Abstract
The NF1 gene product (neurofibromin) is known to act as a tumor suppressor protein by inactivating ras. The best documented factors involved in urinary bladder transitional cell carcinoma (TCC) are ras proto-oncogene activation and p53 suppressor gene mutations. This is the first study reporting alterations in NF1 gene expression in TCC. We examined NF1 gene expression in a total of 29 surgical urinary bladder TCC specimens representing grades 1 to 3 and in three cell lines, RT4, 5637, and T24 (representing grades 1 to 3, respectively). Decreased NF1 gene expression was observed in 23 of 29 (83%) TCC specimens as estimated by immunohistochemistry, the decrease being more pronounced in high-grade tumors. NF1 mRNA levels were markedly lower in TCC tissue compared with adjacent non-neoplastic urothelium, as studied by in situ hybridization for grade 3 TCC. Immunohistochemistry and Western blotting demonstrated that TCC cell lines expressed NF1 protein at different levels, expression being almost undetectable in T24 (grade 3) cells. Northern blotting for cell lines demonstrated reduced NF1 mRNA levels in grade 3 TCC cells. Reverse transcription polymerase chain reaction for cell lines and selected grade 2 and grade 3 tissue samples demonstrated NF1 type II mRNA isoform predominance in all samples studied. Our results show that both NF1 mRNA and protein levels are decreased in high-grade TCC, suggesting that alterations of NF1 gene expression may be involved in bladder TCC carcinogenesis.
Neurofibromin is a 250- to 280-kd tumor suppressor protein coded by the NF1 gene. 1,2 The mutations of the NF1 gene cause type 1 neurofibromatosis, 3-5 which is characterized with multiple neurofibromas, café au lait pigment spots, and an increased risk to develop certain malignancies. Interestingly, somatic mutations of NF1 gene have also been found in malignant tissues of otherwise healthy persons. Specifically, NF1 gene mutations have been found in colon adenocarcinoma, myelodysplastic syndrome, anaplastic astrocytoma, and neuroblastoma and in cell lines cultured from malignant melanoma. 6-9 Elevated NF1 mRNA steady-state levels have been found in astrocytic tumors. 10 Furthermore, the amount of neurofibromin has been reported to be altered in certain proliferative diseases, such as basal cell carcinoma, pheochromocytoma, meningioma, and psoriasis. 11-14
NF1 mRNA is ubiquitously expressed as estimated by reverse transcription polymerase chain reaction (RT-PCR) of rat tissues. 15,16 In humans, the tissue distribution of neurofibromin is less well known. To our knowledge, NF1 mRNA or protein levels have not been investigated either in human or in rodent urinary bladder. In one report on bladder cancer, potential mutations of codon 1423 of the NF1 gene were studied. In this codon, mutations have previously been reported in certain malignancies, 6 but no mutations were observed in 31 bladder cancer specimens studied by Uchida et al. 17 Neurofibromin contains a domain that is related to the GTPase-activating protein (GAP) and accelerates the inactivation of proto-oncogene ras in various cell types 18 and apparently interacts with microtubules. 19,20 Thus, neurofibromin is likely to function as a regulator of cell growth and differentiation. Alternative splicing results in formation of different isoforms of neurofibromin mRNAs (types I to IV). 21,22 Both type I and type II neurofibromin isoforms have an effect on ras inhibition, although type II is less potent. 21
Ras refers to 21-kd proteins, which are products of the ras proto-oncogene superfamily in mammalians (H-ras, N-ras, and K-ras). Ras is present at the protein level in all cells, the highest levels being found in immature and proliferating cells. 23,24 Ras functions as a GTP-binding protein (G-protein), which is necessary for normal cell growth and differentiation by connecting receptor signals to intracellular events. 24,25 Ras cycles between an inactive GDP-bound state and an active GTP-bound state, 26 which is controlled by inactivating signals through GTPase-activating proteins (GAPs) and activating signals through guanine-nucleotide-exchange factors (GEFs). 27 GAPs trigger a 1 × 105-fold enhancement in the activity of intrinsic ras GTPase and thus inactivates ras. 28 Mammalian cells have two ras-GAPs, namely, neurofibromin and p120-GAP. 29 The balance of ras activity can be disturbed by alterations affecting any of these activating or inactivating proteins.
In the Finnish male population, urinary bladder cancer was third in incidence (15.8) after prostate cancer (61.4) and lung cancer (41.6) in 1995. 30 In the Finnish female population, bladder cancer was less common, the incidence being 3.6 in 1995. A decade earlier, the incidence of bladder cancer in males was 13.0 and in females 2.4. The incidence of bladder cancer varies markedly throughout the world. For instance, in Canada, the incidence was 21.0 in males and 5.7 in females, and in Osaka, Japan, the incidence was 8.2 in males and 2.0 in females 31 in the middle of the past decade. Transitional cell carcinoma (TCC) is the most common cancer type of the urinary bladder, representing approximately 90% of all cases. The risk factors for bladder carcinogenesis have remained largely unsolved, but smoking seems to be one among others. ras is the best characterized proto-oncogene involved in bladder carcinogenesis. ras mutations have been found in 40% of bladder carcinomas using PCR-based assays. 32 There has been a wide interest in finding tumor suppressor proteins or other factors involved in bladder carcinogenesis. To date, mutations of the p53 suppressor gene are well documented factors to be involved in human urothelial carcinogenesis. 33,34
In this study we have evaluated the expression of the NF1 gene in human bladder cancer cells of different grades both in vivo and in vitro using immunolabeling, Northern and Western transfer analyses, and in situ hybridization. The results indicate that NF1 gene expression is dramatically decreased during carcinogenesis, the expression being lowest in high-grade invasive carcinomas. We have also investigated the ratio of neurofibromin type I versus type II mRNA isoforms by RT-PCR and found that type II predominated in all samples studied.
Materials and Methods
Tissues and Cell Lines
A total of 29 grades 1 to 3 TCCs of the urinary bladder were obtained from Turku University Central Hospital, Department of Pathology (Table 1) ▶ , with appropriate approval of the Joint Ethical Committee of the Turku University Central Hospital and the University of Turku, Finland. The material included a total of 12 grade 3 TCC samples, 10 grade 2 samples, and 7 grade 1 samples (Table 1) ▶ . Most of the samples included both normal, or non-neoplastic, epithelium and TCC tissue. Two pathologists studied the samples independently, graded them, and ruled out possible necrosis in the samples. The TCC tumors were evaluated by the degree of nuclear atypia and epithelial disorganization of tumor cells, employing three grades (grades 1, 2, and 3) according to the World Health Organization classification of urinary bladder tumors. 35 The papillary or invasive growth pattern of urinary bladder carcinomas was designated independently of grading of anaplasia, and the invasive growth pattern of a TCC sample is indicated in Table 1 ▶ . For the present study, we have used the terms low-grade carcinoma and high-grade carcinoma to indicate grade 1 carcinoma and grade 2 or 3 carcinoma, respectively. Frozen tissue was available from 4 of 29 tumors, and 1 frozen tumor was obtained separately. Frozen tumor tissues were used as sources of RNA for RT-PCR.
Table 1.
NF1 Tumor Suppressor Protein Expression in Transitional Cell Carcinomas
| Sample | Grade (1 to 3) and possible invasion | Intensity score (1 to 4)* |
|---|---|---|
| N1 | ++++ | |
| N2 | ++++ | |
| T1N | 1 | ++++ |
| T2 | 1 | ++++ |
| T3 | 1 | ++ |
| T4N | 1 | ++++ |
| T5 | 1 | ++ |
| T6N | 1 | ++++ |
| T7N | 1 | ++ |
| T8 | 2 (invasive) | + |
| T9 | 2 | ++ |
| T10 | 2 | ++ |
| T11 | 2 | ++ |
| T12 | 2 | ++ |
| T13N | 2 (invasive) | ++++ |
| T14 | 2 | + |
| T15N | 2 | ++++ |
| T16 | 2 | ++ |
| T17 | 2 | ++ |
| T18N | 3 (invasive) | + |
| T19N | 3 | ++ |
| T20N | 3 (invasive) | ++ |
| T21 | 3 (invasive) | ++ |
| T22N | 3 (invasive) | ++ |
| T23N | 3 (invasive) | + |
| T24N | 3 | ++ |
| T25N | 3 | +++ |
| T26N | 3 (invasive) | +++ |
| T27N | 3 (invasive) | + |
| T28N | 3 (invasive) | + |
| T29N | 3 (invasive) | + |
N1 and N2, representative non-neoplastic bladder epithelium; T1 to T29, tumor samples; Nnon-neoplastic urothelium included in tissue section.
