Abstract
Dimethylbenzanthracene (DMBA) induces pancreatic adenocarcinomas in rats 9 months after carcinogen exposure, with precursor lesions (tubular complexes) developing 1 month after initiation of treatment. Because previous studies have suggested an acinar cell of origin for these tumors, we investigated the expression pattern of ductal, acinar, and islet cell markers in these cancers to gain insight into their phenotype and cell of origin. Pancreatic neoplasms were induced in rats by implantation of DMBA into the head of the pancreas. Lesions studied included 10 early tubular complexes (DMBA for 2 weeks), 8 tubular complexes (DMBA for 1 month), and 10 adenocarcinomas (DMBA for 9 months). Normal rat pancreas served as a control. For comparison, 5 human ductal adenocarcinomas were also evaluated. Immunohistochemistry with ductal (keratin, cytokeratin 19, cytokeratin 20), acinar (chymotrypsin), and islet (chromogranin A) cell markers was performed to analyze the tissues. Rat tubular complexes and adenocarcinomas revealed strong expression of keratin, cytokeratin 19, and cytokeratin 20 in the cytoplasm of all neoplastic cells, absence of chymotrypsin, and rare immunoreactivity to chromogranin A. Human adenocarcinomas showed strong expression of keratin and cytokeratin 19 in all neoplastic cells, expression of cytokeratin 20 in 5–20% of cells, and absence of chymotrypsin and chromogranin A. Pancreatic adenocarcinomas induced by DMBA in rats express markers consistent with a ductal phenotype, as observed in human tumors. Ductal marker expression in early tumor stages suggests a ductal cell of origin.
Chemical carcinogenesis using dimethylbenzanthracene (DMBA) has been shown to induce pancreatic tumors in rats with potential for local invasion and peritoneal metastases. 1 Tumors produced show a duct-like phenotype reminiscent of pancreatic neoplasms in man. Moreover, surrounding pancreatic ducts display features of hyperplasia, dysplasia, and carcinoma in situ, suggesting a gradual transition from normal duct epithelium to invasive carcinoma.
Despite the ductal phenotype of most human pancreatic carcinomas, there is still considerable debate as to the cell of origin of these tumors. Most adenocarcinomas are presumed to originate from ductal cells because they show mucin production, lack secretory granules, and have morphological features of ductal cells. However, cases of human ductal pancreatic neoplasms with prominent neuroendocrine and acinar differentiation have been described in the literature, suggesting a pluripotent cell of origin. 2,3
Experimental rodent models of pancreatic carcinoma have also contributed to the existing debate. Duct-like cell lines have been derived from a transplantable acinar cell carcinoma induced by asazerine in rats. 4 The authors suggest that some pancreatic carcinomas with a ductal phenotype might result from transformation, dedifferentiation, and/or metaplasia of acinar cells. In a different model, islet cells have been implicated as cells of origin for pancreatic neoplasms induced by N-nitroso-bis(2-oxypropyl) amine (BOP) in Syrian female hamsters. 5 Neoplastic duct-like complexes have been observed in the vicinity of islets of Langerhans in hamster pancreata exposed to BOP, suggesting a role for neuroendocrine cells in pancreatic tumorigenesis. These investigators have also observed differentiation of cultured islet cells into ductular and acinar cells and have used this information to explain their findings in BOP-induced tumors. 6
To further characterize DMBA pancreatic tumors in rats, we studied the pattern of expression of various ductal, acinar and islet cell markers in these lesions. Our goals were to determine whether DMBA tumors express markers consistent with a ductal phenotype, to determine whether tumor marker expression is consistent with human pancreatic adenocarcinomas, and to look at marker expression in early tumor stages to gain insight into the cell of origin of these neoplasms.
Cytokeratins were selected as ductal markers for this study. Cytokeratins are constituents of the intermediate-sized filaments of epithelial cells. 7 Epithelial cells express characteristic combinations of cytokeratins according to their location and degree of differentiation. Differential cytokeratin expression by immunohistochemistry allows differentiation of ductal cells from acinar or islet cells in the normal pancreas. 8,9 Moreover, during transformation of normal epithelia to malignant cells, the cell-type specificity of cytokeratins is largely conserved. 10 This property makes them valuable clinical tools for histological tumor diagnosis, providing key information pertaining to tumor origin and degree of differentiation. 11-13
Cytokeratin 19 (400 amino acids, 44.1 kd) is a widely distributed cytokeratin expressed in many simple epithelia. Cytokeratins 7 and 19 are the classic pancreatic ductal cell intermediate filaments and have been used in many studies to identify normal as well as neoplastic pancreatic ducts. 14 In contrast, cytokeratin 20 (424 amino acids, 48.6 kd) is a recently discovered intermediate filament, distinct from other cytokeratins and expressed only in gastrointestinal epithelia, the urothelium, and Merkel cells. It has significant value in tumor histodiagnosis because it is expressed mainly in carcinomas derived from cytokeratin 20-positive epithelia, being absent in most other carcinomas. As such, it has been shown to be very useful in the diagnosis of bile tract and pancreatic neoplasms. 15
For pancreatic acinar cells, chymotrypsin was chosen as marker molecule. Chymotrypsin is a pancreatic enzyme involved in protein digestion. This enzyme is expressed within the secretory granules on the apical aspect of acinar cells. Chymotrypsin has been used immunohistochemically to characterize human tumors of acinar or mixed acinar-endocrine origin. 16
Finally, chromogranin A was used as an islet cell marker. Chromogranin A is well established as a marker of neuroendocrine cells. 17 It is a glycoprotein involved in the sorting of peptide hormones and neurotransmitters to secretory granules within cells. In normal tissues it is expressed in adrenal chromaffin cells, anterior pituitary cells, gut enterochromaffin cells, pancreatic islet cells, thyroid C cells, and parathyroid cells. The specificity of expression of chromogranin A makes it a valuable tool for classification of pancreatic tumors of neuroendocrine cell origin. 18
Materials and Methods
Tumor Induction and Tissue Harvest
Tumors were induced in rats according to our previously established protocol. 1 Briefly, male Sprague-Dawley rats weighing approximately 150 g underwent a midline laparotomy with exposure of the pancreatic head. The common bile duct was identified and a 5-mm incision was made parallel to the course of the duct. A pocket in the pancreatic parenchyma was developed at the incision site, where 5 mg of DMBA crystals were implanted and secured in place by means of a 6–0 prolene pursestring suture. Following surgery, rats were housed in cages in our animal care facility. Animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85–23, 1985). The rats were allowed to have food and water ad libitum and were exposed to 12-hour light and dark cycles. Rat food consisted of Laboratory Rodent Diet 5001 from Purina Mills, Inc. (St. Louis, MO). Postoperative care consisted of monthly weight measurements, wound inspection, and abdominal palpation.
