Abstract
Glutaraldehyde-treated porcine aortic valve xenografts frequently fail due to calcification. Calcification in the prostheses begins intracellularly. In a previous study, various types of cell injury to canine valvular fibroblasts, including glutaraldehyde treatment, led to calcification. An influx of extracellular Ca2+ into the phosphate-rich cytosol was theorized to be the mechanism of calcification. To test the Ca2+ influx theory, cytosolic Ca2+ and Pi concentrations were assessed in glutaraldehyde-treated porcine aortic valve fibroblasts, and their relationship to a subsequent calcification was studied. Glutaraldehyde caused an immediate and sustained massive cytosolic Ca2+ increase that was dose dependent and a several-fold increase in Pi. Calcification of cells followed within a week. The earliest calcification was observed in blebs formed on glutaraldehyde-treated cells. Live control cells or cells fixed with glutaraldehyde in Ca2+-free solution did not calcify under the same conditions. Concomitant increases in Ca2+ and Pi in glutaraldehyde-treated cells appear to underlie the mechanism of calcification, and the presence of extracellular Ca2+ during glutaraldehyde fixation promotes calcification.
Calcification in human aortic valves (AVs) occurs commonly. It begins at approximately the age of 20 years, increases progressively with age, and gives rise to calcific aortic stenosis in 2.9% of the elderly population. 1,2 Calcification is enhanced in damaged valves such as those with congenital anomalies or rheumatic valvulitis. Because of the shortage of human replacement prosthetic heart valves, aortic valves with calcific stenosis have been replaced with glutaraldehyde (GA)-fixed valvular prostheses (GFVPs) from animals. GA fixation eliminates antigenicity and provides GFVPs with tensile strength and pliability. Unfortunately, one-third of GFVPs in elderly recipients fail within 10 years mainly due to calcification. 3,4 Calcification of GFVPs in children, like other pathological calcifications in general, progresses more rapidly, resulting in their failure in 2 to 5 years. 5 Forty-two percent of GFVP failures occur in patients younger than 40 years of age. 6 The mechanism of calcification in GFVPs is incompletely understood. There are currently four mechanistic theories that have been proposed to explain the cause of GFVP calcification: 1) glutaraldehyde fixation, 2) organic matrix composition, 3) mechanical stress, and 4) cell injury theories.
There has been a view that GA molecules retained in GFVPs may cause calcification. GFVPs are known to calcify in rat subcutis, whereas fresh valves provoke inflammation but do not calcify. 7 The amount of GA in bovine pericardium rat implants has also been shown to have a quantitative relationship with calcific deposits. 8 However, there have been other reports that question the role of GA in calcification. Fifty percent of GA leaches out of GFVPs in rat subcutis over a period of weeks, and in failed human GFVP xenografts, acid hydrolyzable GA is known to decrease with the graft’s duration. 9,10 Paradoxically, fixation of porcine aortae in a higher concentration of GA diminishes calcification in rat subcutis. 11
The role of the extracellular matrix in calcification has also been extensively studied. Collagen, as in other calcifying tissues, has been implicated as a nucleator of apatite in GFVPs. 12 Osteocalcin and osteopontin have been isolated from calcified tissues, including GFVPs, suggesting they may play a role in calcification. 7,13 However, a study with isolated matrix demonstrated that osteopontin and osteocalcin did not nucleate apatite in vitro. 14 Other noncollagenous proteins, especially phosphoproteins, have been implicated in GFVP calcification. 15
In orthotopically grafted GFVPs, calcific deposits have been found selectively in the areas of mechanical stress. 16 Insudation of the plasma contents into the stressed areas may have a role in calcification. 17 Alternatively, relentless oscillations of the valves may let calcified particles migrate and accumulate in the stressed areas.
Calcification in xenografted GFVPs has been shown to begin intracellularly, 18 and several types of cell injury, including GA treatment, result in canine aortic valve (AV) fibroblast calcification. 19 Furthermore, removal of cells from GFVPs with detergents and lipid solvents has been shown to prevent calcification. 20-22 Nevertheless, the cell’s role in GFVP calcification has not been studied in detail. Cell injury has long been known to cause an influx of Ca2+ into the cell. 23 Recently, there has been increasing evidence supporting that cell injury also increases cytosolic phosphate ([Pi]i). 24-26 Concomitant increases in intracellular Ca2+ ([Ca2+]i) and [Pi]i have been theorized to be the underlying mechanism of calcification. 27 In the present study, we have tested the hypothesis that calcification in GFVPs results from GA-induced increases in [Ca2+]i and [Pi]i during the fixation process. Porcine AV fibroblasts were grown in culture and subsequently fixed with GA while measuring cellular and media Ca2+ and Pi changes immediately after fixation and over a period of 7 days. GA was found to cause a massive increase in [Ca2+]i and several-fold increases in [Pi]i followed by calcification of the cells.
Materials and Methods
The acetoxymethyl esters of Fura-2, Indo-1, Fluo-3, Calcium Green-1, and Oregon Green 488 BAPTA-1 (fura-2/AM, indo-1/AM, fluo-3/AM, CaGr-1/AM, and OrGr-1/AM) were purchased from Molecular Probes (Eugene, OR), and GA (50%) was from E. F. Fullam (Schenectady, NY). Fetal bovine serum was obtained from GIBCO (Grand Island, NY). Tissue culture media and analytic grade chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell Culture
Porcine aortic valves were obtained from a local slaughterhouse within 30 minutes of sacrifice. Valves for cell culture were transported in ice-chilled RPMI 1640 with 20% fetal bovine serum, 1.25 μg/ml amphotericin B, and 50 μg/ml gentamicin sulfate. The valves were treated with 15% collagenase for 15 minutes, endothelial cells were removed with a scalpel, and the remaining tissue was rinsed twice. The tissue was minced with scalpels, explanted, and subcultured in minimal essential medium supplemented with 10% fetal bovine serum, 100 IU of penicillin, and 100 μg/ml streptomycin under 5% CO2 at 37°C and 90% humidity.
Measurement of [Ca2+]i
Dual-excitation ratiometry of fura-2-loaded cells was performed with a model RM-D Deltascan photometry system (Photon Technology International, Brunswick, NJ). Dual-emission ratiometry of indo-1-loaded cells and single-wavelength fluorescence image analysis of [Ca2+]i were performed with a Bio-Rad MRC-1000 confocal microscope (Bio-Rad Microscience, Herts, UK) with a Diaphot inverted microscope (Nikon, Garden City, NY) with krypton-argon and ultraviolet argon ion laser attachments (Coherent Enterprise, Salt Lake City, UT). LaserSharp 1024 Time Course software (Bio-Rad) and a 40× Fluor oil emulsion objective lens with NA of 1.3 (Nikon) were used.
