In Alzheimer’s disease (AD) pathogenesis, an underlying βAPP/Aβ dysmetabolism leading to neuronal toxicity is considered the essential abnormality by most investigators. 1-4 Hyperphosphorylation and filamentogenesis of tau into paired helical filaments (PHFs) is also thought to be important 5,6 and apolipoprotein E4 genotype is considered an aggravating factor. 7 The various hypotheses for sporadic AD (s-AD) acknowledge the milieu of aging brain cells but usually omit consideration of an initial sparking event.
For several years it was presumed that βAPP/Aβ and tau abnormalities occur only in the brain (and its blood vessels in regard to βAPP/Aβ), until βAPP, Aβ, and PHFs containing hyperphosphorylated tau were shown to accumulate pathologically within abnormal muscle fibers of two closely related, progressively disabling muscle diseases, sporadic inclusion-body myositis (s-IBM) and hereditary inclusion-body myopathy (h-IBM). 8-10 s-IBM is a disease of aging muscle with clinical onset nearly always at age 50 or older; h-IBM begins clinically at ages 15–30 in young adult muscle. 11 The autosomal-recessive form of h-IBM is caused by a yet-undetermined gene on chromosome 9 p1-q1, 12,13 not a defect of the βAPP gene on chromosome 21; in two families with the autosomal-dominant form of h-IBM, no defect of the βAPP-gene was demonstrable. 14 s-IBM is the most common muscle disease in older patients. h-IBM is rather rare, but probably more common than hereditary AD (h-AD).
In s-IBM and h-IBM it was shown that βAPP-mRNA was overexpressed in muscle fibers 15 (but not exclusively; cellular prion protein and its mRNA were also overexpressed). 16 Several other “Alzheimer characteristic” proteins, including presenilin-1 and proteins related to oxidative stress, also accumulated within abnormal muscle fibers of s-IBM and h-IBM. 11,17,18
Intracellular congophilic amyloid deposits are another characteristic and consistent feature of s-IBM muscle fibers. 8,19 In h-IBM they are very rare in younger patients, but their occurrence, especially in autosomal-dominant forms, increases in older patients. 11 The intracellular amyloid of the IBMs is of two structural-chemical types: collections of 15- to 21-nm PHFs containing immunoreactive tau (and other components, but not Aβ), and collections (“microplaquettes”) containing 6- to 10-nm filaments immunoreactive for Aβ. 11
Because the same proteins accumulate within s- and h-IBM muscle fibers as accumulate in the brains of patients with sporadic and hereditary forms of AD, the muscle and brain diseases might share certain pathogenic steps and knowledge of one disease might help elucidate the other. Cellular aging and evidence of oxidative stress are associated with the IBMs and the ADs. The IBMs and the ADs are both multifactorial and polygenetic. The respective cascades of events leading to the specific form of AD-like IBM muscle fiber degeneration and the similar specific features in AD brain are not understood. Within both the IBM and the AD categories, the pathological phenotypes of sporadic and hereditary forms are very similar, despite the different direct causes being mainly nongenetic versus mainly genetic. Therefore, in each disease category it has been proposed, by our group for the IBMs 20,21 and by others for the ADs, 22,23 that different etiologies including different genetic defects in the hereditary forms lead to the same upstream step, which then promotes the final common downstream pathogenic cascade of events resulting in the specific cellular deterioration. Moreover, the intracellular pathogenic cascades of the IBMs and the ADs might have strong similarities following determination by yet-unknown factors of the initial tissue selectivity in each category, muscle versus brain.