*Overall cytoplasmic intensity of NF1 tumor suppressor protein immunosignal compared with immunosignal of non-neoplastic bladder epithelium (for details, see Materials and Methods). Score values in this table indicated as + signs.
RT4, 5637, and T24 human urinary bladder cancer cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD). RT4 is a bladder transitional cell papilloma (grade 1 carcinoma). Cell line 5637 represents grade 2, and T24 represents grade 3 carcinoma. The cells were grown on 56-cm 2 petri dishes or in 80-cm 2 cell culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum. When cells reached full confluency, total RNA was isolated or the cells were lysed for Western transfer analysis (see below). The medium was changed the day before harvesting of the cells.
Antibodies
Immunohistochemical analyses and immunoprecipitation were carried out using affinity-purified rabbit polyclonal antibody NF1GRP (D) raised against synthetic peptide corresponding to amino acids 2798 to 2818 of the predicted human neurofibromin (catalog item sc-67, Santa Cruz Biotechnology, Santa Cruz, CA). 2 Western blotting was performed using rabbit polyclonal antibody NF1GRP (N), which is raised against the epitope corresponding to amino acids 509 to 528 of the predicted human neurofibromin (catalog item sc-68, Santa Cruz Biotechnology). 2 Secondary antibodies used in immunohistochemical analyses and Western transfer analysis are described below.
Avidin-Biotin Immunolabeling
The avidin-biotin immunolabeling was carried out using a Vectastain ABC Kit (Vector Laboratories, Burlingame, CA). Formalin-fixed and paraffin-embedded specimens were cut to 5-μm sections, deparaffinized, rehydrated, and incubated with 0.4% pepsin (Sigma Chemical Co., St. Louis, MO) in 0.01 mol/L HCl at 37°C for 45 minutes. Slides were incubated with 0.3% H2O2 in methanol for 30 minutes to reduce endogenous peroxidase activity. Nonspecific binding was blocked using normal goat serum diluted 1:100 in 0.05 mol/L Tris-buffered saline (TBS, pH 7.5) for 2 hours. The sections were incubated with primary antibody NF1GRP (D) diluted 1:250 in TBS with 1% bovine serum albumin (BSA) (Sigma Chemical Co.) at 4°C overnight and washed three times in TBS at room temperature. The samples were subsequently incubated with nonimmune goat serum with the same dilution as earlier for 20 minutes and exposed to biotinylated anti-rabbit IgG secondary antibody (1:220 in TBS with 1% bovine serum albumin) for 30 minutes and to the avidin-peroxidase complex for 30 minutes. Samples were then incubated for 5 minutes with 3,3′-diaminobenzidine tetrahydrochloride (0.05%) in TBS with 0.07% imidazole (Merck, Darmstadt, Germany) and 0.01% H2O2 to visualize the antibody localization. Finally, the slides were dehydrated and mounted. Control immunoreactions included the following: 1) primary antibody was replaced with 1% BSA/TBS, and 2) primary antibody was preabsorbed with a fivefold weight excess of synthetic peptide used for immunization as recommended by the manufacturer. In all controls, a faint uniform background reaction was observed. The samples were observed and photographed with a Leitz Aristoplan microscope equipped with a camera attachment using Fuji Superia film for color prints.
All tissue sections were treated identically, and the intensity of immunosignal in TCC was compared with non-neoplastic urothelium. Most of the tissue sections contained both TCC and non-neoplastic epithelium, which showed almost identical immunosignal in all samples. Those samples that did not contain non-neoplastic tissue (see Table 1 ▶ ) were compared with the same representative immunolabeled non-neoplastic epithelium (samples N1 and N2 in Table 1 ▶ ). The samples were scored for overall intensity of cytoplasmic immunoreactivity. As almost all non-neoplastic urothelium samples showed similar immunosignal with staining of all epithelial cells, this signal was scored as having an intensity value of 4. TCC tissue was scored on a four-tiered scale for immunosignal, comparing TCC with non-neoplastic epithelium: similar overall immunosignal, 4; moderate overall reduction, 3; marked reduction, 2; absent or very weak immunosignal, 1 (Table 1) ▶ .
In Situ Hybridization Analysis
For detection of NF1 mRNA by in situ hybridization, an NF1 sequence-specific cDNA was amplified by RT-PCR and subcloned to pBluescript vector. Specifically, primers 5′ CAGAATTCCCCCCTCAACTTCGAAGT 3′ and 5′ TGCGTGCTGCATCAAAGTTGCTTTTCCAC 3′ 36 were used to amplify 303-bp and 366-bp sequences corresponding to types I and II neurofibromin, respectively. The 303-bp PCR product was purified from 3% agarose gels, digested with EcoRI and SmaI, and ligated to pBluescript vector. The identity of the sequence was confirmed by sequence analysis. In situ hybridizations were conducted according to previously described protocols with minor modifications. 37,38 Here, the technique is described briefly. Linearized pBluescript plasmid containing the NF1 cDNA insert was used as a template. In vitro transcription reactions were performed in the presence of [35S](thio)UTP (>1000 Ci/mmol; Amersham International, Little Chalfont, UK) by using trans-Probe-T kit (Pharmacia LKB, Uppsala, Sweden) to produce the antisense strand (T7 RNA polymerase, plasmid linearized with ClaI), which was used as a probe, and the corresponding sense strand (T3 RNA polymerase, plasmid linearized with SacI), which was used in negative control hybridizations. After DNAse I digestion, the reactions were heated at 65°C for 15 minutes, cooled on ice, and fractionated on a Sephadex column G-50 (Pharmacia LKB). Surgical tumor samples were fixed in formalin immediately after resection and embedded in paraffin for sectioning. The 4-μm-thick sections were cut on silanated object slides and acetylated. The hybridizations were performed at 52°C for 18 hours with sense or antisense (cRNA) probes prepared as described above. The 35S-labeled cRNA-mRNA hybrids were detected by dipping the samples into NTB-3 autoradiography emulsion (Eastman Kodak, Rochester, NY) and exposing them in a desiccant-containing box for at 4°C for 10 to 36 days. The samples were developed with Kodak D-19 developer (Kodak-Pathé, Chalon-Sur-Saone, France), fixed with Agefix (Agfa-Gevaert, Leverkusen, Germany), stained with hematoxylin, dehydrated with ethanol, cleared with xylene, and mounted. For grain counting, sections were analyzed using a Nikon Optiphot II microscope equipped with Nikon 40× E Plan 0.65Na objective. The images were acquired with a DageMTI 72 CCD camera. Grain counting was performed with MCID-M4 image analysis software (Imaging Research, St. Catherines, Canada) after applying the target accentuation image-processing filter to each image. The grains were determined by placing a rectangular scan tool of 30 × 30 μm randomly over non-neoplastic urothelium, cancerous tissue, and background area that did not contain any of the tissue section.
Indirect Immunofluorescence
Glass slides covered with cultured cells were washed with phosphate-buffered saline (PBS), and samples were fixed with ethanol at −20°C for 20 minutes. The samples were incubated with 1% BSA in PBS at room temperature for 30 minutes to reduce nonspecific binding of the antibodies. Primary antibody NF1GRP (D) (diluted 1:30 in 1% BSA in PBS) was incubated on the samples at 4°C overnight. Slides were washed three times with PBS, and a second blocking of nonspecific binding was performed as previously. The slides were incubated with tetramethylrhodamine isothiocyanate (TRITC)-conjugated swine anti-rabbit IgG diluted 1:100 with 1% BSA in PBS (Dakopatts, Glostrup, Denmark) at room temperature for 60 minutes, washed, rinsed with distilled water, and mounted with Glycergel (Dako, Carpinteria, CA). In control immunoreactions, primary antibody was replaced with 1% BSA/PBS. Only faint background fluorescence was observed in all control samples. The samples were observed and photographed with a Leitz Aristoplan epifluorescence microscope equipped with filters for TRITC fluorescence using Kodak T-Max film. For quantitation of the immunosignal, immunolabeled microscope slides were photographed to black and white negatives, and the optical densities of the negatives were analyzed by digital image analysis system MCID-M4. Negatives were digitized with a DageMTI 72E CCD camera and MicroNikkor 55-mm objective over a precision light table (Northern Light, Imaging Research). After the optical density calibration (with a Kodak 911ST602 intensity wedge), the negative frames from different cell lines and unexposed negative frames as background area were analyzed. The background was subtracted from the intensity values.