Semiconfluent cells on 25-mm round coverslips were mounted on an Attofluor cell chamber (Molecular Probes) and were loaded with 5 μmol/L indo-1/AM in phenol-red-free Hanks’ balanced salt solution with 25 mmol/L HEPES, and the total calcium was adjusted to 2.0 mmol/L with CaCl2, pH 7.4 (HBSS2.0), for 30 minutes at 37°C. The cells were allowed to de-esterify for an additional 30 minutes. Alternatively, the cells were loaded with fura-2/AM for 20 minutes and de-esterified for 20 minutes at 37°C or loaded with 5 μmol/L fluo-3, 5 μmol/L CaGr-1/AM, or 5 μmol/L OrGr-1/AM for 30 minutes at room temperature and de-esterified for 30 minutes. The de-esterification step was omitted for fluo-3. Ratiometry of indo-1-loaded cells was performed at an excitation wavelength (WLEX) of 351 nm and emission wavelengths (WLEM) of 460 and 405 nm, and with fura-2, at WLEX of 340 and 380 nm and WLEM of 510 nm. Single-wavelength [Ca2+]i measurements were performed at WLEX of 488 nm and WLEM of 531 nm for CaGr-1 and 523 nm for fluo-3 and OrGr-1.
[Ca2+]i in CaGr-1-loaded cells was measured either in HBSS2.0 or in HBSS with the serum level (2.5 mmol/L) of calcium (HBSS2.5). [Ca2+]i in selected samples was measured in Ca2+-free HBSS (HBSS-0) or in HBSS2.0 with 10 mmol/L EGTA to determine possible intracellular redistribution of Ca2+ caused by GA. To convert fluorescence intensity (FI) to nanomoles per liter, [Ca2+]i measurement with CaGr-1 was calibrated using a calibration kit (Bio-Rad, 170-3141) according to the formula [Ca2+]i = Kd[F − Fmin]/[Fmax − F], where Kd is the dissociation constant of CaGr-1, F is the fluorescence of the indicator at the experimental calcium level, and Fmin is the fluorescence in the absence of extracellular Ca2+ ([Ca2+]o); Fmax was determined by treating CaGr-1-loaded cells with 1.66 μmol/L Ca2+ ionophore 4-Br-A23187. 28 The LaserSharp software has a built-in calibration program. To simulate GFVP preparation, [Ca2+]i in cells treated with 0.2% to 0.6% GA was measured at room temperature. Optimal loading of the dyes and the topography of GA-induced changes in [Ca2+]i were assessed with fluorescence images. Cells with mitochondrial loading of the dyes and signs of injury, ie, blebs, were excluded from the study. To illustrate the distribution of [Ca2+]i increase in GA-treated cells, 12-band pseudocolors ranging from blue to white were assigned to 0 to 255 gray scales of captured images with the LaserSharp program.
[Pi]i Measurement
A total of 5 × 10 5 cells suspended by trypsinization in triplicate were fixed with 0.6% GA in HBSS-0 or HBSS2.5 at room temperature, rinsed three times with 25 mmol/L HEPES in 0.9% saline solution, pH 7.4, by centrifugation, and pelleted in microfuge tubes. The pellets were ground with a motorized pestle, dissolved in 0.25 ml of 0.6 mol/L perchloric acid, neutralized with 1.25 ml of 3 mol/L KOH, and centrifuged at 16,000 × g for 5 minutes. The supernatants were stored at −80°C for up to a week. Pi in the supernatant was measured by an ammonium molybdate color reaction at 720 nm by the method of Ohnishi et al. 29 The extraction procedures were carried out on ice in a cold room.
Calcification in Vitro
Confluent cells in 25-cm 2 flasks in triplicate were treated with 0.6% GA in HBSS2.5 for 24 hours at room temperature. After rinsing away the GA, cells were incubated in 10 ml of HBSS2.5 without GA for 7 days at 37°C. Live cells incubated in minimal essential medium with 2.5 mmol/L total calcium (MEM2.5) in a CO2 incubator or cells fixed with 0.6% GA in HBSS-0 and incubated in HBSS2.5 served as controls. Samples were harvested on days 1, 2, 3, and 7. Free Ca2+ in the supernatant was measured with an ion-selective electrode (Radiometer, Cleveland, OH). Pi was measured by an ammonium molybdate color reaction at 720 nm. 13 The cells were subsequently fixed in 10% formalin in phosphate-buffered saline for 3 minutes and stained with 0.03% calcein for 1 minute. Calcein is a fluorochrome that has been used to fluoresce calcium phosphate deposits in mineralizing cell cultures, 30 and fluorescence densitometry of calcein-stained samples has been shown to be a suitable technique for quantification of calcific deposits in fibroblasts. 19 Fluorescence densitometry was performed on 10 tandem areas, using a 10× objective lens at WLEX of 488 nm and WLEM of 520 nm. To capture detached cells and blebs, the supernatant was filtered through a polycarbonate membrane filter with pore size of 0.2 μm using a syringe. The surface area of the filter was corrected for 25 cm2, and the FI was added to that of attached cells. Polycarbonate membrane is neither autofluorescent nor stainable with calcein.
Electron Microscopy
Cells suspended by trypsinization were fixed with 0.6% GA and incubated in HBSS2.5 in airtight plastic tubes on a rotary drum at 37°C for a week. Cell pellets were embedded in agar, cut into 1-mm 3 cubes and fixed in 4% GA in cacodylate buffer, post-fixed in OsO4 in s-collidine buffer, dehydrated in ethanol and propylene oxide, and embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate and examined with a Philips 200 microscope. Selected area electron diffraction was performed at 80 kV. The diffraction pattern was measured according to d = D/K, where d is interplanar spacing, D is diameter of the powder pattern, and K is the camera constant; K is determined by diffraction of ThCl sputtered grids at each diffraction of the sample. 31 Crystals were identified with the Inorganic Powder Diffraction Files, Joint Committee on Powder Diffraction Standards (Philadelphia, PA).
Statistical Analysis
Statistical analysis was performed using the SigmaStat software (SPSS, Chicago, IL).