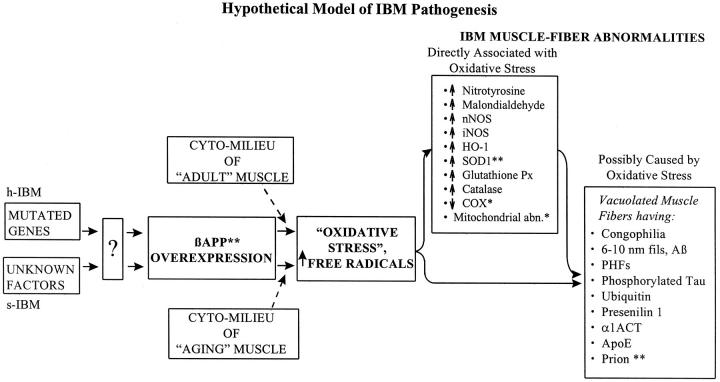
In both s-IBM and h-IBM, it is of particular interest that individual muscle fibers accumulate N- and C-terminal βAPP and Aβ, and βAPP-mRNA, before other abnormalities are evident. 8-11 We hypothesized that overexpression of βAPP might be upstream to other cellular abnormalities, including oxidative stress and mitochondrial abnormalities (Figure 1) ▶ . 21 To test this hypothesis, wild-type full-length 751 βAPP was overexpressed long-term in cultured normal human muscle. It produced within muscle fibers several aspects of the IBM cellular phenotype, including vacuolization, congophilic amyloid inclusions (as microplaquettes) in a small percentage of muscle fibers, cytoplasmic 6- to 10-nm amyloid-like filaments, nuclear PHFs, mitochondrial cytochrome oxidase deficiency, and mitochondrial morphological abnormalities. 24,25
Figure 1.
Genetic defects in h-IBM and various factors (including a putative virus) in s-IBM lead to presently unknown mechanisms [“?” box in diagram]. These up-regulate βAPP transcription, resulting in βAPP overexpression. In the milieu of aging muscle of s-IBM or of adult muscle of h-IBM, overexpression producing excessive βAPP (the whole molecule or its Aβ fragment) leads to oxidative stress with increased free radicals, which contribute to producing the demonstrated IBM muscle fiber abnormalities. Those abnormalities are either directly associated with oxidative stress or possibly caused by oxidative stress. Perhaps some of the former contribute to producing, directly or indirectly, some of the latter. *, present in virtually all s-IBM patients and in the older h-IBM patients; **, both mRNA.
Transgenic Mice
This approach of overexpressing βAPP in muscle has now been extended in two very interesting studies involving transgenic mice, reported in this issue of the Journal. Fukuchi et al overexpressed a wild-type 99-amino acid C-terminal sequence of βAPP, 26 and Jin et al 27 overexpressed a C-terminal 99-amino acid sequence of βAPP containing the substitution of lysine-612 to valine (K612V) resulting in a α-secretase-resistant mutation intended to increase Aβ production. The groups reached some important parallel conclusions.
(1) Within muscle fibers, some aspects of the IBM pathology were produced, including vacuolization and abnormal intracellular accumulation of C-terminal βAPP and Aβ and intracellular amyloid deposits. Whereas Jin et al reported intracellular thioflavin-s-positive amyloid deposits in one-third of the muscle fibers, Fukuchi et al observed polarized-light congo-red positivity in only a few muscle fibers. It remains to be determined whether this difference is due to use of a different βAPP sequence, mutant versus wild, to generate the transgenic mice or to different staining techniques to identify amyloid deposits. In IBM muscle fibers, because of the small size of amyloid inclusions, evaluating congo-red staining through polarizing filters is often not satisfactory. Using ultraviolet illumination and Texas-red filters greatly increases detection of congophilic amyloid, 28 an approach that might be helpful in further studies of the Fukuchi model.
(2) The muscle abnormalities were evident only in aging mice, not being detectable until the mice were 24 months of age or older. In the transgenic mice generated by Fukuchi et al, possibly there was strain-susceptibility.
Additional interesting findings by Jin et al included amyloid-like 6- to 8-nm fibrils within muscle fibers by electron microscopy that closely resemble those present in IBM, and lymphocytic inflammation comparable to that seen in s-IBM muscle (but usually not in h-IBM). Although in s-IBM the lymphocytic response in muscle can be moderate to prominent, the patients respond only slightly or not at all to anti-dysimmune treatments, suggesting that the major causes of clinical weakness are noninflammatory intracellular malfunctions resulting in muscle-fiber vacuolation and atrophy. 29 The results reported by Jin et al now suggest that overexpression of βAPP can produce, directly or indirectly, a lymphocytic response in aging mouse muscle, raising the possibility that in s-IBM the lymphocytic inflammation might not be a primary phenomenon. (The term “inflammation” in reference to s- and h-AD pathology is used quite differently and denotes a response not of circulating lymphocytes or granulocytes but of local microglia and astrocytes.)