Three groups of carcinogen-treated animals were established. Rats in the first group were killed 9 months after carcinogen implantation, or earlier if moribund. At 9 months, approximately 30% of DMBA-treated animals developed pancreatic adenocarcinomas. 1 Rats in the second group were killed 1 month after carcinogen implantation, when preneoplastic lesions known as tubular complexes occur. 19 The final group consisted of animals sacrificed 2 weeks after carcinogen exposure, when the initial stages of tubular complex formation are presumed to take place. Following euthanasia, animals underwent abdominal exploration with close attention to the area of the pancreatic head. Pancreatic tumors were identified and representative samples fixed in formalin or snap-frozen in Tissue-Tek O.C.T. compound, (Sakura Finetek, Torrance, CA) at −70°C. Normal rat pancreatic tissue to be used as a control was obtained from weight-matched untreated animals. The study was approved by the subcommittee on animal research at our institution.
In addition to rat neoplasms, we also obtained frozen samples of three normal human pancreata and five human pancreatic cancers. The cancer tissues originated from five different patients who underwent pancreaticoduodenectomy for symptomatic tumors in the head of the pancreas. Clinical details regarding age, sex, and final pathological diagnosis for these patients are summarized in Table 1 ▶ .
Table 1.
Human Pancreatic Tumors Analyzed
| Specimen no. | Age/sex | Pathological diagnosis | Cytokeratin 19 | Cytokeratin 20 |
|---|---|---|---|---|
| 1 | 71 /M | 1.5 cm ductal adenocarcinoma G2-3, 1/10 postive LN | ++++ | + |
| 2 | 78 /F | 1.1 cm ductal adenocarcinoma G2, 0/8 positive LN | ++++ | ++ |
| 3 | 68 /F | 2.3 cm ductal adenocarcinoma G2, 9/13 postive LN | ++++ | ++ |
| 4 | 66 /M | 2.5 cm ductal adenocarcinoma G3, 5/25 postive LN | ++++ | + |
| 5 | 68 /M | 3 cm ductal adenocarcinoma G3, 2/13 postive LN | ++++ | + |
M or F, male or female; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated; LN, lymph node(s); +, 5–20% positive cells; ++, 20–50% positive cells; +++, 50–90% positive cells; ++++, >90% positive cells.
Histology and Immunohistochemistry
For routine histology, 5-μm sections of formalin-fixed, paraffin-embedded tissue were prepared and stained with hematoxylin and eosin. All slides were reviewed in a blinded fashion by a single pathologist and assessed for the presence of precursor lesions (tubular complexes) or invasive pancreatic ductal adenocarcinoma. A diagnosis of invasive adenocarcinoma required the presence of infiltrating irregular neoplastic glands within a desmoplastic stroma. The neoplastic epithelium was assessed for nuclear crowding, hyperchromatism, and mitotic activity to determine the degree of tumor differentiation.
For immunohistochemistry, the following primary antibodies were obtained as ductal cell markers: anti-cytokeratin 19 (mouse monoclonal, RPN 1165, dilution 1:10, Amersham, Buckinghamshire, UK), anti-cytokeratin 20 (mouse monoclonal, Ks20.8, dilution 1:50, Dako, Carpinteria, CA), and anti-keratin, wide spectrum screening (rabbit polyclonal, dilution 1:500, Dako). Primary antibodies obtained against acinar cell were anti-chymotrypsin (mouse monoclonal, 4E1, dilution 1:1000, Biogenesis, Sandown, NH) and against neuroendocrine cells, anti-chromogranin A (rabbit polyclonal, SP-1, dilution 1:500, Incstar, Stillwater, MN).
Anti-cytokeratin 19 and 20 antibodies were applicable only to acetone-fixed cryostat sections. All other antibodies were used in paraffin-embedded tissues.