Results
In fura-2- and indo-1-loaded fibroblasts, GA treatment caused an immediate increase in the fluorescence ratio that was soon followed by a decrease in FI (data not shown). In contrast, when 0.6% GA was added to 1 μmol/L potassium salt of fura-2 in cell-free 6 mmol/L HEPES buffer containing 2 mmol/L CaCl2, pH 7.4, and the ratiometry was repeated, GA completely abolished fura-2’s fluorescence within minutes. The quenching of fluorescence was less pronounced in cells loaded with fluo-3 or OrGr-1. No such fluorescence depression was noted in CaGr-1-loaded cells, although the spike in FI still occurred. The spike was produced even when CaGr-1-loaded cells were in HBSS-0 or in HBSS2.0 with 10 mmol/L EGTA. Because GA appeared to have quenching effects on fura-2, indo-1, OrGr-1, and fluo-3 and because CaGr-1 fluorescence did not appear to be affected by GA, CaGr-1 was chosen for [Ca2+]i measurements.
When CaGr-1 was used, GA caused a progressive rise in FI for over 1 hour and in a dose-dependent manner. FI of cells treated with 0.6% GA approached the Fmax obtained from the cells treated with Ca2+ ionophore (1.66 μmol/L 4-Br-A23187). Throughout the experiment, the intensity of cell-free background changed little or none. CaGr-1 FI was converted to [Ca2+]i using the aforementioned BioRad kit and fluorescence equation. Photometric data for CaGr-1-loaded cells are shown in Figure 1 ▶ . [Ca2+]i tended to be higher when measured in HBSS2.5 than in HBSS2.0. Otherwise, the [Ca2+]i response to GA was similar in both solutions (data not shown). Pseudocolored images demonstrated the increase of [Ca2+]i especially in the perinuclear area (Figure 2) ▶ .
Figure 1.
Photometric data of [Ca2+]i response to 0.2% to 0.6% GA treatment in CaGr-1-loaded porcine AV fibroblasts. GA caused an immediate increase in [Ca2+]i followed by progressive increases in [Ca2+]i in a dose-dependent manner.
Figure 2.
A montage of pseudocolored images of a chain of CaGr-1-loaded fibroblasts treated with 0.6% GA demonstrating increases in [Ca2+]i. Images were captured at time zero and every 12 minutes. [Ca2+]i increase is seen mainly in perinuclear areas. Pseudocolors ranging from blue to white were assigned to 0 to 255 gray scales.
GA also caused several-fold increases in [Pi]i within hours. It remained elevated for 24 hours and returned to the ground status in a week. The increase in [Pi]i was greater in cells fixed with 0.6% GA in HBSS2.5 than those fixed in HBSS-0 (Figure 3) ▶ . A slight decrease in Ca2+ and an increase in Pi in the supernatant during GA fixation for over 24 hours was noted.
Figure 3.
[Pi]i in cells treated with 0.6% GA in HBSS2.5 (solid bars) and in HBSS-0 (gray bars). GA caused an increase in [Pi]i within hours especially in cells fixed in the presence of [Ca2+]o. [Pi]i remained elevated for 24 hours and returned to the ground status in a week. Error bars indicate SEM.
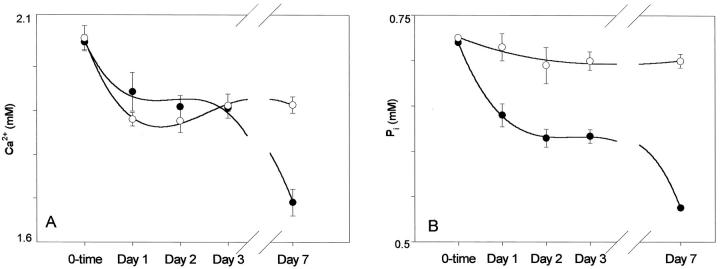
Cells fixed with 0.6% GA and incubated in HBSS2.5 progressively depleted Ca2+ and Pi from the supernatant, whereas cells fixed in HBSS-0 did not (Figure 4) ▶ . After an initial decline on day 1, the slopes of ion deple- tions leveled off for a few days followed by a precipitous decline in day 7. No ion depletion from the supernatant of the unfixed live control cells was noted for a week. In the supernatant of cells fixed in HBSS-0, the precipitous decline did not occur at day 7 and ion concentrations remained level.
Figure 4.
A: Depletions of Ca2+ from HBSS2.5 by cells fixed with 0.6% GA in HBSS2.5 (•) and in HBSS-0 (○). HBSS2.5 Ca2+ leveled off for a few days after an initial drop. A precipitous decline of Ca2+ occurred with cells previously fixed in HBSS2.5, whereas Ca2+ remained level with cells previously fixed in HBSS-0. B: A progressive depletion of Pi from HBSS2.5 was caused by cells fixed with 0.6% GA in HBSS2.5. Cells fixed in HBSS-0 and incubated in HBSS2.5 did not deplete Pi.
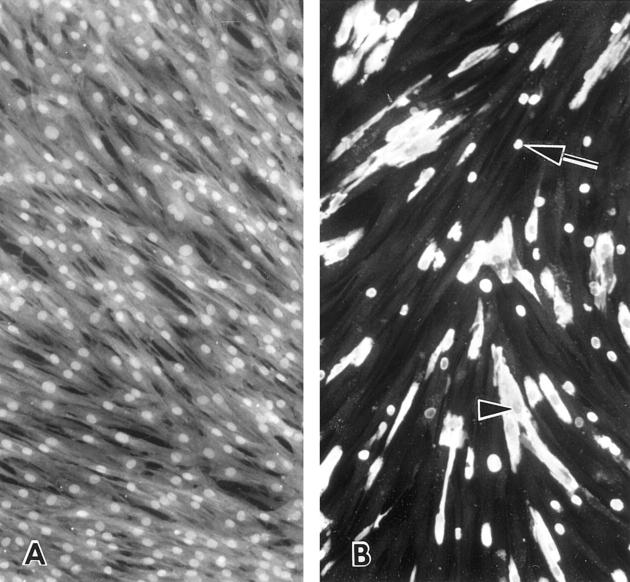
Calcein has been shown to have a greater sensitivity for calcium detection than tetracycline or alizarin red-S. 19 When stained with calcein, the control cells without GA treatment displayed a higher FI in nuclei than the cytoplasm. The higher nuclear FI was also seen in routinely cultured cells without incubation in MEM2.5. Cells fixed with 0.6% GA in HBSS2.5 began to form blebs with increased calcein FI during the fixation period. Blebs frequently detached from the cells and floated in the supernatant. Compared with other types of cell injury, 27 the size and the number of blebs formed by GA-treated cells were smaller. Most blebs were smaller than the corresponding cellular nuclei. The number of calcified blebs increased mildly during the incubation in HBSS2.5 followed by calcification of the entire cells by day 7. What appeared to be mitochondria frequently emitted more intense fluorescence in the earlier stages of the incubation. Nuclei were usually spared from calcification in heavily calcified cells (Figure 5) ▶ . Calcified cells also tended to detach from the flask surface. A comparison of media Ca2+ depletion with cellular calcein FI revealed an inverse linear relationship with a regression coefficient of 0.79 (Figure 6) ▶ . Blebs were scanty to absent and calcification was not evident in cells fixed in HBSS-0, incubated in HBSS2.5, and stained with calcein.