Another abnormality described by Jin et al that is similar to that typical of IBM muscle 11,17 is the presence of small, angular, atrophic muscle fibers; in muscle pathology these are usually considered to indicate a denervation component. This feature is especially interesting in view of the recent studies in cultured human muscle fibers demonstrating that experimental overexpression of βAPP prevents formation of neuromuscular junctions, apparently by rendering those cultured muscle fibers incapable of becoming innervated. 30 Cultured h-IBM muscle fibers also were not able to be innervated, possibly due to their demonstrated overexpression of βAPP/Aβ. 30 A synaptopathic mechanism of βAPP/Aβ overexpression might also be responsible for denervation (and possibly more subtle synaptic abnormalities) seen in IBM patients in vivo and in the βAPP-transgenic mouse. Likewise, in AD, βAPP/Aβ overexpression might be a direct intracellular cause of the known synaptic abnormalities, considered by some to be the most important substratum of the dementia. 31,32
In summary, overexpression of βAPP/Aβ in transgenic mice and in cultured human muscle produces changes similar to those in IBM muscle. This is the scenario we have hypothesized (Figure 1) ▶ . 21 In some transgenic mice the changes also included lymphocytic inflammation. That the IBM-like changes in the mice were not due to a nonspecific effect of an overexpressed gene is supported by a previous report indicating that in transgenic mice overexpressing the cellular prion protein gene, there was a morphologically nonspecific myopathy that lacked characteristic features of the IBM phenotype. 33
Questions
If overexpression of βAPP/Aβ is a key pathogenic step in the IBMs, several questions become important.
(1) How does excessive βAPP/Aβ cause other changes further downstream in mouse and human muscle? Does it directly or indirectly affect transcription, translation, or protein function of other genes, unleashing a cascade of cellular malfunction? Several of the proteins, including some associated with oxidative stress, that accumulate extrajunctionally within IBM muscle fibers are found in normal human muscle only at the postsynaptic region of the neuromuscular junctions. 34 These junctionally concentrated proteins include βAPP and its mRNA and presenilin-1, but not tau. 34 We therefore proposed, as an aspect of the pathogenic cascade, an upstream junctionalization phenomenon involving initial overexpression of a putative junctionalizing master gene (jmg). 17,34 The βAPP/Aβ overexpression might either activate a jmg or have a jmg function itself.
(2) If aging of the patient is a key prerequisite for developing either s-IBM or s-AD, in what manner might an aging cellular milieu foster the pathogenic mechanism? This is not known but in general, possible mechanisms, such as more easily induced oxidative stress or mitochondrial dysfunction, might involve decrease of youthful protective influences and/or increase of susceptibility factors.
(3) Are there present within muscle fibers of transgenic mice mitochondrial abnormalities, such as absent cytochrome oxidase activity and ultrastructural distortions and increased markers of oxidative stress, like those found in s-IBM biopsied abnormal muscle fibers 11,18,20 and in cultured normal human muscle overexpressing the transferred βAPP gene? 11,24
(4) In s-IBM, what is the initial event activating the putatively βAPP/Aβ -mediated pathogenic cascade? A virus-induced mechanism has been proposed. 17,29 Because only a small percent of aging persons develop s-IBM, there could be a human strain-susceptibility to either viral infection or viral-induced expression of βAPP/Aβ (eg, by a viral protein acting directly or indirectly on the enhancer/promoter of the βAPP gene or on its mRNA) or to consequences thereof. This could be analogous to the possible strain-susceptibility of the transgenic mice proposed by Fukuchi et al and reported previously in relation to Alzheimer models of βAPP-overexpressing transgenic mice. 35,36 Whether a virus might be the initiating spark of s-AD remains to be determined. In contrast to Alzheimer’s disease, ApoE4 is not a risk factor for the IBMs, 37,38 but an increased frequency of the DRB1 0301/0302 allele corresponding to the DR3 HLA haplotype was reported in two studies. 39,40
(5) In the h-IBMs, how might a non-βAPP gene defect cause βAPP/Aβ overexpression? The same general question applies to the h-AD patients having a non-βAPP mutation of presenilin-1 or presenilin-2. 41,42 A general possibility is that the non-βAPP mutant gene product might directly or indirectly affect βAPP/Aβ expression and/or processing. For example, presenilin-1 and presenilin-2 mutations lead to overproduction of Aβ, as do mutations of βAPP. 1-3,41,42
(6) Is the pathogenesis of the IBMs essentially intracellular? We have been proposing this for several years and suggested an upstream intracellular pathogenic role of overexpressed βAPP/Aβ, through modulation of expression of other genes and induction of the demonstrated oxidative-stress and mitochondrial abnormalities. 17,18,20 The βAPP-overexpressing cultured human muscle and transgenic mice support an upstream role for intracellularly overexpressed βAPP/Aβ. In the IBMs, extracellular amyloid plaques do not form. Even though normal muscle fibers in culture secrete Aβ 40 and Aβ 42 and those secretions are greatly increased in βAPP-overexpressing cultured muscle fibers (Xie, McFerrin, Engel, Selkoe, and Askanas, unpublished observations, 1997), medium from the latter is not myotoxic. 24,25 In vivo, it is possible that clearance of extracellular Aβ is more effective from muscle than from brain.