For immunohistochemical staining of 5-μm paraffin or cryostat sections, the avidin-biotin-peroxidase complex (ABC) method was applied. Paraffin sections were initially deparaffinized, rehydrated, incubated in H2O2, and subjected to antigen retrieval by heating in 0.1 mmol/L citrate buffer. Likewise, cryostat sections were prepared by fixing in acetone and blocking endogenous peroxidase activity with H2O2. Both paraffin and cryostat sections were then processed in identical fashion. To block nonspecific antibody binding, blocking serum (Vector ABC Kit, Vector Lab, Burlingame, CA) was applied for 20 minutes, then washed off. Primary antibodies in appropriate dilutions were then applied and slides were incubated overnight in a moist chamber at 4°C. Controls were incubated with either phosphate buffered saline and/or purified mouse IgG standards in the same dilution as the primary antibodies. The next day unbound antibody was washed off and slides were incubated in secondary biotinylated anti-mouse or anti-rabbit antibodies, followed by ABC reagent (Vector ABC Kit). Slides were then developed in AEC solution (80 mg of 3-amino-9-ethylcarbazole in 10 ml of NN-dimethyl formamide diluted in 29.6 ml of 0.2 mol/L acetic acid, 70.4 ml of 0.2 mol/L sodium acetate, 0.4 ml of H2O2, and 100 ml of distilled water). Following fixation, slides were counterstained with Lerner’s no. 1 hematoxylin and mounted on glycergel (DAKO) for light microscopy.
Results
Normal Pancreas (Control)
In normal rat pancreatic tissue, antibodies against cytokeratin 19 selectively stained the cytoplasm of all ductal cells. Staining with polyclonal anti-keratin and monoclonal anti-cytokeratin 19 antibodies was identical in the normal pancreas. In contrast, anti-cytokeratin 20 antibodies labeled a subpopulation of ductal cells, having stronger reactivity with intralobular and interlobular ductules (>50% positive cells), and weaker reactivity in scattered cells of large and intermediate-sized pancreatic ducts (5–20% positive cells). None of these ductal markers showed cross-reactivity with acinar or islet cells.
Chymotrypsin immunolocalization was in the granules of the apical cytoplasm of acinar cells. Staining was strong and granular. Anti-chromogranin A antibodies selectively labeled individual cells within the islets of Langerhans. Not all cells within the islets stained with anti-chromogranin A antibodies, and most positive cells were found along the periphery of the islets. Antibodies against chymotrypsin did not react with ductal or islet cells, whereas antibodies against chromogranin A showed no cross-reactivity with ductal or acinar cells.
Three specimens of normal human pancreatic tissue were also evaluated with the ductal markers. The results confirmed the observations made in normal rat pancreatic tissue as described above.
Rat Pancreatic Adenocarcinomas
Table 2 ▶ demonstrates the laparotomy findings, gross pathology, and histological diagnosis of the 10 tumors selected for the study. Most tumors were harvested 9 months after carcinogen exposure, but animal no. 9 had a sizable mass after only 5 months. Almost all tumors were >2 cm in diameter and demonstrated considerable gastric outlet and biliary ductal obstruction. Overall, histology revealed ductal adenocarcinoma with mucin production and intestinal differentiation. Most neoplasms showed extensive local growth with occasional invasion of adjacent organs; three specimens showed intra-abdominal lymph node metastasis.
Table 2.
Gross and Microscopic Findings of Tumors Analyzed
| Rat no. | Survival (days) | Gross pathology | Microscopic diagnosis | CK-19 | CK-20 |
|---|---|---|---|---|---|
| 1 | 270 | 3.8× 4× 2.7 cm mass HOP, LN metastasis | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 2 | 270 | 5× 3.5× 3 cm mass HOP | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 3 | 270 | 3× 2× 2.2 cm mass HOP invading duodenum and colon | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 4 | 270 | 2× 1.8× 2 cm mass HOP | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 5 | 215 | 3.5× 3× 4 cm mass HOP, LN metastasis | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 6 | 249 | 4.5× 4× 3.5 cm mass HOP | G1 ductal adenocarcinoma | ++++ | ++++ |
| 7 | 250 | 6.5× 6× 5 cm mass HOP invading liver and stomach, LN metastasis | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 8 | 249 | 5× 4.5× 3 cm mass HOP | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 9 | 158 | 3× 3× 3 cm mass HOP | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
| 10 | 231 | 5× 7 mm mass HOP | G2 mucinous ductal adenocarcinoma | ++++ | ++++ |
HOP, head of the pancreas; LN, lymph node; G1, grade 1 or well-differentiated; G2, grade 2 or moderately differentiated; CK, cytokeratin; ++++, >90% positive cells.
Immunohistochemistry with keratin, cytokeratin 19, and cytokeratin 20 antibodies showed strong cytoplasmic staining in >90% of the cells comprising the epithelia of the neoplastic glands, as seen in Figure 1, B and C ▶ . No difference in expression of ductal markers, especially between cytokeratin 19 and 20, was noted within tumors.
Figure 1.