Figure 5.
A: Calcein fluorescence micrographs of live control cells incubated in MEM2.5 for a week. Nuclei are brighter than the cytoplasm. B: Calcein-stained cells fixed in 0.6% GA in HBSS2.5 for 24 hours and incubated in HBSS2.5 for a week. Calcified cells and blebs (arrow) emitted strong fluorescence. Nuclei of heavily calcified cells are usually spared from calcification (arrowhead). The pale, noncalcified cells in the background are due to automated camera exposure time. Magnification, ×300.
Figure 6.
A: Calcein fluorescence of cells treated with 0.6% GA and incubated in HBSS2.5 showed an inverse relationship with Ca2+ depletion. B: Regression analysis yielded a coefficient of 0.79. Elimination of the outlier on day 1 increased the coefficient to 0.99.
Electron microscopy of cells fixed in 0.6% GA and incubated in HBSS2.5 frequently revealed blebs. The earliest (least) calcific deposits were seen in blebs, either in apposition to the inner surface of plasma membrane or in what appeared to be swollen mitochondria (Figure 7) ▶ . Blebs detached from the cells frequently contained large amounts of needle-shaped apatite. In heavily calcified cells, apatite deposited diffusely in the cytoplasm in apposition to the surface of organelles. In occasional cells, only nuclei were selectively calcified. Although a limited number of cells were examined, cells with nuclear calcification did not appear to form calcifying blebs. Cells fixed in HBSS-0 showed only ruffling of the plasma membrane resulting from trypsinization but no blebs or calcific deposits. Electron diffraction of calcified cells yielded the powder pattern consistent with hydroxyapatite.
Figure 7.

Electron micrographs of suspended cells fixed in 0.6% GA for 24 hours and incubated in HBSS2.5 for a week. A: A cell with calcified blebs. No calcific deposits are seen in the cytoplasm. Magnification, ×7500. B: A closer view of the tip of a bleb indicated by the arrowhead in A. Needle-shaped apatite crystals abut against the inner surface of the membrane. Magnification, ×32,500. C: A calcific deposit in what appears to be a swollen mitochondrion in a bleb. Magnification, ×8300. Mitochondria outside of the bleb show matrix condensation but no apatite. Radiating needles are seen in a closer view of the deposit (inset, ×34,000). D: A heavily calcified cell with diffuse apatite deposition in the cytoplasm. The nucleus did not calcify. Magnification, ×7500. E: A higher magnification of the perinuclear area in D. Needle-shaped apatite crystals are apparent. Magnification, ×32,500. F: Electron diffraction of a calcified cell yielded the powder pattern consistent with hydroxyapatite. G: In occasional cells, the deposits were selectively seen in nuclei. No calcified blebs but a ruffled plasma membrane due to trypsinization were present. Magnification, ×8500. H: A closer view of an area in G demonstrates needle-shaped apatite in the nucleus. Magnification, ×25,000.
Discussion
Crystals are formed by the orderly packing of atoms and molecules that are mainly brought together by van der Waals attractive forces. 32 The attractive forces are weaker than chemical bonds, and crystal nucleation requires supersaturation of lattice ions. The kinetics of crystal formation differ from that of chemical reactions in that once nucleation takes place after a latent period, the subsequent crystal growth is autocatalytic and occurs rapidly. Crystal nucleation can be entirely dependent on lattice ion concentrations, in which case it is referred to as homogeneous nucleation, or the nucleation may be catalyzed by solid particles, a process known as heterogeneous nucleation. 33,34 Because of the abundance of solids that exist in cells, Ca2+ and Pi crystallization in vivo most likely occurs by heterogeneous nucleation. As a consequence, calcific deposits in vivo are frequently observed in apposition to membranous surfaces. 27 Under normal conditions, the serum Ca2+ and Pi concentrations are low enough that their solubility coefficient is not exceeded, and homogeneous nucleation does not occur. The addition of a minute particle of apatite to serum, however, results in a rapid proliferation of apatite crystals until a new Ca2+ and Pi equilibrium is reached at a value approximately one-third the normal serum level. 35 Thus, calcific deposits in vivo appear to exist in a disequilibrium with serum Ca2+ and Pi. Apatite nucleation is prevented by plasmalemmal partitioning of the high [Ca2+]o away from the high [Pi]i. Live tissues also apparently have a capacity to curb the spread of calcification probably by secreting inhibitors for apatite growth; hence, osteocytes survive in densely calcified bone.
In our studies, CaGr-1 was used to assess the influx of Ca2+ into AV fibroblasts during fixation with GA. [Ca2+]i in resting valvular fibroblasts ranges between 100 and 150 nmol/L, whereas [Ca2+]o is approximately 1.5 mmol/L. Thus, there is a steep electrochemical gradient of Ca2+ across the plasmalemma. GA fixation led to dose-dependent, progressive increases in [Ca2+]i over a period of 1 hour (see Figures 1 and 2 ▶ ▶ ). After an initial immediate increase in [Ca2+]i, there was a relatively slow and sustained increase when compared with the transient surges usually associated with signal transduction. This increase eventually reached a value comparable to that obtained when unfixed porcine AV fibroblasts were treated with Ca2+ ionophore 4-Br-A23187 and subsequently allowed to equilibrate with [Ca2+]o. These observations suggest that GA initially permeabilizes the plasmalemma to Ca2+ and allows leakage into the cell. Intracellular Ca2+ overload exerts many deleterious effects to the cell and is considered to be the underlying mechanism of cell death, both apoptosis and necrosis. 23,36 Physiological increases of [Ca2+]i stimulate mitochondrial ATP synthesis, but a massive increase in mitochondrial Ca2+ collapses the proton-motive electric potential of the mitochondrial inner membrane and arrests ATP synthesis. 37 In acute cell injury, mitochondria themselves frequently calcify.