IBM muscle fiber nuclei do not show apoptotic changes by the TUNEL. 43-45 However, in all s-IBM patients, 2–3% of the muscle nuclei manifest at least a few disease-characteristic PHFs in addition to those in the cytoplasm, 11 and some autosomal-dominant h-IBM nuclei possess many tau-containing PHFs. 46 These indicate a disturbance of nuclear metabolism that could be accompanying malfunction of DNA and/or RNA. The cultured human muscle experimentally overexpressing the βAPP gene had a few of those intranuclear PHFs. 25
By analogy with IBM, we raise the possibility that in AD there might be similar mechanisms of intracellularly overexpressed βAPP/Aβ directly inducing abnormality in neurons to cause the essential damage. Although AD investigators generally have favored an initially extracellular role of Aβ toxicity, 1-4,31,42 recently some have endorsed intracellular mechanisms. 47 Because extracellular Aβ experimentally can be neurotoxic, 2,4 it is possible that extracellular Aβ in AD can exert an additional adverse effect.
In IBM muscle fibers, apolipoprotein-E (ApoE) is pathologically localized on the Aβ-containing 6- to 10-nm filaments and the tau-containing PHFs, 48 but it has not yet been studied in the IBM transgenic mice. In AD, astrocyte-derived intraneuronal ApoE is considered to aggravate neurotoxicity and to be isoform-dependent, ie, more prominent with ApoE4. 7 In both IBM and AD, an ApoE aggravation of cytotoxicity might occur by enhancing intracellular damage caused by overexpressed neuronal βAPP/Aβ. Perhaps ApoE also facilitates formation of intracellular 6- to 10-nm fibrils and PHFs.
An obvious corollary of the hypothetically intracellular mechanisms of IBMs and ADs is that a therapeutic drug must enter or at least modulate the intracellular milieu. The two new transgenic mouse models of IBM, like our βAPP/Aβ-overexpressing human muscle cultures, may now be used to explore intracellular pathogenic mechanisms and proposed treatments of βAPP/Aβ overexpression in muscle fibers.
(7) To support a central role for βAPP/Aβ in the pathogenesis of s- and h-IBM, is more evidence needed in the βAPP overexpression models involving cultured human muscle and the transgenic mice regarding formation of PHFs, accumulation of tau and other Alzheimer-characteristic proteins, and muscle weakness in the transgenic mice? Such evidence would certainly support the hypothesis. In the βAPP-overexpressing transgenic mice, the question of hyperphosphorylated tau accumulation was not addressed, and ultrastructural PHFs in muscle were not found. In cultured human muscle overexpressing the transferred βAPP gene, PHFs like those in s-and h-IBM were present in muscle nuclei but not yet found in the muscle cytoplasm. 25 Perhaps longer-cultured, longer-overexpressing, older muscle fibers will also manifest the cytoplasmic tau-containing PHFs typical of the IBMs. It is reported that Aβ up-regulates tau protein kinase I (TPK-I), resulting in hyperphosphorylated tau, which can lead to PHF formation 49 (Aβ-up-regulated TPK-I can also phosphorylate, and thereby inactivate, mitochondrial pyruvate dehydrogenase, resulting in cellular depletion of acetyl CoA and consequently reduced ATP synthesis). 49 From another aspect, one can ask whether in IBM and AD the accumulated hyperphosphorylated-tau PHFs contribute early and significantly to cellular malfunction, or are simply diagnostically interesting parallel markers, somewhat later-appearing and themselves relatively harmless? If not critically pathogenic, is PHF formation actually necessary to enhance validity of the models?