Immunoprecipitation and Western Transfer Analysis
Cells were rinsed twice with PBS at 4°C and lysed with 2 ml of lysis buffer (50 mmol/L Tris/HCl, pH 7.5, 0.15 mol/L NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, and 2 mmol/L EGTA) containing proteinase inhibitors (2 mmol/L phenylmethylsulfonyl fluoride, 2 mmol/L p-aminobenzamidine with N-ethylmaleimide, and 5 mmol/L EDTA-Na) for 30 minutes. After incubation, cells were harvested, and cellular debris was removed by centrifugation (10,000 rpm at 4°C for 10 minutes). Protein concentration was measured using Bio-Rad Dc Protein Assay kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. For immunoprecipitation, equal amounts of protein (4 mg) were incubated with 1 μg of polyclonal antibody NF1GRP (D) at 4°C for 24 hours and subsequently with 90 μl of protein A/Sepharose (CL-4B, Pharmacia LKB) at 4°C for 2 hours and centrifuged at 14,000 rpm for 5 minutes. The supernatant was removed, and the pellet was washed two times with 1 ml of washing buffer 1 (1% SDS, 0.5% sodium deoxycholate, 1% Triton-X-100, 1 mmol/L EDTA, and PBS, pH 7.5), two times with washing buffer 2 (0.25 mol/L sodium chloride, 1% Nonidet P-40, 0.1% SDS, and 0.02 mol/L Tris/HCl, pH 8.3), and subsequently two times with washing buffer 1. After the last wash, supernatant was removed, and 50 μl of solution containing 62.5 mmol/L Tris/HCl, pH 6.8, 2.3% SDS, and 8% glycerol was added. For Western transfer analysis, the immunoprecipitated samples were reduced and subjected to 5% SDS-polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred to a methanol-soaked Immobilon-P membrane (Millipore Co., Bedford, MA) in Tris/glycine buffer using 500 mA of current for 18 hours in a tank system (LKB Bromma 2005 Transphor electroblotting unit, LKB-Produkter, Bromma, Sweden). After transfer, the membrane was blocked by air drying at room temperature for 2 hours and incubated with polyclonal antibody NF1GRP (N) diluted 1:1000 in PBS with 1% BSA and 0.05% Tween (Tween 20, Fluka Chemie, Buchs, Switzerland) at 4°C overnight. The membrane was washed two times with PBS containing 0.05% Tween for 15 minutes and exposed to secondary antibody (donkey anti-rabbit IgG horseradish-peroxidase-linked whole antibody; code NA 934, Amersham International) in PBS containing 1% BSA and 0.05% Tween for 1 hour. The membrane was washed with PBS/0.05% Tween two times for 15 minutes, and the bound peroxidase activity was detected using an ECL kit (Amersham International, Poole, UK) as recommended by the manufacturer. Chemiluminescence was detected with Kodak X-OMAT film (Kodak, Rochester, NY), and the signal was quantitated using MCID-M4 laser scanning densitometry (Imaging Research). As a control, the primary antibody was preabsorbed with a 10-fold weight excess of synthetic peptide used for immunization. No signal was observed in the control lanes.
Isolation of RNA and Northern Blot Analysis
Isolation of total RNA from cultured bladder cancer cells was performed as described previously. 39 The cells were lysed in 2.7 ml of 4 mol/L guanidine thiocyanate, 25 mmol/L sodium acetate, and β-mercaptoethanol. The lysates were layered onto a CsCl cushion (5.7 mol/L CsCl and 25 mmol/L sodium acetate) and centrifuged in a SW-55 rotor (Beckman, Palo Alto, CA) at 35,000 rpm at 20°C for 21 hours. Thereafter, the aqueous guanidine thiocyanate solution and CsCl were removed, and the RNA pellets were washed with 200 μl of 99.5% ethanol. The pellets were dissolved in 300 μl of RNAse-free water, RNA was extracted with 300 μl of phenol/chloroform (1:1), and the aqueous phase was precipitated with a 1:10 vol of 3 mol/L sodium acetate and 750 μl of 99.5% ethanol at −20°C. RNA pellets were dried and finally dissolved into RNAse-free water. Isolation of RNA from surgical bladder TCC specimens was performed as follows. Specimens were homogenized using an Ultra-Turrax homogenizer (Janke and Kunkel, Staufen, Germany) in 3.2 ml of 4 mol/L guanidinium thiocyanate, 25 mmol/L sodium acetate, and β-mercaptoethanol. The lysates were centrifuged for 20 minutes at 10,000 rpm. Isolation of RNA from supernatant proceeded as described for cultured cells.
Aliquots (30 μg) of total RNA were fractioned electrophoretically on 1% agarose gels after denaturation with formalin and transferred to Zeta-probe blotting membrane (Biorad). RNA was immobilized to membrane by baking at 80°C for 30 minutes. The membrane was prehybridized for 2 hours in a solution containing 50% formamide, 0.25 mol/L Na2PO4·H20 (pH 7.2), 0.25 mol/L NaCl, 7% SDS, and 1 mmol/L EDTA. Hybridization was performed for 20 hours in the same solution containing a radiolabeled human neurofibromin-specific 1.2-kb cDNA probe that was produced by PCR, as described earlier, 36 subcloned in a pBluescript vector, and sequenced. The probe was radiolabeled with [α-32P]dCTP using a random primed DNA labeling kit (Boehringer, Mannheim, Germany). After the hybridization, the membrane was washed two times in 2X SSC/0.1% SDS at room temperature for 10 minutes and two times in 0.1X SSC/0.1% SDS at 52°C for 15 minutes. Kodak Biomax films were exposed to membranes at −70°C for 7 to 21 days.
Reverse Transcriptase Polymerase Chain Reaction
Total RNA (5 μg) isolated from cultured bladder cancer cells or from surgical tissue samples was transcribed into single-stranded DNA in a 20-μl reaction volume containing 200 U of murine Moloney leukemia virus reverse transcriptase enzyme (GIBCO, Grand Island, NY), first-strand buffer (250 mmol/L Tris/HCl, pH 8.3, 375 mmol/L KCl, and 15 mmol/L MgCl2), 0.5 mmol/L of each of the four deoxynucleotides, 10 mmol/L dithiothreitol, 20 U of RNasin (Promega Corp., Madison, WI), and 60 pmol of random primers (Promega Corp.). The reaction was allowed to proceed at 37°C for 90 minutes. For PCR, 2 μl of the RT reaction product was used as a template in a 50-μl reaction volume containing 15 pmol of neurofibromin-specific oligonucleotide primers NF1G.C and NF1G.D 36 (sense, 5′-CAGAATTCCCCCCTCAACTTCGAAGT-3′; antisense, 5′-TGCGTGCTGCATCAAAGTTGCTTTTCAC-3′), 10 pmol of glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers (sense, 5′-TCTCATGGTTCACACCCATGACGAACATG-3′; antisense, 5′-AAGAAGATGCGGCTGACTGTCGAGCCACAT-3′), 10 nmol/L of each nucleotide, 1 U of Dynazyme (Thermus brockianus strain F500, Finnzymes, Espoo, Finland), 5 μl of 10X Dynazyme buffer (Finnzymes), and 39.5 μl of H20. Amplification was performed by incubating the mixture for 30 cycles of denaturation (60 seconds at 94°C), annealing (60 seconds at 64°C), and extension (60 seconds at 72°C). In the negative control samples, the template was omitted, and the PCR was performed in the same conditions as other samples. The PCR products were fractionated electrophoretically on 3% agarose gels and stained with ethidium bromide.