The mechanism of GA fixation is complex and incompletely understood. GA is believed to cross-link proteins mainly by piperidine ring formation via a Schiff base reaction. 38 Despite its extensive use for fixation of tissues for electron microscopy, relatively little is known about the detailed effects of GA on cell metabolism. As it diffuses into the cell, GA is likely to inactivate many enzymes. The progressive increase of [Ca2+]i in GA-treated cells suggests that Ca2+-Mg2+ ATPase of the plasma membrane no longer functions. GA has been shown to bind specifically to the ATP-binding sites of sarcoplasmic reticulum Ca2+ pumps and to abolish the pump function. 38 A similar inactivation of the plasma membrane Ca2+-Mg2+ ATPase seems likely. The cessation of ATP synthesis by overloaded [Ca2+]i and a generalized devitalization of the cell by GA are likely to contribute further to the abolition of the plasma membrane Ca2+ pump activity. On devitalization of the cell, [Ca2+]i will equilibrate with [Ca2+]o. Inhibition of plasma membrane Ca2+ pumps has been shown to induce calcification of canine AV fibroblasts. 19
In contrast to Ca2+, Pi is mainly intracellular. It exists mostly in organic forms. The [Pi]i in resting cells is estimated to range between 0.5 and 2 mmol/L by 31P nuclear magnetic resonance (NMR). 39 NMR tends to underestimate [Pi]i, detecting only one-third of the values obtained by chemical analysis. 24 [Pi]i is closely linked to hydrolysis of ATP, and in metabolically active cells, eg, rat skeletal muscle, it can be as high as 8 mmol/L. 40 With the recent advent of NMR, it became apparent that [Pi]i increases during cell injury, usually in association with a reversal of the ATP/Pi ratio. 24-26 In our GA-treated AV fibroblasts, GA led to a several-fold increase in [Pi]i over a period of hours that remained elevated for at least 24 hours (see Figure 3 ▶ ). In addition, the presence of Ca2+ in GA solution was associated with a greater increase in the [Pi]i than when GA solution contained no Ca2+. Overloaded [Ca2+]i has been shown to activate cell enzymes, ie, phospholipases and endonucleases. 36 The additional increase of [Pi]i in GA-treated cells can be attributed to the activation of hydrolytic enzymes for organic phosphates by Ca2+ and the arrest of ATP synthesis in Ca2+-overloaded mitochondria. The occurrence of calcification in mitochondria in GA-treated cells concurs with such Ca2+ increases in mitochondria. Alkaline phosphatase has been implicated to have a role in calcification, especially of matrix vesicles. 41 The enzyme may hydrolyze phosphoesters and thereby contribute to the increase in [Pi]i in GA-treated cells. The enzyme has been shown to persist in cells of GFVPs. 42-44 The gradual decline of [Pi]i in a week after a several-fold increase in [Pi]i in hours in GA-treated cells is evidently due to its leakage into the supernatant. Pi in the supernatant increases in association with the decrease in [Pi]i (results not shown). In our study, the [Ca2+]i × [Pi]i products in GA-treated cells exceed by several times that of serum, reaching values sufficient for heterogeneous nucleation of apatite. Valvular fibroblasts not treated with GA and maintained under otherwise similar conditions did not calcify. The occurrence of calcification in GA-treated cells but not in live control cells under similar conditions attests that the influx of [Ca2+]o into the Pi-rich cytosol is most likely the mechanism of calcification. The absence of calcification in cells fixed in HBSS-0 in comparison with cells fixed in HBSS2.5 is consistent with a necessary Ca2+ influx during the early stage of GA fixation, presumably when the [Ca2+]i · [Pi]i products are at the peak, leading to subsequent calcification of the cells. GA-treated cells apparently remain permeable to Ca2+ as evidenced by the progressive depletion of Ca2+ from HBSS2.5 (see Figure 4A ▶ ). In addition to the progressive Ca2+ depletion from HBSS2.5, the Pi content of the buffer also diminished with time, suggesting intracellular nucleation of Ca2+ and Pi was occurring in GA-treated fibroblasts.
After an initial relatively rapid depletion rate, HBSS2.5 ion depletions lagged for a few days, then progressed rapidly once again. These observations suggest that the intracellular apatite mass grows to a critical size and levels off before additional, more rapid extraction occurs from the buffer. There was also observed a relative delay in HBSS2.5 Pi depletion, when compared with Ca2+. This delay was most likely attributable to a higher [Pi]i than [Ca2+]i found initially in cells. Cells fixed in Ca2+-free media did not deplete Pi (see Figure 4B ▶ ). Calcein fluorescence densitometry studies of cells and blebs revealed that the earliest calcification preceded the aforementioned lag phase and occurred in blebs and mitochondria. This was followed by intense FI in the fibroblast soma (see Figure 5B ▶ ). HBSS2.5 Ca2+ depletion significantly and inversely correlated with cellular calcein FI, further indicating that intracellular nucleation occurred concomitantly with media ion depletion (see Figure 6 ▶ ). Interestingly, the ion depletion lag phase is not observed in other types of cell injury such as anoxia or repeated freezing and thawing, 19 presumably because membranes are damaged. Membranes are well preserved after GA treatment (see Figure 7 ▶ ). The mechanism of Pi entry into GA-treated cells was not addressed in the present study, but in functioning cells, Pi is co-transported along with Na+. 39 In this study, HBSS2.5 is tailored for the short-term experiments of cellular calcification, and the [Ca2+] was higher, whereas [Pi] was lower than in serum. This medium, however, allowed the identification of calcified cells within a week.