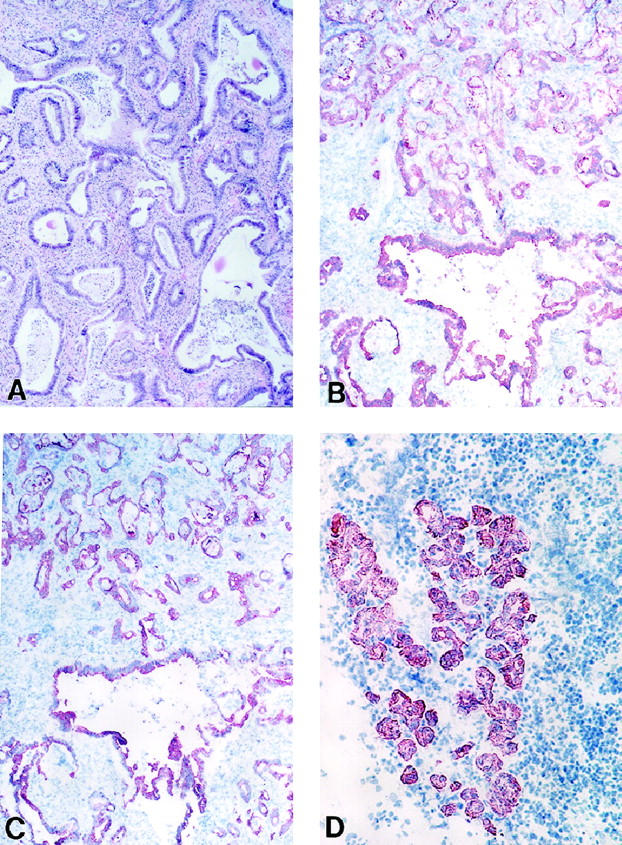
Ductal cell markers in DMBA-induced rat pancreatic neoplasms. A: Adenocarcinoma (hematoxylin and eosin stain, paraffin section, original magnification, ×100). B: Adenocarcinoma, cytokeratin 19 (cryostat section, ABC reaction, original magnification, ×100). C: Adenocarcinoma, same tumor as in B now stained for cytokeratin 20 (cryostat section, ABC reaction, original magnification, ×100). D: Tubular complex at 1 month, cytokeratin 19 (cryostat section, ABC reaction, original magnification, ×200).
Chymotrypsin immunoreactivity was absent in all tumors studied, as seen in Figure 2A ▶ .
Figure 2.
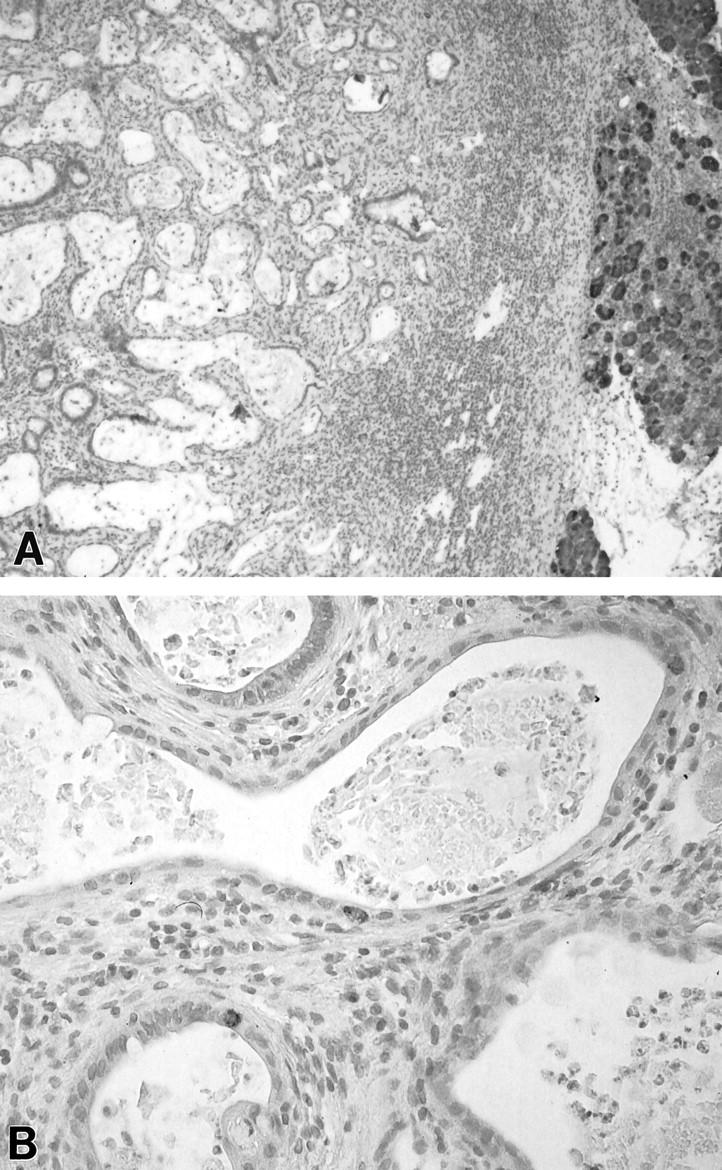
Acinar and islet cell markers in DMBA adenocarcinomas. A: Chymotrypsin. Note absence of staining in tumor (left) and positive staining in surrounding normal pancreas (right) (paraffin section, ABC reaction, original magnification, ×50). B: Chromogranin A, scattered single cells staining within neoplastic epithelium (paraffin section, ABC reaction, original magnification, ×200).
Anti-chromogranin A antibodies labeled single scattered cells within the neoplastic epithelia as seen in Figure 2B ▶ . These cells accounted for <2% of the cells in the transformed glands and were more prevalent in well differentiated than in moderately differentiated tumors.
Preneoplastic Changes 1 Month after DMBA Implantation
Precursor lesions of DMBA adenocarcinomas, known as tubular complexes, were collected from rats 1 month after carcinogen implantation. Eight different lesions were selected for the study. Histological evaluation revealed multiple conglomerates of tubular structures at a distance of 1–4 mm from implantation sites, with significant associated inflammation and early fibrosis. Cellular mitoses were scant and zymogen granules were not detectable. Most lesions were pathologically graded as ductular hyperplasias, but at least two contained carcinoma in situ. Immunohistochemistry with ductal markers revealed strong staining in all cells forming tubular complexes (Figure 1D) ▶ . Intensity of staining did not depend on radial distance from the DMBA pocket, and all tubular complexes identified stained positive for cytokeratin 19, cytokeratin 20, and keratin. Staining for chymotrypsin or chromogranin A was absent within the tubular complexes, but present in the surrounding normal pancreas within acinar cells and islet cells respectively.
