E. A. Kurt-Jones, R. W. Finberg, L. J. Anderson, and R. A. Tripp. 2001. Involvement of Toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 75:10730-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendry, R. M., E. Godfrey, L. J. Anderson, B. F. Fernie, and K. McIntosh. 1985. Quantification of respiratory syncytial virus polypeptides in nasal secretions by monoclonal antibodies. J. Gen. Virol. 66:1705-1714. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, S., C. Oliver, G. A. Prince, V. G. Hemming, D. S. Pfarr, S. C. Wang, M. Dormitzer, J. O'Grady, S. Koenig, J. K. Tamura, R. Woods, G. Bansal, D. Couchenour, E. Tsao, W. C. Hall, and J. F. Young. 1997. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 176:1215-1224. [DOI] [PubMed] [Google Scholar]
- 19.Kerem, E., T. Bistritzer, A. Hanukoglu, T. Hofmann, Z. Zhou, W. Bennett, E. MacLaughlin, P. Barker, M. Nash, L. Quittell, R. Boucher, and M. R. Knowles. 1999. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N. Engl. J. Med. 341:156-162. [DOI] [PubMed] [Google Scholar]
- 20.Kunzelmann, K., A. H. Beesley, N. J. King, G. Karupiah, J. A. Young, and D. I. Cook. 2000. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc. Natl. Acad. Sci. USA 97:10282-10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunzelmann, K., J. König, D. Markovich, N. King, G. Karupiah, and D. I. Cook. 2004. Acute effects of parainfluenza virus on epithelial electrolyte transport. J. Biol. Chem. 279:48760-48766. [DOI] [PubMed] [Google Scholar]
- 22.Kunzelmann, K., K. Scheidt, B. Scharf, J. Ousingsawat, R. Schreiber, B. J. Wainwright, and B. McMorran. 2006. Pseudomonas flagellin inhibits Na+ transport in airway epithelia. FASEB J. 20:545-546. [DOI] [PubMed] [Google Scholar]
- 23.Kunzelmann, K., R. Schreiber, and D. I. Cook. 2002. Mechanisms for the inhibition of amiloride-sensitive Na+ absorption by extracellular nucleotides in mouse trachea. Pfluegers Arch. 444:220-226. [DOI] [PubMed] [Google Scholar]
- 24.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 25.Lambert, L. C., H. Q. Trummell, A. Singh, G. H. Cassell, and R. J. Bridges. 1998. Mycoplasma pulmonis inhibits electrogenic ion transport across murine tracheal epithelial cell monolayers. Infect. Immun. 66:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan, R. S., G. A. Stewart, R. G. Goldie, and P. J. Henry. 2004. Altered expression and in vivo lung function of protease-activated receptors during influenza A virus infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L388-L398. [DOI] [PubMed] [Google Scholar]
- 27.Lindgren, C., L. Jing, B. Graham, J. Grogaard, and H. Sundell. 1992. Respiratory syncytial virus infection reinforces reflex apnea in young lambs. Pediatr. Res. 31:381-385. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig, S., O. Planz, S. Pleschka, and T. Wolff. 2003. Influenza-virus-induced signaling cascades: targets for antiviral therapy? Trends Mol. Med. 9:46-52. [DOI] [PubMed] [Google Scholar]
- 29.Mall, M., B. R. Grubb, J. R. Harkema, W. K. O'Neal, and R. C. Boucher. 2004. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 10:487-493. [DOI] [PubMed] [Google Scholar]
- 30.Malley, R., L. Vernacchio, J. Devincenzo, O. Ramilo, P. H. Dennehy, H. C. Meissner, W. C. Gruber, H. S. Jafri, P. J. Sanchez, K. Macdonald, J. B. Montana, C. M. Thompson, and D. M. Ambrosino. 2000. Enzyme-linked immunosorbent assay to assess respiratory syncytial virus concentration and correlate results with inflammatory mediators in tracheal secretions. Pediatr. Infect. Dis. J. 19:1-7. [DOI] [PubMed] [Google Scholar]
- 31.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 32.Sartori, C., H. Duplain, M. Lepori, M. Egli, M. Maggiorini, P. Nicod, and U. Scherrer. 2004. High altitude impairs nasal transepithelial sodium transport in HAPE-prone subjects. Eur. Respir. J. 23:916-920. [DOI] [PubMed] [Google Scholar]
- 33.Soong, G., B. Reddy, S. Sokol, R. Adamo, and A. Prince. 2004. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J. Clin. Investig. 113:1482-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stutts, M. J., J. H. Schwab, M. G. Chen, M. R. Knowles, and R. C. Boucher. 1986. Effects of Pseudomonas aeruginosa on bronchial epithelial ion transport. Am. Rev. Respir. Dis. 134:17-21. [DOI] [PubMed] [Google Scholar]
- 35.Tamaoki, J., N. Sakai, K. Isono, T. Kanemura, K. Takeyama, and T. Takizawa. 1991. Lipopolysaccharide from Klebsiella pneumoniae inhibits Na+ absorption in canine tracheal epithelium. Infect. Immun. 59:716-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomlinson, L. A., T. C. Carpenter, E. H. Baker, J. B. Bridges, and J. V. Weil. 1999. Hypoxia reduces airway epithelial sodium transport in rats. Am. J. Physiol. 277:L881-L886. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, M., K. J. Kim, D. Iyer, Y. Lin, J. Belisle, K. McEnery, E. D. Crandall, and P. F. Barnes. 1997. Effects of Mycobacterium tuberculosis on the bioelectric properties of the alveolar epithelium. Infect. Immun. 65:692-698. [DOI] [PMC free article] [PubMed] [Google Scholar]