Results
Immunolabeling for NF1 Tumor Suppressor Protein in TCC and Non-Neoplastic Urothelium
For immunolocalization of NF1 protein in paraffin-embedded TCC samples, avidin-biotin immunolabeling was performed. Most samples included both neoplastic and non-neoplastic tissue. Samples were scored as described in Materials and Methods. Non-neoplastic transitional cell epithelium displayed an intense immunoreaction with the NF1GRP (D) antibody (Figure 1, E and F) ▶ . In grade 1 TCC samples, four of seven showed a similar intense staining pattern as seen in normal or non-neoplastic urothelium, whereas three of seven showed a marked reduction in NF1 protein immunosignal. In grade 2 TCC, 6 of 10 samples showed marked reduction, and in 2 of 10 samples, immunosignal was very weak or absent. In 2 grade 2 TCC samples, NF1 protein was expressed in similar intensity as seen in non-neoplastic epithelium. In grade 3 TCC samples, 5 of 12 showed weak or absent immunoreaction for NF1 protein, 5 of 12 samples showed marked reduction, and 2 of 12 samples a moderate reduction in NF1 protein expression. In conclusion, the intensity of NF1 protein immunosignal showed reduction in 24 of 29 samples (83%), but generally the reduction in immunosignal was more pronounced in grade 3 and in grade 2 than in grade 1. In all grade 3 TCC samples, the reduction of NF1 immunosignal was observed, whereas some grades 1 and 2 TCC samples displayed NF1-specific immunosignal that was comparable to that of the non-neoplastic control urothelium. Representative samples of grade 1 and grade 3 TCCs are presented in Figure 1 ▶ .
Figure 1.
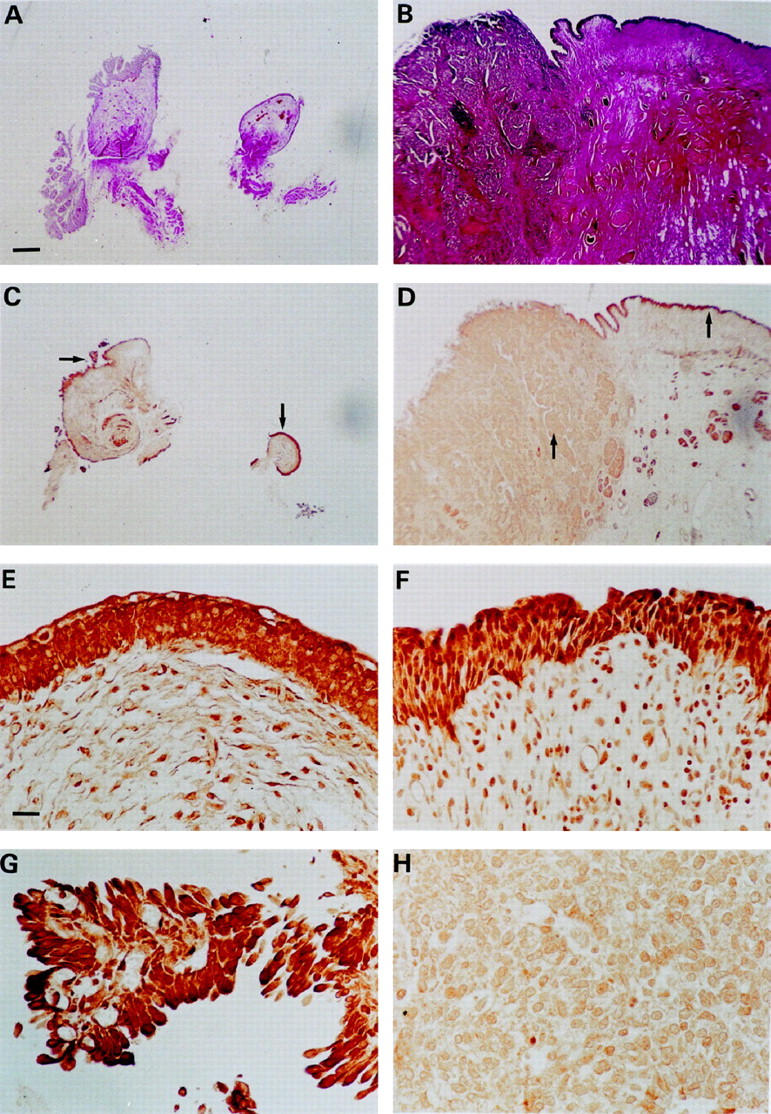
Immunolocalization of neurofibromin in grade 1 (C, E, and G; T1 in Table 1 ▶ ) and grade 3 (D, F, and H; T27 in Table 1 ▶ ) urinary bladder transitional cell carcinoma samples. A: Low-magnification photomicrograph of tissue harboring grade 1 TCC (van Gieson staining). C: Immunolabeling for neurofibromin in the same specimen as in A; arrows point to areas of higher magnification in E and G. E and G: A higher magnification representing non-neoplastic urothelium and grade 1 TCC, respectively. B: Low-magnification photomicrograph of tissue harboring grade 3 TCC (van Gieson staining). D: Immunolabeling for neurofibromin in the same specimen as in B; arrows point to areas of higher magnification in F and H. F and H: A higher magnification representing non-neoplastic urothelium and grade 3 TCC, respectively. Magnification, ×2 (A and B) and ×40 (C to H); scale bar, 500 μm (A to D) and 25 μm (E to H).
Positive immunosignal for NF1 protein seen in fibroblasts, lymphocytes, and endothelial and smooth muscle cells is in good agreement with previous studies on NF1 gene expression by these cells. 40-43 These results show that neurofibromin levels decrease during carcinogenesis from normal epithelium to poorly differentiated carcinoma.
In Situ Hybridization Analysis of NF1 mRNA Expression in TCC and Non-Neoplastic Urothelium
In situ hybridization analysis was performed on selected grade 3 TCC specimens to study potential differences in NF1 mRNA expression in TCC tissue and non-neoplastic transitional epithelium in the same tissue sections (Figure 2) ▶ . The autoradiographic grains representative of radiolabeled neurofibromin-specific cRNA-mRNA hybrids were quantitated as described in Materials and Methods. When comparing random scan areas over background and cancer and non-neoplastic urothelium, in situ hybridization for NF1 mRNA revealed mean grain counts of 36, 52, and 217, respectively. When a mean grain size of 6 μm 2 was applied, the estimated grain counts were 38, 58, and 426, respectively. In control sections, sense probe was used and only a faint background noise was observed. These results suggest that the NF1 mRNA levels in grade 3 TCC were 20% of those of non-neoplastic urothelium in the samples studied.
Figure 2.
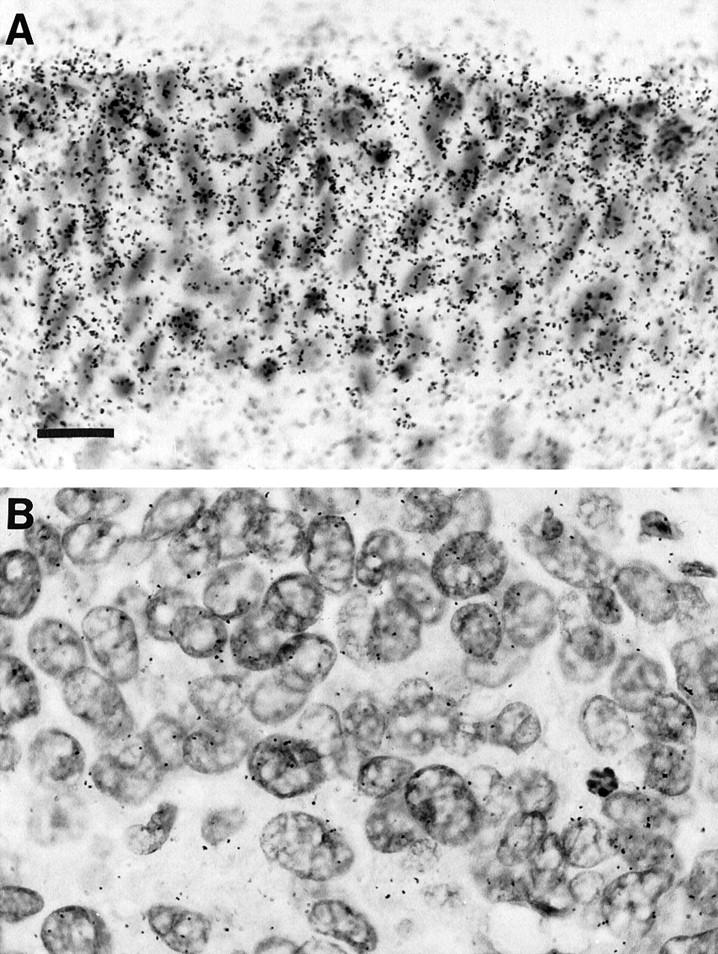
NF1 gene expression in grade 3 urinary bladder transitional cell carcinoma, as visualized by in situ hybridization. A: Normal urinary bladder epithelium. B: Area of carcinoma within the same tissue section. The tissue specimen is the same as in Figure 1B ▶ (T27 in Table 1 ▶ ). Radioactively labeled cRNA-mRNA hybrids were detected by autoradiography. The autoradiographic grains were quantitated using MDID-M4 image analysis software (see Results). Hematoxylin counterstain; magnification, ×50; scale bar, 15 μm.