Preneoplastic Changes 2 Weeks after DMBA Treatment
Early stages of tubular complex formation were studied by analyzing pancreata two weeks after DMBA implantation. Ten lesions from 10 different animals were evaluated. Histology revealed aggregates of tubular structures with identical characteristics to lesions observed at 1 month (Figure 3A) ▶ . In comparison to lesions at 1 month, these tubular complexes were smaller in size and included fewer tubules per complex. Early tubular complexes showed strong keratin expression by immunohistochemistry, irrespective of their radial distance from the center of the carcinogen implantation site (Figure 3B) ▶ . No chymotrypsin or chromogranin A immunoreactivity was noted within any of the tubular complexes identified (Figure 3C) ▶ . Apoptotic acinar cells with residual chymotrypsin expression were observed within areas of inflammation and early fibrosis, but these were never observed in tubular configuration.
Figure 3.
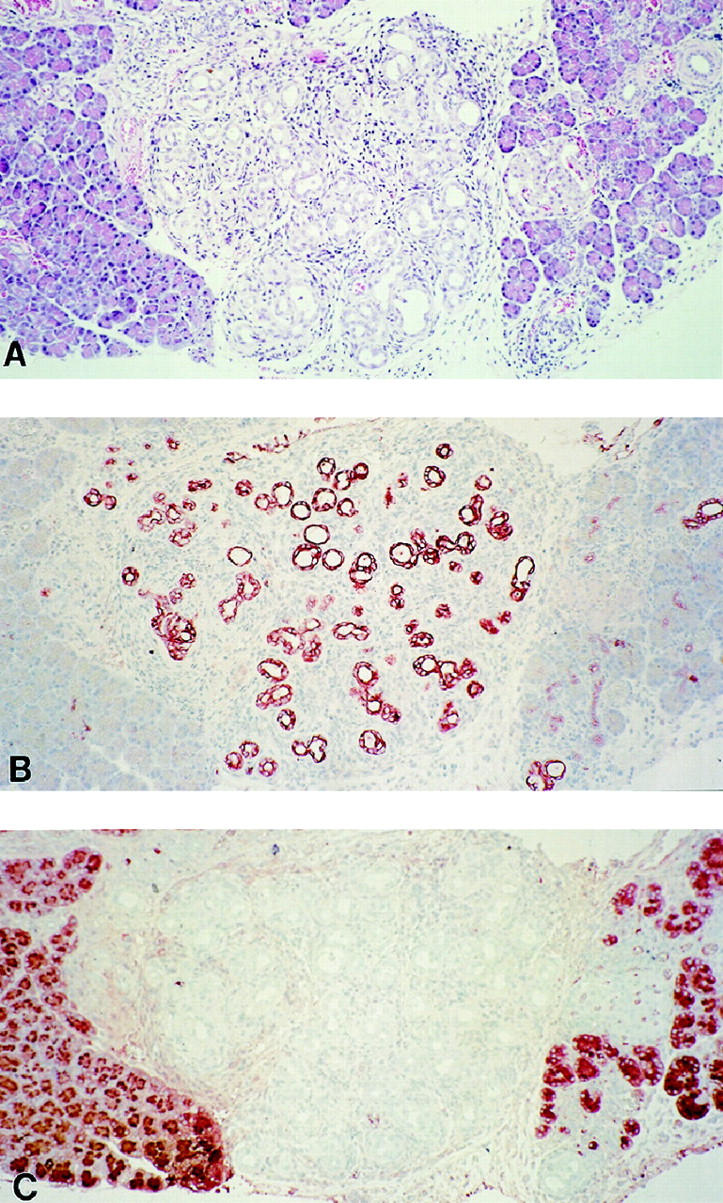
Immunohistochemistry of rat tubular complex developing 2 weeks after DMBA implantation. A: Tubular complex is in the center of the photograph flanked by normal pancreatic tissue on either side (hematoxylin and eosin stain, paraffin section, original magnification, ×150). B: Keratin. Note strong staining of tubular complex epithelium and small ducts within surrounding pancreatic parenchyma on the right. (paraffin section, ABC reaction, original magnification, ×150). C: Chymotrypsin (paraffin section, ABC reaction, original magnification, ×150).
Human Pancreatic Adenocarcinomas
Five human pancreatic adenocarcinomas were tested for markers of ductal or acinar cells; results are summarized in Table 1 ▶ . Keratin and cytokeratin 19 antibodies labeled >90% of the neoplastic epithelium in all tumors studied. Staining was strong and cytoplasmic. In contrast, cytokeratin 20 expression was heterogenous, being present in 10 to 50% of the neoplastic epithelia. Staining was strong, but restricted to single cells or group of cells within neoplastic glands. No immunoreactivity to chymotrypsin or chromogranin A was observed in any of the tumors studied.
Discussion
Pancreatic cancer is the fifth leading cause of cancer-related mortality in the United States. 20 It is a devastating disease with a 5-year survival rate of less than 3%. Factors involved in this extremely poor prognosis include late presentation, unavailable screening protocols, and limited therapeutic options. As opposed to colon or breast cancer, precursor lesions in pancreatic carcinoma are poorly understood, curtailing attempts at early tumor detection. In this setting, experimental models offer a good method to study the evolution, natural history, and effective therapy of cancer of the pancreas.