Conclusion
The two new reports of muscle abnormality in transgenic mice overexpressing βAPP provide exciting data and intellectual stimulation for further studies of the intracellular molecular pathogenic mechanisms of βAPP/Aβ, which may relate to both the IBMs and the ADs.
Footnotes
Address reprint requests to Valerie Askanas, MD, PhD, USC Neuromuscular Center, 637 South Lucas Avenue, Los Angeles, CA 90017-1912. E-mail: askanas@hsc.usc.edu.
References
- 1.Selkoe DJ: Amyloid β-protein, and the genetics of Alzheimer’s disease. J Biol Chem 1996, 271:18295-18298 [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ: Alzheimer’s disease: genotypes, phenotype, and treatments. Science 1997, 275:630-631 [DOI] [PubMed] [Google Scholar]
- 3.Hardy J: The Alzheimer family of diseases: many etiologies, one pathogenesis? Proc Natl Acad Sci USA 1997, 94:2095-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geula C, Wu C-K, Saroff D, Lorenzo A, Yuan M, Yankner BA: Aging renders the brain vulnerable to amyloid β-protein neurotoxicity. Nat Med 1998, 4:827-831 [DOI] [PubMed] [Google Scholar]
- 5.Terry RD: The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol 1996, 55:1023-1025 [PubMed] [Google Scholar]
- 6.Wisniewski HM, Wegiel J, Kotula L: Some neuropathological aspects of Alzheimer’s disease and its relevance to other disciplines. Neuropathol Appl Neurobiol 1996, 22:3-11 [DOI] [PubMed] [Google Scholar]
- 7.Roses AD, Saunders AM: ApoE, Alzheimer’s disease, and recovery from brain stress. Ann NY Acad Sci 1977, 826:200-212 [DOI] [PubMed] [Google Scholar]
- 8.Askanas V, Engel WK, Alvarez RB: Light- and electronmicroscopic localization of β-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol 1992, 141:31-36 [PMC free article] [PubMed] [Google Scholar]
- 9.Askanas V, Alvarez RB, Engel W: β-amyloid precursor epitopes in muscle fibers of inclusion-body myositis. Ann Neurol 1993, 34:551-560 [DOI] [PubMed] [Google Scholar]
- 10.Askanas V, Engel WK, Bilak M, Alvarez RB, Selkoe DJ: Twisted tubulofilaments of inclusion-body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol 1994, 144:177-187 [PMC free article] [PubMed] [Google Scholar]
- 11.Askanas V, Engel WK: Newest approaches to diagnosis and pathogenesis of sporadic inclusion-body myositis and hereditary inclusion-body myopathies, including molecular-pathologic similarities to Alzheimer disease. Askanas V Serratrice G Engel WK eds. Inclusion-Body Myositis and Myopathies. 1998, :pp 3-78 Cambridge University Press, Cambridge [Google Scholar]
- 12.Mitrani-Rosenbaum S, Argov Z, Blumenfeld A, Seidman CD, Seidman JG: Hereditary inclusion body myopathy maps to chromosome 9p1–q1. Hum Mol Genet 1996, 5:159-163 [DOI] [PubMed] [Google Scholar]
- 13.Middleton LT, Christodoulou K, Askanas V, Engel WK, McFerrin J, Kyriakides T, Zamba E, Papadopoulou E: Molecular genetics of autosomal-recessive hereditary inclusion-body myopathy (AR-IBM). Ann Neurol 1997, 42:414 [Google Scholar]
- 14.Sivakumar K, Cervenáková L, Dalakas MC, Leon-Monzon M, Isaacson SH, Nagle JW, Vasconcelos O, Goldfarb LG: Exons 16, and 17 of the amyloid precursor protein gene in familial inclusion body myopathy. Ann Neurol 1995, 38:267-269 [DOI] [PubMed] [Google Scholar]
- 15.Sarkozi E, Askanas V, Johnson SA, Engel WK, Alvarez RB: β -amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. NeuroReport 1993, 4:815-818 [DOI] [PubMed] [Google Scholar]
- 16.Sarkozi E, Askanas V, Engel WK: Abnormal accumulation of prion protein mRNA in muscle fibers of patients with sporadic inclusion-body myositis and hereditary inclusion-body myopathy. Am J Pathol 1994, 145:1280-1284 [PMC free article] [PubMed] [Google Scholar]