In Vitro Immunolabeling for NF1 Protein in TCC Cell Lines
In additional studies, three well characterized cell lines originally derived from carcinoma tissue corresponding to grades 1 to 3 were studied. The grade 1 RT4 cell line displayed an intense immunoreaction for NF1 protein (Figure 3A) ▶ . The immunosignal was markedly decreased in the 5637 carcinoma cell line (grade 2) compared with the RT4 cell line (Figure 3B) ▶ . In the grade 3 T24 carcinoma cell line, the immunosignal for neurofibromin was barely detectable (Figure 3C) ▶ . The intensity of immunosignal for NF1 protein in each culture was quantitated as described in Materials and Methods. The results showed that RT4, 5637, and T24 cells expressed NF1 protein in a 32:15:1 ratio. These results are consistent with the results obtained from tissue immunolabelings demonstrating that poorly differentiated carcinoma cells display very low levels of the tumor suppressor protein neurofibromin. Furthermore, these results suggest that TCC cell lines RT4, 5637, and T24 are useful tools in studies elucidating the expression of the NF1 tumor suppressor gene.
Figure 3.
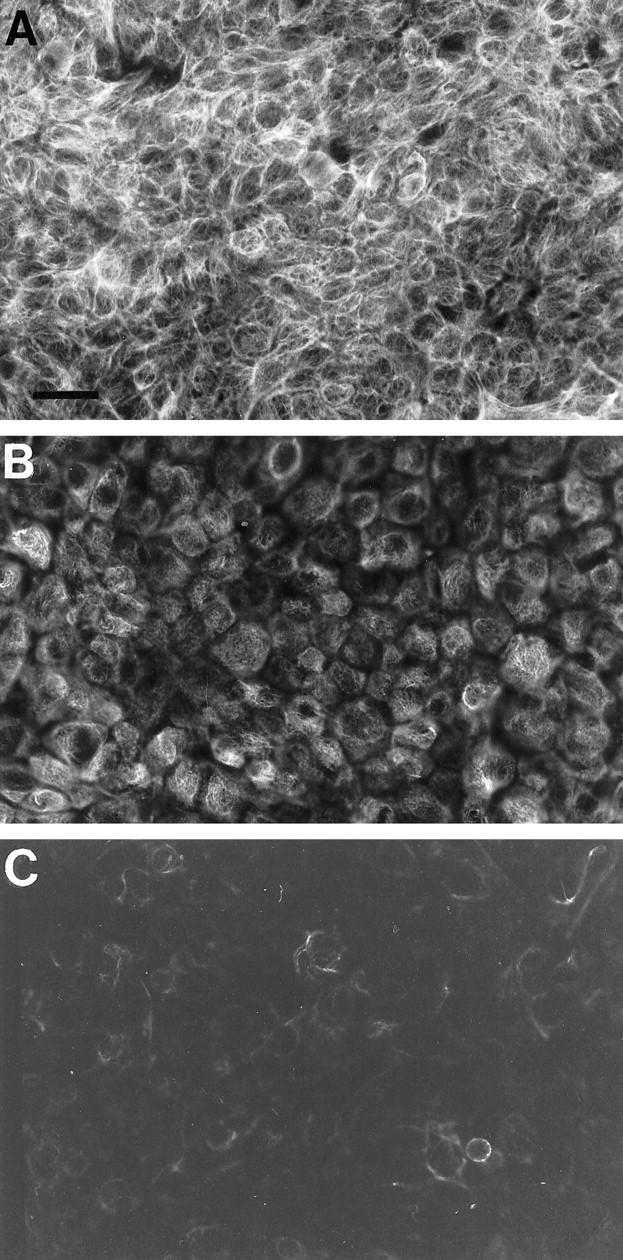
Indirect immunofluorescence labeling of cultured urinary bladder cancer cells with neurofibromin-specific antibody. Representative areas of each specimen were photographed using the same exposure times, and the photomicrographs were reproduced under identical conditions. A: RT4 cells originating from grade 1 urinary bladder carcinoma. B: 5637 grade 2 cancer cells. C: T24 grade 3 cancer cells. The intensity of the immunosignals was quantitated using digital image analysis system MCID-M4 (see Results). Magnification, ×40; scale bar, 25 μm.
Western Transfer Analysis of NF1 Protein Expression in Cultured TCC Cell Lines
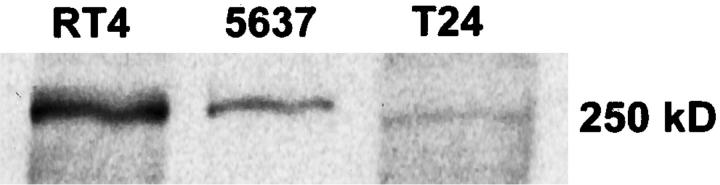
Western blotting was performed as described in Materials and Methods, and the specific neurofibromin NF1GRP(N)-antibody-labeled bands (Figure 4) ▶ were analyzed with the MCID-M4 imaging system. The amount of neurofibromin was estimated by background corrected area-under-curve (AUC) values, which were 120.9, 26.2, and 8.2 for cell lines RT4, 5637, and T24, respectively. The relative amounts of NF1 protein in cell lines RT4, 5637, and T24 were 15:3:1, respectively. These results show significant reduction in neurofibromin levels in poorly differentiated urinary bladder carcinoma cell lines.
Figure 4.
Neurofibromin expression of cultured urinary bladder cancer cells as measured by immunoprecipitation followed by Western blotting. The cells were lysed and immunoprecipitated with neurofibromin-specific NF1GRP(D) antibody. The samples were subsequently subjected to SDS-5% PAGE, transferred to Immobilon-P membrane and incubated with NF1GRP(N) antibody. Specific 250-kd neurofibromin bands were detected using an ECL kit. The neurofibromin-specific signals were quantitated by MCID-M4 laser scanning densitometry (see Results).
Expression of NF1 mRNA in TCC Cell Lines
Under the cell culture conditions used, the NF1 mRNA levels in RT4 and 5637 cell lines were essentially the same, as estimated by Northern blotting. The T24 cell line showed a marked reduction in NF1 mRNA levels compared with the other two cell lines studied (Figure 5) ▶ .
Figure 5.