It has been difficult to develop an animal model resembling human pancreatic cancer, which appears to be ductal adenocarcinoma. Chemical carcinogenesis using azaserine in rats has been plagued by the development of acinar cell carcinomas. 21 Transgenic mice models, although very successful in targeting and inducing pancreatic neoplasms, have resulted mostly in acinar and mixed acinar-ductal carcinomas. 22 Models involving xenografting of human tissue in nude mice bypass the process of multistage carcinogenesis, and eliminate immunological modulation of tumor growth and spread. 23 At the moment, the most successful model involves chemical carcinogenesis using BOP in hamsters. BOP has been shown to induce ductal pancreatic adenocarcinomas 24 with genetic mutations analogous to their human counterparts. 25
The only model of pancreatic ductal adenocarcinoma in the common laboratory rat involves DMBA carcinogenesis. 26 Recent studies from our laboratory demonstrated that DMBA was the only known carcinogenic agent that reliably induces ductal pancreatic cancers in rats. 1 At present, the DMBA model has been characterized by plain histology and by a complex series of structural-morphological studies by Bockman in 1981. 27 These studies involved three-dimensional cast reconstructions of normal pancreas and DMBA neoplasms, and electron microscopic analysis. Bockman concluded that DMBA tumors were not ductal in origin, but resulted from dedifferentiation of acinar cells. Through dedifferentiation, a process involving halting of cellular synthesis and zymogen granule disappearance, acinar cells were thought to take on the appearance of ducts or ductules. 28
The present study employs immunohistochemistry to characterize these tumors in more detail. We demonstrate that pancreatic adenocarcinomas induced by DMBA in rats express cytokeratins 19 and 20, markers of ductal cells in the normal pancreas. Cytokeratin 19 and 20 expression has been reported in human pancreatic ductal adenocarcinomas 7,15 and was confirmed by immunohistochemistry of five different human pancreatic tumors in this study. Ductal marker expression, therefore, is similar in the pancreatic neoplasms of humans and DMBA-treated rats.
The presence of ductal markers and absence of acinar markers are consistent with a ductal cell of origin for DMBA carcinomas, if we assume that the cytokeratin pattern of the cell of origin is preserved in the process of neoplasia. 11-13 It is possible, however, that ductal cell markers are acquired by acinar cells during the development of carcinoma. Ductal metaplasia has been described in other models of pancreatic cancer, particularly in association with azaserine-induced pancreatic carcinomas in the rat and acinar cell carcinomas in the Ela-1-myc transgenic mice. 4,22 To address this issue, we examined cell marker expression during early tumor stages. The premise for this study is that the identification of antigenic phenotypes in early tumor stages may provide insights into the cell of origin for these tumors. If acinar cells, along with ductal cells, participate in the origin of DMBA tumors by dedifferentiation or metaplasia, it should be possible to identify intermediate cells containing both acinar and ductal markers.
The earliest DMBA precursor lesions described in the medical literature are known as tubular complexes, appearing 1–3 months after carcinogen treatment. 19 Our results confirm the presence of these precursor lesions following DMBA exposure 1 month in duration. Theoretically, tubular complexes could originate from ductal cells, acinar cells, or a combination of these two cell types. To study the process of tubular complex formation, we analyzed pancreatic changes occurring 2 weeks after DMBA implantation. Although it is likely that these complexes begin to form immediately after DMBA treatment, attempts at evaluating lesions earlier than 2 weeks were uninformative due to superimposed chemical pancreatitis. It is significant that other studies have described tubular complexes in association with acute and chronic pancreatitis, suggesting that inflammation may also play a role in the formation of these structures. 29-31
Our results demonstrate that tubular complexes express the same cytokeratins as normal pancreatic ducts and advanced DMBA tumors. A ductal phenotype was observed in precursor lesions as early as 2 weeks after carcinogen exposure, irrespective of their radial distance from the implantation site. In contrast, chymotrypsin expression was not observed within tubular complexes, and apoptotic acinar cells were noted to blend with the ongoing pancreatic fibrosis without degeneration into duct-like structures. These results suggest targeting of ductal cells at the initial stages of DMBA carcinogenesis, without evidence for metaplasia and dedifferentiation in the process. Similar patterns of ductal cytokeratin expression have been described in pancreatic tumors and premalignant lesions induced by BOP in hamsters and have been used to support the hypothesis of a ductal cell of origin. 32 However, because we were unable to study the earliest effects of DMBA, a determination of the cell of origin of tubular complexes cannot conclusively be made at this time and awaits analysis of pancreatic changes occurring during the first days after carcinogen treatment.
It is interesting that a subpopulation of ductal cells was recognized by cytokeratin 20 antibodies in the normal pancreas, with a predilection for small intralobular/interlobular ductules over larger pancreatic ducts. This observation was previously reported in the original paper describing the characterization of cytokeratin 20. 15 Because cytokeratin 20 is known to be present mostly in malignancies derived from cytokeratin 20-positive precursor cells, the expression of this keratin by DMBA tumors suggests a ductular cell of origin. Indeed, because ductular cells comprise approximately 80% of the cells in the pancreatic duct system of the rat, they would appear, at least statistically, to be the most likely targets for DMBA-induced carcinogenesis. 33 Similarly, the difference in expression of cytokeratin 20 between rat and human tumors could be a reflection of slightly different cells of origin for these neoplasms (ductular versus duct cells respectively).