- 17.Askanas V, Engel WK: New advances in the understanding of sporadic inclusion-body myositis and hereditary inclusion-body myopathies. Curr Opin Rheumatol 1995, 7:486-496 [DOI] [PubMed] [Google Scholar]
- 18.Askanas V, Engel WK: Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol 1998, 10:530-542 [DOI] [PubMed] [Google Scholar]
- 19.Mendell JR, Sahenk Z, Gales T, Paul L: Amyloid filaments in inclusion body myositis. Arch Neurol 1991, 48:1229-1234 [DOI] [PubMed] [Google Scholar]
- 20.Askanas V: New developments in hereditary inclusion-body myopathies. Ann Neurol 1997, 41:421-422 [DOI] [PubMed] [Google Scholar]
- 21.Askanas V, Engel WK: Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: diseases of oxidative stress and aging? Arch Neurol 1998, 55:915-920 [DOI] [PubMed] [Google Scholar]
- 22.Lippa CF, Saunders AM, Smith TW, Swearer JM, Drachman DA, Ghetti B, Nee L, Pulaski-Salo, Dickson D, Robitaille Y, Bergeron C, Crain B, Benson MD, Farlow M, Hyman BT, St. George-Hyslop P, Roses AD, Pollen DA: Familial and sporadic Alzheimer’s disease: neuropathology cannot exclude a final common pathway. Neurology 1996, 46:406-412 [DOI] [PubMed] [Google Scholar]
- 23.Hyman BT: Alzheimer’s disease or Alzheimer’s diseases? clues from molecular epidemiology. Ann Neurol 1996, 40:135-136 [DOI] [PubMed] [Google Scholar]
- 24.Askanas V, McFerrin J, Baqué S, Alvarez RB, Sarkozi E, Engel WK: Transfer of β-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc Natl Acad Sci USA 1996, 93:1314-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Askanas V, McFerrin J, Alvarez RB, Baqué S, Engel WK: βAPP gene transfer into cultured human muscle induces inclusion-body myositis aspects. NeuroReport 1997, 8:2155-2158 [DOI] [PubMed] [Google Scholar]
- 26.Fukuchi K-I, Pham D, Hart M, Li L, Lindsey JR: Amyloid-β deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am J Pathol 1998, 153:XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin L-W, Hearn MG, Ogburn CE, Dang N, Nochlin D, Ladiges WC, Martin GM: Transgenic mice over-expressing the C-99 fragment of β PP with an α-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol 1998, 153:XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askanas V, Engel WK, Alvarez RB: Enhanced detection of congo-red-positive amyloid deposits in muscle fibers of inclusion-body myositis and brain of Alzheimer disease using fluorescence technique. Neurology 1993, 43:1265-1267 [DOI] [PubMed] [Google Scholar]
- 29.Engel WK, Askanas V: Treatment of inclusion-body myositis and hereditary inclusion-body myopathy with reference to pathogenic mechanisms. Personal experience. Askanas V Serratrice G Engel WK eds. Inclusion-Body Myositis and Myopathies. 1998, :pp 351-382 Cambridge University Press, Cambridge [Google Scholar]
- 30.McFerrin J, Engel WK, Askanas V: Impaired innervation of cultured human muscle overexpressing βAPP experimentally and genetically: relevance to inclusion-body myopathies. NeuroReport 1998, 9:3201-3205 [DOI] [PubMed] [Google Scholar]
- 31.Masliah E: Biology of disease: role of amyloid precursor protein in the mechanisms of neurodegeneration in Alzheimer’s disease. Lab Invest 1997, 77:197-209 [PubMed] [Google Scholar]
- 32.Hansen LA, Terry RD: Position paper on diagnostic criteria for Alzheimer disease. Neurobiol Aging 1997, 18:S71-S73 [DOI] [PubMed] [Google Scholar]
- 33.Westaway D, DeArmond SJ, Cayetano-Canlas J, Groth D, Foster D, Yang S-L, Torchia M, Carlson GA, Prusiner SB: Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell 1994, 76:117-129 [DOI] [PubMed] [Google Scholar]