We also observed that the nuclei of control valvular fibroblasts exhibited a stronger calcein fluorescence than the cytosol. The nuclear concentration of Ca2+ is higher or lower than the cytosol depending on the type and the physiological status of the cells and is believed to have a role in cell proliferation. The nuclear membrane, which is continuous with the endoplasmic reticulum membrane has its own Ca2+ transport mechanisms, including inward Ca2+ pumps and inositol 1,4,5-trisphosphate receptors. 45 The observation of nuclear calcification indicates that nuclear Ca2+ and Pi can reach sufficiently high levels for apatite nucleation. Nuclear calcification has been described in calcification of GFVPs in vivo. 18
In addition to nuclear calcification, calcification may be promoted in injured cells through blebbing. Blebbing has long been known to occur in cell injury. 46 Blebs have been shown to be associated with Ca2+ overload in injured cells and to contain higher concentrations of Ca2+ than the remaining cytosol. 47,48 Cells apparently have a capacity to condense overloaded Ca2+ into blebs. Blebbing may be a cellular defense mechanism to protect against Ca2+ overload. Isolated blebs formed by senescent canine AV fibroblasts have been shown to readily calcify in MEM2.5, 19 and calcification in membranous cell debris has been observed in GFVPs removed from human hearts. 49 In a variety of human calcinoses, including aging aortic valves, bleb-like membranous vesicles are the predominant foci of calcification regardless of the type of tissues involved. 27 It has been proposed that matrix vesicles are blebs formed by apoptotic cells. For example, hypertrophic chondrocytes of the epiphysis, the major producer of matrix vesicles, undergo apoptosis.see 27
Our in vitro data are consistent with the hypothesis that blebbing may contribute to calcification in GFVPs. Calcein fluorescence of fibroblasts fixed in HBSS2.5 containing GA frequently revealed cellular blebbing (Figure 5) ▶ , and the earliest calcific deposits were observed in these blebs. Electron microscopy showed these deposits were usually found either in close apposition to the inner surface of the plasma membrane or in swollen mitochondria. Cells and blebs that became detached often contained large amounts of needle-shaped crystals that were confirmed by electron diffraction to be hydroxyapatite (Figure 7) ▶ . By the 7th day after GA exposure, calcification of entire cells was observed. In heavily calcified cells, apatite was deposited throughout the cytoplasm but usually in apposition to organelle surfaces. Blebs were scanty to absent, and calcification was not evident in cells fixed in HBSS-0 containing GA.
Thus, our studies of porcine AV fibroblasts suggest that the GA fixation process itself may be an important contributor to the calcification that occurs following after with GFVP. The data suggest that GA fixation leads to an early, sustained influx of Ca2+ into the valvular cell followed by apatite nucleation. In addition to the fixation process, others have provided evidence that tissue injury before GA fixation may affect the subsequent calcification of GFVPs. A delay in GA fixation of bovine pericardium and porcine aorta intensifies calcification of GFVPs in rat subcutis. 11,50 Furthermore, anoxic exposure at room temperature before GA fixation resulted in a heavy calcification of GFVPs, whereas the exposure at 4°C significantly reduced calcification in rat subcutis. 51 It has been theorized that GA preserves certain structures that promote calcification during the early stage of fixation, thus resulting in delayed calcification. 52 The major difficulty of the Ca2+ influx theory is linking GA-induced cell calcification, or any short-term calcification, with the slow progression of calcification of GFVPs that occurs in vivo. Calcification in GFVPs xenografted in human hearts progresses over years 4,5 and, in aging human aortic valves, over decades. 1
Several explanations can be offered for the delayed calcification in vivo. First, a variety of calcification inhibitors have been identified in tissue fluid. 53 Fibroblasts inhibit calcification of bone cells cultured in the same dish. 54 Cryopreserved AV allografts with viable cells survive twice as long as GFVPs in human hearts. 55 Thus, calcification of GFVPs in vivo appears to be delayed by cellular inhibitors. Second, GFVPs are usually fixed in a Ca2+-free solution. Therefore, GFVP extracellular Ca2+ is likely diluted by the fixative. This could lead to less Ca2+ influx into valvular cells during fixation and delayed calcification. Third, nucleated apatite during GA fixation of GFVPs is likely to be dissolved during the storage in Ca2+-free solutions. It has been shown that when calcified rat aortae were decalcified with EDTA, they calcify more readily than the aorta without previous calcification (unpublished data). Once tissues have been calcified in GA, they appear to retain a template for heterogeneous nucleation of apatite. If such a template exists in GFVPs, it could predispose the valves to delayed calcification after grafting. Fourth, cellular membranes may cause delayed calcification. Liposomes made of phosphatidylserine and red cell ghosts readily calcify in serum, suggesting the phospholipid bilayer may serve as a nidus for heterogeneous nucleation. 56 This may be one of the reasons that removal of cells from GFVPs prevents calcification. 19-21 In addition, a complex and poorly understood interaction exists between plasma membrane and the extracellular matrix. 57 The extracellular matrix may play the role of diffusion barrier for both fixative and ions and thus delay calcification. At the same time, the matrix may predispose valvular grafts to this delayed calcification. For example, collagen is known to stimulate calcification in matrix vesicles isolated from epiphyseal growth plates. 58
Along with tetracycline, calcein has been used for the study of new bone deposition. In view of its relative inertness, acetoxymethyl ester of calcein has also been used as a vital dye. Although calcein has a strong affinity for Ca,2+ it also reacts with a variety of divalent metals. However, as opposed to Ca2+ and Mg2+, binding of other metal ions quenches the fluorescence of calcein. 59 This property of the dye has allowed the measurement of labile iron pools in certain cells. 60 Calcific deposits in GFVPs are usually quantified by atomic absorption spectrophotometry of charred samples. The method is destructive and ignores topography of the deposits. Findings in this study further support that calcein can be useful for monitoring the progress of calcification, especially in cell cultures. Plasma membrane is impermeable to calcein. Fixation of the cells, eg, with formalin, is usually required for staining of cells with calcein. GA-treated cells obviates the need for the fixation. A small amount of calcein (0.15 μmol/L) added to HBSS2.5 allows a continuous monitoring of the progress of calcification in the same cells (results not shown).
In conclusion, cultured porcine AV fibroblasts appear to be a useful model for studies of the Ca2+ influx theory of GFVP calcification. GA treatment of cultured fibroblasts led to increases in [Ca2+]i and [Pi]i and intracellular heterogeneous nucleation of apatite. Early cellular blebbing appeared to play a significant role in the calcification process.
Acknowledgments
We thank Mrs. Barbara Rodriguez for her technical support in electron microscopy.
Footnotes
Address reprint requests to Dr. Kookmin M. Kim, Pathology and Laboratory Medicine Service, V.A. Medical Center, 510 Stoner Avenue, Shreveport, LA 71101-4295.
Supported in part by the American Heart Association, Louisiana Affiliate, grant LA97GS08.