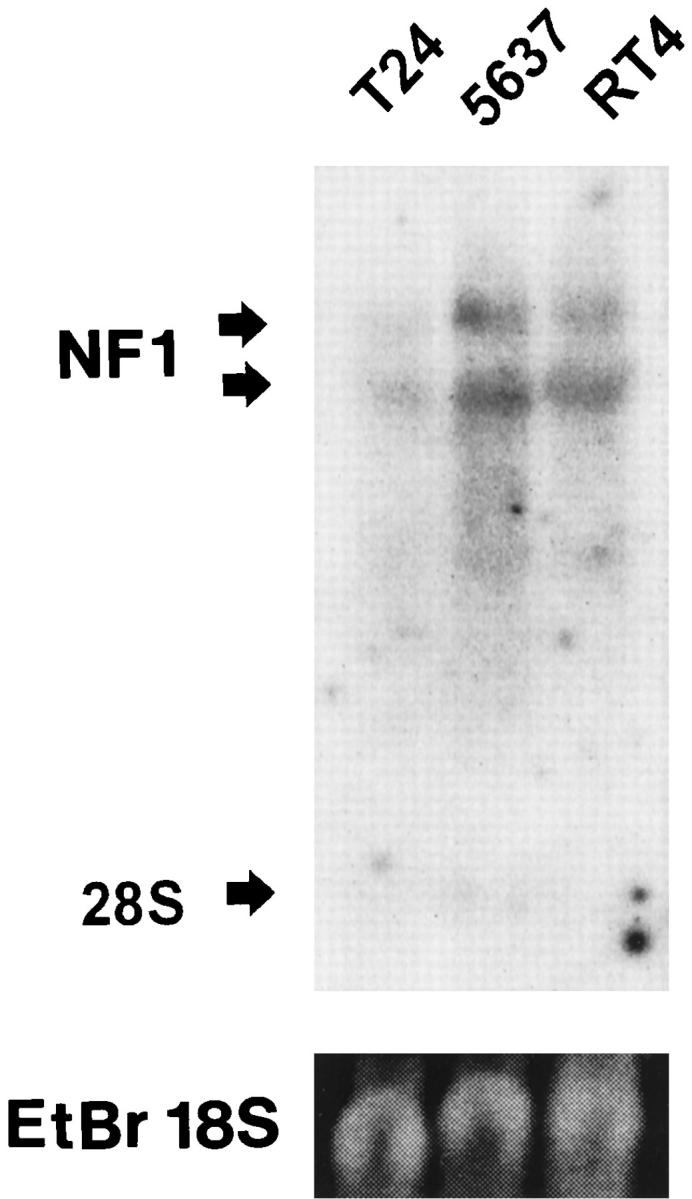
Northern blot analysis of neurofibromin mRNA from cultured TCC cells: T24 cells from grade 3 TCC, 5637 cells from grade 2 TCC, and RT4 cells from grade 1 TCC. Arrows indicate the 11- to 13-kb NF1 mRNA and the migration position of the 28 S ribosomal unit. In the lower panel, ethidium bromide staining of the gel visualizes the 18 S ribosomal unit.
RT-PCR Analysis of NF1 Type I/II mRNA Ratio in TCC Tissues and Cell Lines
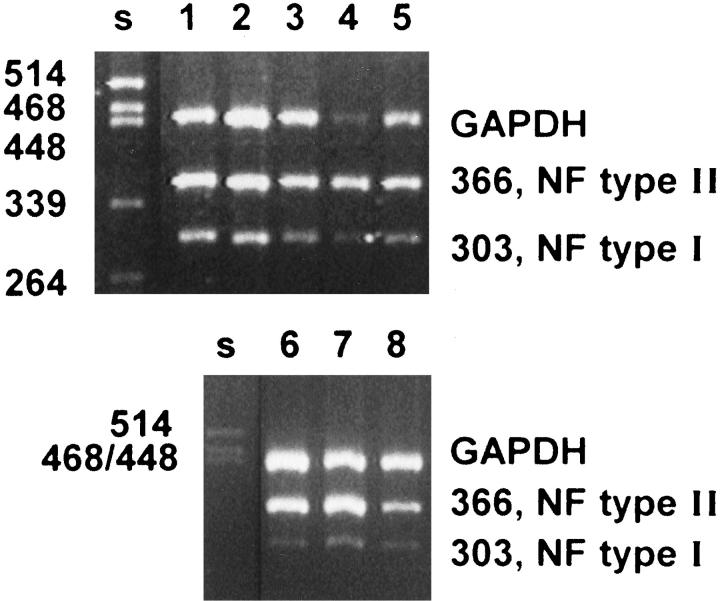
To determine the ratio of type I versus type II NF1 mRNA, RT-PCR analysis was performed for all three urinary bladder carcinoma cell lines studied and for one grade 2 and four grade 3 TCC tissue samples. GAPDH primers were used to enhance semiquantitative evaluation of the RT-PCR analysis. The NF1 type II mRNA predominance was evident in all samples, being more pronounced in mRNA derived from cell culture samples. The type I versus type II NF1 mRNA ratio was apparently the same in all samples representing different grades of malignancy (Figure 6) ▶ .
Figure 6.
Demonstration of type I and II neurofibromin mRNA isoforms and GAPDH mRNA by RT-PCR in TCC tissue samples and cultured TCC cell lines. Primers for NF1 mRNA amplify both type I and type II isoforms. A second primer pair was used to detect GAPDH mRNA in the same samples. Lanes 1 to 4, analyses on mRNAs isolated from four different grade 3 TCCs; lane 5, grade 2 TCC; lanes 6 to 8, RT-PCR of mRNAs derived from cell lines T24, 5637, and RT4, respectively. PstI-digested λ-DNA was used as DNA size marker (s).
Discussion
In many cases, carcinogenesis of the urinary bladder includes alterations in proto-oncogene and tumor suppressor gene functions. The best documented proto-oncogene involved in bladder carcinogenesis is ras. 44,45 Mutations of the p53 tumor suppressor gene have been documented as being involved in urinary bladder carcinoma with a high incidence. 33,34 Alterations of chromosome 17 in bladder neoplasia have recently been documented to be predominantly confined to regions p12–13, q22–11, and q24–25. 46 The facts that the NF1 tumor suppressor gene is located in 17q11.2 and that alterations in NF1 gene expression are involved in several malignancies led us to study the potential role of NF1 gene expression in bladder transitional cell carcinogenesis. Previously, Uchida et al 17 studied a total of 31 TCC samples with respect to mutations in the NF1 gene, but none were found in their material. 17 In the present study, we focused on finding possible alterations in NF1 gene expression in TCC either at the mRNA or at the protein level.
Our first approach was to evaluate the level of NF1 gene expression in surgical TCC tumor samples using immunohistochemistry. The results showed that NF1 gene was expressed at a readily detectable level in non-neoplastic urothelium. In contrast, the TCC showed marked reduction in NF1 gene expression compared with normal urothelium. Furthermore, this reduction was more significant in grades 2 and 3 than in grade 1 TCC. These results suggest that bladder carcinogenesis is associated with reduction of NF1 tumor suppressor gene expression. In situ hybridization analysis of selected grade 3 TCC samples revealed that NF1 mRNA levels were decreased in these samples compared with adjacent non-neoplastic urothelium. Thus, both mRNA and protein levels were decreased in TCC tissues analyzed in the present study.
In additional studies, we used three well documented cell lines representing different grades of TCC. Immunohistochemical and Western transfer analyses demonstrated that NF1 protein expression was almost undetectable in T24 cells representing grade 3 TCC. In contrast, NF1 protein was readily detectable in RT4 cells (grade 1) and 5637 (grade 2). Data obtained from these analyses were quantitated by image analysis, and results were compared as numerical ratios. These studies demonstrated that the intensity of NF1 immunosignal in RT4 cells (grade 1) was 32-fold compared with that of T24 cells (grade 3). Immunoprecipitation followed by Western blotting and subsequent quantitation of the chemiluminescence reaction showed a 15-fold difference in the same cells. Even though these two techniques did not give identical results, both demonstrated markedly higher levels of NF1 protein in RT4 cells (grade 1) than in T24 cells (grade 3). Northern transfer analysis of RNAs isolated from the three different TCC cell lines showed that the steady-state levels of NF1 mRNA in RT4 cells were also higher than in T24 cells. However, we have taken a critical view in the interpretation of the data obtained from Northern transfer analysis of cultured TCC cells. Specifically, our studies concerning TCC have shown that NF1 mRNA levels may oscillate significantly within hours depending on cell culture conditions (unpublished data).
Previous studies have shown that alterations in the type I versus type II NF1 mRNA ratio can be associated with the development of certain malignancies. 47 We used RT-PCR to study three TCC cell lines and five surgical patient samples for possible alterations in the type I versus type II NF1 mRNA ratio. All samples showed type II predominance, and there was no significant difference in the ratio between the cell lines studied. The predominance was observed also in patient samples, but it was not as pronounced as that seen in vitro.
The best characterized role of NF1 protein is its GTPase-accelerating function, which leads to inactivation of ras. 29 There are also reports that NF1 protein can inhibit ras-dependent growth by alternative ways independent of its GAP function. 9,48 The reduction of NF1 gene expression reported here may thus lead to tumor promotion by affecting the ras-MAPK pathway in various ways. This requires additional studies on TCC, especially concentrating on ras and raf activity.