The significance of neuroendocrine cells detected within the neoplastic epithelium of DMBA tumors is unknown. Scattered goblet, brush, and neuroendocrine cells are known to be present among the principal cells of pancreatic ducts. 34 In addition, scattered neuroendocrine cells have been observed in some human pancreatic ductal adenocarcinomas, especially well-differentiated neoplasms. 35,36 DMBA tumors are usually well to moderately differentiated, and the presence of neuroendocrine cells correlates with the level of differentiation. Overall, neuroendocrine cells were observed infrequently and their role, if any, in the pathogenesis of these tumors remains to be elucidated.
In summary, our study demonstrates that DMBA induces duct-like neoplasms in the rat pancreas with protein expression concordant with human pancreatic tumors. Evaluation of precursor lesions suggests that these tumors arise from ductal cell transformation. Further characterization is ongoing in our laboratory to determine whether genetic/mutational changes (K-ras, p16, p53) in DMBA tumors recapitulate their human counterparts. Preliminary data already demonstrates the presence of activating codon 12 K-ras mutations. 37 If other mutational changes in these tumors prove similar to those found in human malignancies, the model could be uniquely useful in the study of the origin, biology, metastasis, and treatment of pancreatic carcinoma.
Footnotes
Address reprint requests to Carlos Fernandez-del Castillo, M.D., Associate Professor of Surgery, WACC 336, Massachusetts General Hospital, Boston, MA 02114.
Supported by the Marshall K. Bartlett, M.D. Resident Research Fellowship, Harvard Medical School (to R. E. J.) and by grants from the Swiss National Foundation (to K. Z.) and the Swiss Cancer Society (BIL SKL 415–1-97 to K. Z.).
Results were presented at the 1998 meeting of the American Gastroenterological Association (New Orleans, May 17–20) and the 1998 meeting of the American Pancreatic Association (Chicago, November 5–6).
References
- 1.Rivera JA, Graeme-Cook F, Werner J, Z’graggen K, Rustgi AK, Rattner DW, Warshaw AL, Fernandez-del Castillo C: A rat model of pancreatic ductal adenocarcinoma: targetting chemical carcinogens. Surgery 1997, 122:82-90 [DOI] [PubMed] [Google Scholar]
- 2.Schron DS, Mendelsohn G: Pancreatic carcinoma with duct, endocrine, and acinar differentiation: a histologic, immunocytochemical and ultrastructural study. Cancer 1984, 54:1766-1770 [DOI] [PubMed] [Google Scholar]
- 3.Bosman FT: Neuroendocrine cells in non-neuroendocrine tumors. Falkmer S Hakanson R Sundler F eds. Evolution and Tumor Pathology of the Neuroendocrine System. 1984, :pp 519-543 Elsevier Science Publishers, New York [Google Scholar]
- 4.Pettengill OS, Faris RA, Bell RH, Kuhlmann ET, Longnecker DS: Derivation of ductlike cell lines from a transplantable acinar cell carcinoma of the rat pancreas. Am J Pathol 1993, 143:292-303 [PMC free article] [PubMed] [Google Scholar]
- 5.Pour PM, Weide L, Liu G, Fienhold MA, Sanger W: Evidence for the origin of ductal type adenocarcinoma from the islets of Langerhans. Am J Pathol 1997, 150:2167-2180 [PMC free article] [PubMed] [Google Scholar]
- 6.Pour PM, Liu G, Moyer MP: Differentiation of cultured islet into ductular and acinar cells. Pancreas 1997, 15:424 [Google Scholar]
- 7.Moll R, Franke WW, Schiller DL: The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31:11-24 [DOI] [PubMed] [Google Scholar]
- 8.Real FX, Vila MR, Skoudy A, Ramaekers FCS, Corominas JM: Intermediate filaments as differentiation markers of exocrine pancreas. II Expression of cytokeratins of complex and stratified epithelia in normal pancreas and in pancreas cancer. Int J Cancer 1993, 54:720-727 [DOI] [PubMed] [Google Scholar]
- 9.Schussler MH, Skoudy A, Ramaekers F, Real FX: Intermediate filaments as differentiation markers of normal pancreas and pancreas cancer. Am J Pathol 1992, 140:559-568 [PMC free article] [PubMed] [Google Scholar]
- 10.Moll R: Cytokeratins in the histological diagnosis of malignant tumors. Int J Biol Markers 1994, 9:63-69 [DOI] [PubMed] [Google Scholar]
- 11.Denk H, Krepler R, Lackinger E, Artlieb U, Franke WW: Biochemical and immunocytochemical analysis of the intermediate filament cytoskeleton in human hepatocellular carcinomas and in hepatic neoplastic nodules of mice. Lab Invest 1982, 46:584-596 [PubMed] [Google Scholar]
- 12.Gabbiani G, Kapanci Y, Barrazone P, Franke WW: Immunochemical identification of intermediate-sized filaments in human neoplastic cells: a diagnostic aid for the surgical pathologist. Am J Pathol 1981, 104:206-216 [PMC free article] [PubMed] [Google Scholar]
- 13.Schlegel R, Banks-Schlegel S, McLeod JA, Pinkus GS: Immunoperoxidase localization of keratin in human neoplasms. Am J Pathol 1980, 110:41-49 [PMC free article] [PubMed] [Google Scholar]