References
- 1.Sell S, Scully RE: Aging changes in the aortic and mitral valves: histological and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am J Pathol 1965, 46:345-365 [PMC free article] [PubMed] [Google Scholar]
- 2.Lindroos M, Kupari M, Heikkila J, Tilvis R: Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993, 21:1220-1225 [DOI] [PubMed] [Google Scholar]
- 3.Vongpatanasin W, Hillis D, Lange RA: Prosthetic heart valves. N Engl J Med 1996, 335:407-416 [DOI] [PubMed] [Google Scholar]
- 4.Hammermeister KE, Sethi GK, Henderson WG, Oprian C, Kim T, Rahimtoola S: A comparison of outcome in men 11 years after heart-valve replacement with mechanical valve or bioprosthesis. N Engl J Med 1993, 328:1289-1301 [DOI] [PubMed] [Google Scholar]
- 5.Sanders SP, Levy RJ, Freed MD, Norwood WI, Castaneda AI: Use of Hancock porcine xenografts in children and adolescents. Am J Cardiol 1980, 46:429-438 [DOI] [PubMed] [Google Scholar]
- 6.Grunkmeier GL, Jamieson WRE, Miller DC, Starr A: Actuarial versus actual risk of porcine structural valve deterioration. J Thorac Cardiovasc Surg 1994, 108:709-718 [PubMed] [Google Scholar]
- 7.Levy RJ, Schoen FJ, Levy JT, Nelson AC, Howard SL, Oshry LJ: Biological determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol 1983, 113:143-155 [PMC free article] [PubMed] [Google Scholar]
- 8.Golomb G, Schoen FJ, Smith MS, Linden J, Dixon M, Levy RJ: The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac bioprostheses. Am J Pathol 1987, 127:122-130 [PMC free article] [PubMed] [Google Scholar]
- 9.Webb CL, Schoen FJ, Levy FJ: Covalent binding of aminopropane-hydroxidiphosphonate to glutaraldehyde residues in pericardial bioprosthetic tissue: stability and calcification inhibition studies. Exp Mol Pathol 1989, 50:291-302 [DOI] [PubMed] [Google Scholar]
- 10.Hughes H, Tipton LS, Feuchuk D, Probhakar G, Aboul-Enein HY, Duran CMG: Glutaraldehyde, γ-carboxyglutamic acid and calcium in explanted bioprosthetic heart valves. J Heart Valve Dis 1994, 3:111-116 [PubMed] [Google Scholar]
- 11.Zilla P, Weissenstein C, Bracher M, Zhang Y, Koen W, Human P, van Opel U: High glutaraldehyde concentrations reduce rather than increase the calcification of aortic wall tissue. J Heart Valve Dis 1997, 6:502-509 [PubMed] [Google Scholar]
- 12.Levy RJ, Schoen FJ, Sherman FS, Nichols J, Hawley MA, Lund SA: Calcification of subcutaneously implanted type I collagen sponges: effects of glutaraldehyde and formaldehyde pretreatments. Am J Pathol 1986, 122:71-82 [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM: Osteopontin is expressed in human aortic valvular lesions. Circulation 1995, 92:2163-2168 [DOI] [PubMed] [Google Scholar]
- 14.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA: Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J 1996, 317:59-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gura TA, Wright KL, Veis A, Webb CL: Identification of specific calcium-binding non-collagenous proteins associated with glutaraldehyde-preserved bovine pericardium in the rat subdermal model. J Biomed Mater Res 1997, 35:483-495 [DOI] [PubMed] [Google Scholar]
- 16.Thubrikar MJ, Deck JD, Aouad J, Nolan SP: Role of mechanical stress in calcification of aortic bioprosthetic valves. J Thorac Cardiovasc Surg 1983, 86:115-125 [PubMed] [Google Scholar]
- 17.Hilbert SL, Ferrans VJ, McAllister HA, Cooley DA: Ionescu-Shiley bovine pericardial bioprostheses: histological and ultrastructural studies. Am J Pathol 1992, 140:1195-1204 [PMC free article] [PubMed] [Google Scholar]
- 18.Schoen FJ, Levy RJ, Nelson AC, Bernhard WF, Nashef A, Hawley M: Onset and progression of experimental bioprosthetic heart valve calcification. Lab Invest 1985, 52:523-532 [PubMed] [Google Scholar]
- 19.Kim KM: Cell death and calcification of canine fibroblasts in vitro. Cells Mater 1994, 4:247-261 [Google Scholar]
- 20.Vesely I, Noseworthy R, Pringle G: The hybrid xenograft/autograft bioprosthetic heart valve: in vivo evaluation of tissue extraction. Ann Thorac Surg 1995, 60:S359-S364 [DOI] [PubMed] [Google Scholar]
- 21.Jorge-Herrero E, Fernandez P, de la Torre N, Escudero C, Garcia-Paez JM, Bujan J, Castillo-Olivares JL: Inhibition of the calcification of porcine valve tissue by selective lipid removal. Biomaterials 1994, 15:815-820 [DOI] [PubMed] [Google Scholar]
- 22.Vyavahare N, Hirsch D, Lerner E, Baskin JZ, Schoen FJ, Bianco R, Kruth HS, Zand R, Levy RJ: Prevention of bioprosthetic heart valve calcification by ethanol preincubation: efficacy and mechanisms. Circulation 1997, 95:479-488 [DOI] [PubMed] [Google Scholar]
- 23.Trump BF, Berezesky IR: The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol 1992, 4:89-109 [DOI] [PubMed] [Google Scholar]
- 24.Humphrey SM, Garlick PB: NMR visible ATP and Pi in normoxic and reperfused rat heart: a quantitative study. Am J Physiol 1991, 29:H6-H12 [DOI] [PubMed] [Google Scholar]
- 25.Gasbarini A, Borle AB, Farghali H, Bender C, Francavilla A, Thiel DV: Effect of anoxia on intracellular ATP, Na+i, Ca2+i, Mg2+i, and cytotoxicity in rat hepatocytes. J Biol Chem 1992, 267:6654-6663 [PubMed] [Google Scholar]
- 26.Von Kienlin M, Rösch C, Fur YL, Behr W, Roder F, Haase A, Horn M, Illing B, Hu K, Ertl G, Neubauer S: Three-dimensional 31P magnetic resonance spectroscopic imaging of regional high-energy phosphate metabolism in injured rat heart. Magn Reson Med , 39:731-741 [DOI] [PubMed] [Google Scholar]
- 27.Kim KM: Apoptosis and calcification. Scanning Microsc 1995, 9:1137-1178 [PubMed] [Google Scholar]