It is of interest that NF1 patients do not have an elevated incidence of TCC, as we have found only two case reports of NF1 patients with TCC. 49,50 The same applies to some other malignancies harboring an inactivating mutation in the NF1 gene. Specifically, colon cancer and myelodysplasias are not more frequent in NF1 patients than in the control population. 6 In analogy to the NF1 gene, mutations of the retinoblastoma gene are frequently seen in sporadic small-cell lung cancers (SCLCs), although SCLC does not have a higher incidence among hereditary retinoblastoma patients. 51,52 On the other hand, eg, astrocytomas, which harbor an inactivating NF1 mutation, are seen in higher incidence among NF1 patients. Thus, the NF1 gene could play a different role in tumors originating from neural-crest-derived tissues in contrast to other tumor types, and different mechanisms of tumor growth promotion may be operative in different types of tumors caused by NF1 mutations. 6
A previous study on bladder cancer 17 has reported that no mutations were detected in the NF1 gene in a region around codon 1423 in which somatic mutations have been found in colon adenocarcinoma, myelodysplastic syndrome, and anaplastic astrocytoma. 6 Taken together, it seems unlikely that mutations of the NF1 gene would cause the reduction of NF1 mRNA and protein in high-grade TCCs in vivo and in vitro as reported in the present study. Earlier studies on other malignancies have reported mutations of the NF1 gene leading to loss of gene expression. 7-9 The shortening of the cell cycle in poorly differentiated cancer cells could lead to diminution of primary transcripts from large genes such as NF1. However, this does not seem to be the case, eg, in RB gene expression in TCC, which is a large gene. 53 RB gene expression is thoroughly studied in TCC, and it has been reported to be normal in the majority of the samples, but altered expression was seen in 30% to 40% of the cases. 54,55 Our results suggest that other factors may be involved in bladder TCC leading to a reduction of NF1 gene expression. Mutations in the gene control regions, down-regulation of the gene for some reason, and possible post-transcriptional regulation of NF1 protein remain issues of speculation.
To conclude, we report reduction of NF1 tumor suppressor gene expression in TCC, which may play a role in development of this malignancy. The question of whether the changes in NF1 gene expression in TCC are causal or consequential with respect to the pathogenesis of TCC remains to be elucidated. Nevertheless, we consider these results significant and that the NF1 gene product may play a role in bladder transitional cell carcinogenesis. Furthermore, we envision that TCC cell lines used in this study provide a useful tool to investigate NF1 gene expression in TCC in vitro.
Acknowledgments
We thank P. Lakkisto, A-S. Björkstrand, O. Rahkonen, and H. Salminen for fruitful discussion and H. Pakarinen, L. Salomaa, L. Peltonen, and E. Oja for skillful technical assistance. The cooperation of J. Saarinen and J. Kemppinen, without which this work would not have been possible, is gratefully acknowledged.
Footnotes
Address reprint requests to Dr. Matti Laato, Department of Surgery, Turku University Central Hospital, FIN-20520 Turku, Finland. E-mail: matti.laato@tyks.fi.
Supported by grants from Turku University Central Hospital, Turku University Foundation, PPSHP Grant H01139, and the Academy of Finland.
V. Aaltonen and P. Boström contributed equally to this work.
References
- 1.Marchuk DA, Saulino AM, Tavakkol R, Swaroop M, Wallace MR, Andersen LB, Mitchell AL, Gutmann DH, Boguski M, Collins FS: cDNA cloning of the type 1 neurofibromatosis gene: complete sequence of the NF1 gene product. Genomics 1991, 11:931-940 [DOI] [PubMed] [Google Scholar]
- 2.Gutmann DH, Wood DL, Collins FS: Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci USA 1991, 88:9658-9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D, Bishop DT, Carey J, Baty B, Kivlin J, Willard H, Waye JS, Greig G, Leinwand L, Nakamura Y, O’Connel P, Leppert M, Lalouel J-M, White R, Skolnick M: Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science 1987, 236:1100-1102 [DOI] [PubMed] [Google Scholar]
- 4.von Deimling A, Krone W, Menon AG: Neurofibromatosis type 1: pathology, clinical features and molecular genetics. Brain Pathol 1995, 5:153-162 [DOI] [PubMed] [Google Scholar]
- 5.Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL, Brownstein BH, Collins FS: Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science 1990, 249:181-186 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Bollag G, Clark R, Stevens J, Conroy L, Fults D, Ward K, Friedman E, Samowitz W, Robertson M, Bradley P, McCormick F, White R, Cawthon R: Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell 1992, 69:275-281 [DOI] [PubMed] [Google Scholar]
- 7.The I, Murthy AE, Hannigan GE, Jacoby LB, Menon AG, Gusella JF, Bernards A: Neurofibromatosis type 1 gene mutations in neuroblastoma. Nature Genet 1993, 3:62-66 [DOI] [PubMed] [Google Scholar]
- 8.Andersen LB, Fountain JW, Gutmann DH, Tarlé SA, Glover TW, Dracopoli NC, Housman DE, Collins FS: Mutations in the neurofibromatosis 1 gene in sporadic malignant melanoma cell lines. Nature Genet 1993, 3:118-121 [DOI] [PubMed] [Google Scholar]
- 9.Johnson MR, Look AT, DeClue JE, Valentine MB, Lowy DR: Inactivation of the NF1 gene in human melanoma and neuroblastoma cell lines without impaired regulation of GTP. Ras. Proc Natl Acad Sci USA 1993, 90:5539-5543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutmann DH, Giordano MJ, Mahadeo DK, Lau N, Silbergeld D, Guha A: Increased neurofibromatosis 1 gene expression in astrocytic tumors: positive regulation by p21-ras. Oncogene 1996, 12:2121-2127 [PubMed] [Google Scholar]
- 11.Hermonen J, Hirvonen O, Ylä-Outinen H, Lakkakorpi J, Björkstrand A-S, Laurikainen L, Kallioinen M, Oikarinen A, Peltonen S, Peltonen J: Neurofibromin: expression by normal human keratinocytes in vivo and in vitro and in epidermal malignancies. Lab Invest 1995, 73:221-228 [PubMed] [Google Scholar]
- 12.Gutmann DH, Geist RT, Rose K, Wallin G, Moley JF: Loss of neurofibromatosis type 1 (NF1) gene expression in pheochromocytomas from patients without NF1. Genes Chromosomes & Cancer 1995, 13:104-109 [DOI] [PubMed] [Google Scholar]
- 13.Sundaram V, Lee JH, Harwalkar JA, Stein DJ, Roudebush M, Stacey DW, Golubic M: Reduced expression of neurofibromin in human meningiomas. Br J Cancer 1997, 76:747-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltonen J, Karvonen S-L, Ylä-Outinen H, Hirvonen O, Karvonen J: Lesional psoriatic epidermis displays reduced neurofibromin immunoreactivity. J Invest Dermatol , 105:664-667 [DOI] [PubMed] [Google Scholar]
- 15.Golubic M, Roudebush M, Dobrowolski S, Wolfman A, Stacey DW: Catalytic properties, tissue and intracellular distribution of neurofibromin. Oncogene 1992, 7:2151-2159 [PubMed] [Google Scholar]
- 16.Gutmann DH, Geist RT, Wright DE, Snider WD: Expression of the neurofibromatosis 1 (NF1) isoforms in developing and adult rat tissues. Cell Growth Differ 1995, 6:315-323 [PubMed] [Google Scholar]
- 17.Uchida T, Wada C, Ishida H, Egawa S, Ao T, Yokoyma E, Koshiba K: Infrequent involvement of mutations on neurofibromatosis type 1, H-ras, K-ras and N-ras in urothelial tumors. Urol Int 1995, 55:63-67 [DOI] [PubMed] [Google Scholar]
- 18.Bollag G, McCormick F: NF is enough of GAP. Nature 1992, 356:663-664 [DOI] [PubMed] [Google Scholar]