- 34.Askanas V, Engel WK, Alvarez RB: Fourteen newly recognized proteins at the human neuromuscular junctions and their non-junctional accumulation in inclusion-body myositis. Richman DP eds. Myasthenia Gravis and Related Diseases: Disorders of the Neuromuscular Junction, 1998, vol 841.:pp 28-56 Annals of the New York Academy of Sciences, New York [DOI] [PubMed] [Google Scholar]
- 35.Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK: Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 1997, 6:1951-1959 [DOI] [PubMed] [Google Scholar]
- 36.Greenberg BD, Savage MJ, Howland DS, Ali SM, Siedlak SL, Perry G, Siman R, Scott RW: APP transgenesis: approaches toward the development of animal models for Alzheimer disease neuropathology. Neurobiol Aging 1996, 17:153-171 [DOI] [PubMed] [Google Scholar]
- 37.Harrington CR, Anderson JR, Chan KK: Apolipoprotein E type E4 allele frequency is not increased in patients with sporadic inclusion-body myositis. Neurosci Lett 1995, 183:35-38 [DOI] [PubMed] [Google Scholar]
- 38.Askanas V, Engel WK, Mirabella M, Weisgraber KH, Saunders AM, Roses AD, McFerrin J: Apolipoprotein E alleles in sporadic inclusion-body myositis, and hereditary inclusion-body myopathy. Ann Neurol 1996, 40:264. [DOI] [PubMed] [Google Scholar]
- 39.Garlepp MJ: Genetics of the idiopathic inflammatory myopathies. Curr Opin Rhematol 1996, 8:514-520 [DOI] [PubMed] [Google Scholar]
- 40.Sivakumar K, Semino-Mora C, Dalakas MC: An inflammatory, familial, inclusion body myositis with autoimmune features and a phenotype identical to sporadic inclusion body myositis: studies in three families. Brain 1997, 120:653-661 [DOI] [PubMed] [Google Scholar]
- 41.Hardy J: Amyloid, the presenilins, and Alzheimer’s disease. TINS 1997, 20:154-159 [DOI] [PubMed] [Google Scholar]
- 42.Blacker D, Tanzi RE: The genetics of Alzheimer disease: current status and future prospects. Arch Neurol 1998, 55:294-296 [DOI] [PubMed] [Google Scholar]
- 43.Mirabella M, Engel WK, Passinetti G, Finch CE, Askanas V: Denervation of adult human muscle fibers induces apoptosis, evidenced by fragmentation of nuclear DNA, and increased expression of the clusterin (ApoJ) gene. Neurology 1996, 46:2708559399 [Google Scholar]
- 44.Schneider C, Gold R, Dalakas MC, Schmied M, Lassmann H, Toyka KV, Hartung H-P: MHC class I-mediated cytotoxicity does not induce apoptosis in muscle fibers nor in inflammatory T cells: studies in patients with polymyositis, dermatomyositis and inclusion body myositis. J Neuropathol Exp Neurol 1996, 55:1205-1209 [DOI] [PubMed] [Google Scholar]
- 45.Behrens L, Bender A, Johnson MA, Hohlfeld R: Cytotoxic mechanisms in inflammatory myopathies. Co-expression of Fas and protective Bcl-2 in muscle fibres and inflammatory cells. Brain 1997, 120:929-938 [DOI] [PubMed] [Google Scholar]
- 46.Alvarez RB, Simmons Z, Engel WK, Askanas V: New autosomal-dominant inclusion-body myopathy (AD-IBM) with many congophilic muscle nuclei that contain paired-helical filaments (PHFs) composed of phosphorylated tau. Neurology 1998, 50:204 [Google Scholar]
- 47.Neve RL, Robakis NK: Alzheimer’s disease: a re-examination of the amyloid hypothesis. TINS 1998, 21:15-19 [DOI] [PubMed] [Google Scholar]
- 48.Mirabella M, Alvarez RB, Engel WK, Weisgraber KH, Askanas V: Apolipoprotein E and apolipoprotein E messenger RNA in muscle of inclusion-body myositis and myopathies. Ann Neurol 1996, 40:864-872 [DOI] [PubMed] [Google Scholar]
- 49.Imahori K, Hoshi M, Ishiguro K, Sato K, Takahashi M, Shiurba R, Yamaguchi H, Takashima A, Uchida T: Possible role of tau protein kinases in pathogenesis of Alzheimer’s disease. Neurobiol Aging 1998, 19:S93-S98 [DOI] [PubMed] [Google Scholar]