- 28.Debono M: Synthesis and characterization of halogenated derivatives of the ionophore A23187: enhanced calcium ion transport specificity by 4-bromo derivative. Biochemistry 1981, 20:6865-6871 [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi T, Gall RS, Mayer ML: An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem 1975, 69:261-267 [DOI] [PubMed] [Google Scholar]
- 30.Hock J, Gunness-Hey M, Poser J, Olson H, Bell N, Raisz L: Stimulation of undermineralized matrix formation by 1,25-dyhydroxyvitamin D3 in long bones of rats. Calcif Tissue Int 1986, 38:78-86 [DOI] [PubMed] [Google Scholar]
- 31.Beeston BEP, Horne RW, Markhem R: Electron diffraction and optical diffraction techniques. Glauert AM eds. Practical Methods in Electron Microscopy. 1986, :pp 252-285 Elsevier, New York [Google Scholar]
- 32.Fagan PJ, Ward MD: Building molecular crystals. Sci Am 1992, July 48–54
- 33.Nancollas GH: The kinetics of crystal growth and renal stone formation. Fleisch H Robertson WG Smith LH Wahlensieck W eds. Urolithiasis Research. 1976, :pp 5-23 Plenum Press, New York [Google Scholar]
- 34.Boistelle R: The concept of crystal growth from solution. Adv Nephrol 1986, 15:173-217 [PubMed] [Google Scholar]
- 35.Neuman WF, Neuman MW: The chemical dynamics of bone. 1958:pp 169-187 University of Chicago Press, Chicago
- 36.Nicotera P, Bellomo G, Orrenius S: Calcim mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol 1992, 32:449-470 [DOI] [PubMed] [Google Scholar]
- 37.McCormack JG, Daniel RL, Osbaldeston NJ, Rutter GA, Denton RM: Micochondrial Ca2+ in mammalian tissues. Biochem Soc Trans 1992, 20:153-159 [DOI] [PubMed] [Google Scholar]
- 38.McIntosh DB: Glutaraldehyde cross-links Lys-492 and Arg-678 at the active site of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 1992, 267:22328-22335 [PubMed] [Google Scholar]
- 39.Wehrle JP, Pedersen PL: Topical review: phosphate transport in eukaryotic cells. J Membr Biol 1989, 111:199-213 [DOI] [PubMed] [Google Scholar]
- 40.Veech RL, Lawson JWR, Cornell NW, Krebs HA: Cytosolic phosphorylation potential. J Biol Chem 1979, 254:6536-6547 [PubMed] [Google Scholar]
- 41.Anderson HC: Mechanism of mineral formation in bone. Lab Invest 1989, 60:320-330 [PubMed] [Google Scholar]
- 42.Maranto AR, Schoen FJ: Phosphatase enzyme activity is retained in glutaraldehyde treated bioprosthetic heart valves. ASAIO Transactions 1988, 34:827-830 [PubMed] [Google Scholar]
- 43.Maranto AR, Schoen FJ: Alkaline phosphatase activity of glutaraldehyde-treated bovine pericardium used in bioprosthetic cardiac valves. Circ Res 1988, 63:844-848 [DOI] [PubMed] [Google Scholar]
- 44.Levy RJ, Schoen FJ, Flowers WB, Staelin ST: Initiation of mineralization in bioprosthetic heart valves: studies of alkaline phosphatase activity and its inhibition by AlCl3 or FeCl3 preincubation. J Biomed Mater Res 1991, 25:905-935 [DOI] [PubMed] [Google Scholar]
- 45.Malviya AN, Rogue PJ: “Tell me where is calcium bred”: clarifying the roles of nuclear calcium. Cel l998, 92:17–23 [DOI] [PubMed]
- 46.Zollinger HU: Cytosolic studies with the phase microscope. I. The formation of “blisters” on the cells in suspension (protocytosis), with observations on the nature of the cellular membrane. Am J Pathol 1948, 24:545–567 [PMC free article] [PubMed]
- 47.Smith MW, Phelps PC, Trump BF: Cytosolic Ca2+ deregulation and blebbing after HgCl2 injury to cultured rabbit proximal tubule cells as determined by digital imaging microscopy. Proc Natl Acad Sci USA 1991, 88:4926-4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemaster JJ, DiGuiseppi J, Nieminen A-L, Herman B: Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature 1987, 325:78-81 [DOI] [PubMed] [Google Scholar]
- 49.Valente M, Bortolotti U, Thiene G: Ultrastructural substrates of dystrophic calcification in porcine bioprosthetic valve failure. Am J Pathol 1985, 119:12-21 [PMC free article] [PubMed] [Google Scholar]
- 50.Maranto AR, Schoen FJ: Effect of delay between tissue harvest and glutaraldehyde pretreatment on mineralization of bovine pericardium used in bioprosthetic heart valves. J Biomed Biomater Res 1988, 22:819-825 [DOI] [PubMed] [Google Scholar]
- 51.Neethling WML, van den Heever JJ, Meyer JM, Barnard HC: Processing factors as determinants of tissue valve calcification. J Cardiovasc Surg 1992, 33:285-291 [PubMed] [Google Scholar]
- 52.Levy R: Editorial: glutaraldehyde and the calcification mechanism of bioprosthetic heart valves. J Heart Valve Dis 1994, 3:101-104 [PubMed] [Google Scholar]
- 53.Blumenthal NC: Mechanisms of inhibition of calcification. Clin Orthoped 1989, 247:279-289 [PubMed] [Google Scholar]
- 54.Ogiso B, Hughes FJ, Melcher AH, McCulloch CAG: Fibroblasts inhibit mineralized bone nodule formation by rat bone marrow stromal cells in vitro. J Cell Physiol 1991, 146:442-450 [DOI] [PubMed] [Google Scholar]
- 55.O’Brien MF, Stafford EG, Gardner MAH, Pohlner PG, Tesar PJ, Cochrane AD, Mau TK, Gall KL, Smith SE: Allograft aortic valve replacement: long-term follow-up. Ann Thorac Surg 1995, 60:S65-S70 [DOI] [PubMed] [Google Scholar]
- 56.Kim KM: Calcification of liposomes and red cell ghosts in vitro and in vivo. Cells Mater 1993, 3:293-304 [Google Scholar]
- 57.Chothia C: The molecular structure of cell adhesion molecules. Annu Rev Biochem 1997, 66:823-862 [DOI] [PubMed] [Google Scholar]
- 58.Kirsch T, Wuthier RE: Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagen. J Biol Chem 1994, 269:11462-11469 [PubMed] [Google Scholar]
- 59.Breuer W, Epsztejn S, Millgram P, Cabantchik IZ: Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am J Physiol 1995, 268:C1354-C1361 [DOI] [PubMed] [Google Scholar]
- 60.Breuer W, Epsztejn S, Cabantchik AI: Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron (II). J Biol Chem 1995, 270:24209-24215 [DOI] [PubMed] [Google Scholar]