- 19.Gregory PE, Gutmann DH, Mitchell A, Park S, Boguski M, Jacks T, Wood DL, Jove R, Collins FS: Neurofibromatosis type 1 gene product (neurofibromin) associates with microtubules. Somat Cell Mol Genet 1993, 19:265-274 [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Gutmann DH: Mutations in the GAP-related domain impair the ability of neurofibromin to associate with microtubules. Brain Res 1997, 759:149-152 [DOI] [PubMed] [Google Scholar]
- 21.Andersen LB, Ballester R, Marchuk DA, Chang E, Gutmann DH, Saulino AM, Camonis J, Wigler M, Collins FS: A conserved alternative splice in the von Recklinghausen neurofibromatosis (NF1) gene produces two neurofibromin isoforms, both of which have GTPase-activating protein activity. Moll Cell Biol 1993, 13:487-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutmann DH, Geist RT, Rose K, Wright DE: Expression of two new protein isoforms of the neurofibromatosis type 1 gene product, neurofibromin, in muscle tissues. Dev Dyn 1995, 202:302-311 [DOI] [PubMed] [Google Scholar]
- 23.Furth ME, Aldrich TH, Cordon-Gardo C: Expression of ras proto-oncogene proteins in normal human tissues. Oncogene 1987, 1:47-58 [PubMed] [Google Scholar]
- 24.Denhardt DT: Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammal cell: the potential for multiplex signaling. Biochem J 1996, 318:729-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall MS: Ras target proteins in eukaryotic cells. FASEB J 1995, 9:1311-1318 [DOI] [PubMed] [Google Scholar]
- 26.Barbacid M: ras genes. Annu Rev Biochem 1987, 56:779-827 [DOI] [PubMed] [Google Scholar]
- 27.Quilliam LA, Khosravi-Far R, Huff SY, Der CJ: Guanine nucleotide exchange factors: activators of the Ras superfamily of proteins. Bioessays 1995, 17:395-404 [DOI] [PubMed] [Google Scholar]
- 28.Bokoch GM, Der CJ: Emerging concepts in the Ras superfamily of GTP-binding proteins. FASEB J 1993, 7:750-759 [DOI] [PubMed] [Google Scholar]
- 29.Boguski MS, McCormick F: Proteins regulating Ras and its relatives. Nature 1993, 366:643-654 [DOI] [PubMed] [Google Scholar]
- 30.Cancer Society of Finland: Cancer incidence in Finland 1995: cancer statistics of the National Research and Development Centre for Welfare and Health. Cancer Society of Finland publication 58. Helsinki, Finland Finnish Cancer Registry, 1997
- 31.Parkin DM, Muir CS, Whelan SL, Gao YT, Ferlay J, Powell J (editors): Cancer Incidence in Five Continents, vol 6. Lyon, France, International Agency for Research on Cancer and International Association of Cancer Registries (World Health Organization), 1992, pp 976–977
- 32.Cordon-Cardo C, Sheinfeld J: Molecular and immunopathology studies of oncogenes and tumor-suppressor genes in bladder cancer. World J Urol 1997, 15:112-119 [DOI] [PubMed] [Google Scholar]
- 33.Sidransky D, von Eschenbach A, Tsai YC, Jones P, Summerhayes I, Marshall F, Paul M, Green P, Hamilton SR, Frost P, Vogelstein B: Identification of p53 gene mutations in bladder cancer and urine samples. Science 1991, 252:706-709 [DOI] [PubMed] [Google Scholar]
- 34.Spruck CH, III, Ohneseit PF, Gonzalez-Zulueta M, Esrig D, Miyao N, Tsai YC, Lerner SP, Schmütte C, Yang AS, Cote R, Dubeau L, Nichols PW, Hermann GG, Steven K, Horn T, Skinner DG, Jones PA: Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res 1994, 54:784-788 [PubMed] [Google Scholar]
- 35.Mostofi FK, Sobin LH, Torloni H: Histological typing of urinary bladder tumours. 1973:pp 15-34 World Health Organization, Geneva
- 36.Nishi T, Lee PSY, Oka K, Lewin VA, Tanase S, Morino Y, Saya H: Differential expression of two types of the neurofibromatosis type 1 (NF1) gene transcripts related to neuronal differentiation. Oncogene 1991, 6:1555-1559 [PubMed] [Google Scholar]
- 37.Sandberg M, Vuorio E: Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by Northern and in situ hybridization. J Cell Biol 1987, 104:1077-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ylä-Herttuala S, Roosenfeld ME, Parthasarathy S, Glass CK, Sigal E, Witztum JL, Steinberg D: Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci USA 1990, 87:6959-6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 18:5294-5299 [DOI] [PubMed] [Google Scholar]
- 40.Ylä-Outinen H, Aaltonen V, Björkstrand A-S, Hirvonen O, Lakkakorpi J, Vähä-Kreula M, Laato M, Peltonen J: Up-regulation of tumor suppressor protein neurofibromin in normal human wound healing and in vitro evidence for platelet derived growth factor (PDGF) and transforming growth factor β1 (TGFβ1) elicited increase in neurofibromin mRNA steady-state levels in dermal fibroblasts. J Invest Dermatol 1998, 110:232-237 [DOI] [PubMed] [Google Scholar]
- 41.Hoffmeyer S, Assum G, Griesser J, Kaufmann D, Nurnberg P, Krone W: An unequal allelic expression of the neurofibromin gene in neurofibromatosis type 1. Hum Mol Genet 1995, 4:1262-1272 [DOI] [PubMed] [Google Scholar]
- 42.Boyer MJ, Gutmann DH, Collins FS, Bar-Sagi D: Crosslinking of the surface immunoglobulin receptor in B lymphocytes induces a redistribution of neurofibromin but not p120-GAP. Oncogene 1994, 9:49-357 [PubMed] [Google Scholar]
- 43.Norton KK, Xu J, Gutmann DH: Expression of the neurofibromatosis I gene product, neurofibromin, in blood vessel endothelial cells and smooth muscle. Neurobiol Dis 1995, 2:13-21 [DOI] [PubMed] [Google Scholar]
- 44.Bos JL: ras oncogenes in human cancer: a review. Cancer Res 1989, 49:4682-4689 [PubMed] [Google Scholar]
- 45.Fujita J, Srivastava SK, Kraus MH, Rhim JS, Tronick SR, Aaronson SA: Frequency of molecular alterations affecting ras protooncogenes in human urinary tract tumors. Proc Natl Acad Sci USA 1985, 82:3849-3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaturvedi V, Li L, Hodges S, Johnston D, Ro JY, Logothetis C, von Eschenbach AC, Batsakis JG, Czerniak B: Superimposed histologic and genetic mapping of chromosome 17 alterations in human urinary bladder neoplasia. Oncogene 1997, 14:2059-2070 [DOI] [PubMed] [Google Scholar]
- 47.Burchill SA, Berry PA, Lewis IJ: Changing expression of GTPase activating proteins with differentiation in neuroblastoma. J Neurol Sci 1994, 126:126-132 [DOI] [PubMed] [Google Scholar]
- 48.Johnson MR, DeClue JE, Felzmann S, Vass WC, Xu G, White R, Lowy DR: Neurofibromin can inhibit Ras-dependent growth by a mechanism independent of its GTPase-accelerating function. Mol Cell Biol 1994, 14:641-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jimbo H, Totsuka Y, Mashimo P, Umeyama T, Uehara H, Shinozak T: Bladder tumor associated with von Recklinghausen‘s neurofibromatosis: a case report. Hinyokika-Kiyo 1995, 41:61-64 [PubMed] [Google Scholar]
- 50.Yonemura S, Okuno T, Yamada Y, Uchida K, Arima K, Yanagawa M, Kawamura J: Bladder cancer associated with von Recklinghausen‘s disease: a case report. Hinyokika-Kiyo 1997, 43:585-588 [PubMed] [Google Scholar]
- 51.Harbour JW, Lai SL, Whang-Peng J, Gazdar AF, Minna JD, Kaye FJ: Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science 1988, 241:353-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokota J, Akiyama T, Fung YK, Benedict WF, Namba Y, Hanaoka N, Wada M, Terasaki T, Shimosato Y, Sugimura T, Terada M: Altered expression of the retinoblastoma (RB) gene in small-cell carcinoma of the lung. Oncogene 1988, 3:471-475 [PubMed] [Google Scholar]
- 53.Lee WH, Bookstein R, Hong F, Young LJ, Shew JY, Lee EY: Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science 1987, 235:1394-1399 [DOI] [PubMed] [Google Scholar]
- 54.Cordon-Cardo C, Wartinger D, Petrylak D, Dalbagni G, Fair WR, Fuks Z, Reuter VE: Altered expression of the retinoblastoma gene is a prognostic indicator in bladder cancer. J Natl Cancer Inst 1992, 84:1251-1256 [DOI] [PubMed] [Google Scholar]
- 55.Logothetis CJ, Xu HJ, Ro JY, Hu SX, Sahin A, Ordonez N, Benedict WF: Altered expression of retinoblastoma protein and known prognostic variables in locally advanced bladder cancer. J Natl Cancer Inst 1992, 84:1256-1261 [DOI] [PubMed] [Google Scholar]