- 14.Dabeva MD, Hwang SG, Vasa SRG, Hurston E, Novikoff PM, Hixson DC, Gupta S, Shafritz DA: Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci USA 1997, 94:7356-7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moll R, Lowe A, Laufer J, Franke WW: Cytokeratin 20 in human carcinomas. Am J Pathol 1992, 140:427-447 [PMC free article] [PubMed] [Google Scholar]
- 16.Klimstra DS, Rosai J, Heffess CS: Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol 1994, 18:765-778 [DOI] [PubMed] [Google Scholar]
- 17.Ozawa H, Takata K: The granin family: its role in sorting and secretory granule formation. Cell Struct Funct 1995, 20:415-420 [DOI] [PubMed] [Google Scholar]
- 18.Solcia E, Capella C, Buffa R, Tenti P, Rindi G, Cornaggia M: Antigenic markers of neuroendocrine tumors: their diagnostic and prognostic value. Fenoglio CM Weinstein RS Kaufman N eds. New Concepts in Neoplasia as Applied to Diagnostic Pathology. 1986, :pp 242-261 Williams & Wilkins, Baltimore [PubMed] [Google Scholar]
- 19.Bockman DE, Black O, Jr., Mills LR, Webster PD: Origin of tubular complexes developing during induction of pancreatic adenocarcinoma by 7,12-dimethylbenzanthracene. Am J Pathol 1978, 90:645-658 [PMC free article] [PubMed] [Google Scholar]
- 20.Warshaw AL, Fernandez-del Castillo C: Pancreatic carcinoma. N Engl J Med 1992, 326:455-465 [DOI] [PubMed] [Google Scholar]
- 21.Lilja HS, Hyde E, Longnecker DS, Yager JDJ: DNA damage and repair in rat tissues following administration of azaserine. Cancer Res 1977, 37:3925-3931 [PubMed] [Google Scholar]
- 22.Sandgren EP, Quaife CJ, Paulovich AG, Palmiter RD, Brinster RL: Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci USA 1991, 88:93-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu X, Guadagni F, Hoffman RM: A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA 1992, 89:5645-5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pour PM, Kruger FW, Althoff J, Cardesa A, Mohr U: Cancer of the pancreas induced in the Syrian golden hamster. Am J Pathol 1974, 76:349-358 [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii H, Egami H, Pour P, Pelling J: Pancreatic ductal adenocarcinomas induced in Syrian hamsters by N-nitrosobis(2-oxopropyl)amine contain a c-K-ras oncogene with a point-mutated codon 12. Mol Carcinog 1990, 3:296-301 [DOI] [PubMed] [Google Scholar]
- 26.Dissin J, Mills LR, Mains DL, Black O, Jr, Webster PD: Experimental induction of pancreatic adenocarcinoma in rats. J Natl Cancer Inst 1975, 55:857-864 [DOI] [PubMed] [Google Scholar]
- 27.Bockman DE: Cells of origin of pancreatic cancer: experimental animal tumors related to human pancreas. Cancer 1981, 47:1528-1534 [DOI] [PubMed] [Google Scholar]
- 28.Bockman DE: Alteration in pancreatic structure associated with adenocarcinoma. Scarpelli DG Reddy JK Longnecker DS eds. Experimental Pancreatic Carcinogenesis. 1987, :pp 131-144 CRC Press, Boca Raton [Google Scholar]
- 29.Lechene de la Porte P, Iovanna J, Odaira C, Choux R, Sarles H, Berger Z: Involvement of tubular complexes in pancreatic regeneration after acute necrohemorrhagic pancreatitis. Pancreas 1991, 6:298-306 [DOI] [PubMed] [Google Scholar]
- 30.Walker NI, Winterford CM, Kerr JFR: Ultrastructure of the rat pancreas after experimental duct ligation. II. Duct and stromal cell proliferation, differentiation, and deletion. Pancreas 1992, 7:420-434 [DOI] [PubMed] [Google Scholar]
- 31.Kataoka K, Sasaki T, Yorizumi H, Sakagami J, Kashima K: Pathophysiologic studies of experimental chronic pancreatitis in rats induced by injection of zein-oleic acid-linoleic acid solution into the pancreatic duct. Pancreas 1998, 16:289-299 [DOI] [PubMed] [Google Scholar]
- 32.Bell RH, Jr., Ray MB: Cytokeratin antigen in BOP-induced pancreatic tumors-implications for histogenesis. Carcinogenesis 1987, 8:1563-1566 [DOI] [PubMed] [Google Scholar]
- 33.Kachar B, Taga R, Knievel GA, Sesso A: Morphometric evaluation of the number of exocrine pancreatic cells during early postnatal growth in the rat. Acta Anat 1979, 103:11-15 [DOI] [PubMed] [Google Scholar]
- 34.Madden ME, Sarras MP, Jr: The pancreatic ductal system of the rat: cell diversity, ultrastructure, and innervation. Pancreas 1989, 4:472-485 [DOI] [PubMed] [Google Scholar]
- 35.Kamisawa T, Fukayama M, Tabata I, Isawa T, Tsuruta K, Okamoto A, Koike: Neuroendocrine differentiation in pancreatic duct carcinoma special emphasis on duct-endocrine cell carcinoma of the pancreas. Pathol Res Pract 1996, 192:901-908 [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Baithum SI, Pollock DJ, Berry CL: Argyrophilic and hormone immunoreactive cells in normal and hyperplastic pancreatic ducts and exocrine pancreatic carcinoma. Virchows Arch A 1988, 413:399-405 [DOI] [PubMed] [Google Scholar]
- 37.Z’graggen K, Jimenez RE, Werner J, Graeme-Cook F, Warshaw AL, Fernandez-del Castillo C: Mutations of the K-ras but not the H-ras gene are involved in the carcinogenesis of DMBA-induced ductal pancreatic adenocarcinomas in rats. Pancreas 1998, 17:460


