Abstract
Numerous post mortem studies have demonstrated increased accumulation of lipid peroxidation products in diseased regions of Alzheimer’s disease (AD) brain; however, few have used techniques that quantify the magnitude of lipid peroxidation in vivo. F2-isoprostanes (F2-IsoP’s) are exclusive products of free radical-mediated peroxidation of arachidonic acid, and their quantification has been widely used as an in vivo biomarker of the magnitude of lipid peroxidation. We have determined F2-IsoP concentrations in lateral ventricular fluid (VF) from 23 AD and 12 age-matched controls and correlated these with neuropathological and genetic markers of AD. VF F2-IsoP levels were significantly elevated in AD patients compared with controls (p < 0.01) and were significantly correlated with three different measures of brain degeneration: reduction in brain weight (p < 0.01), degree of cortical atrophy (p < 0.01), and Braak stage (p = 0.02). When analysis was restricted to AD patients only, VF F2-IsoP levels still were significantly correlated to reduction in brain weight and degree of cortical atrophy (p < 0.05). VF F2-IsoP concentrations were not related to density of neuritic plaques or neurofibrillary tangles in seven brain regions, or to the number of ε4 alleles of the apolipoprotein E gene (APOE). These data suggest that the magnitude of brain lipid peroxidation is closely related to the extent of brain degeneration in AD but is not significantly influenced by the density of neuritic plaques or neurofibrillary tangles, or the number of ε4 alleles of APOE.
Numerous post mortem studies have demonstrated regionally increased brain oxidative damage in Alzheimer’s disease (AD) patients compared to age-matched controls. 1-3 These studies have taken a number of experimental approaches, including measuring oxidative damage to nucleic acids, oxidative modification of protein, and consequences of lipid peroxidation. This last process has been proposed to be especially damaging, because it is self-propagating and because brain is relatively enriched in polyunsaturated fatty acids, the substrates for lipid peroxidation.
Several laboratories have reported studies showing increased accumulation of lipid peroxidation products in diseased regions of AD brain. 2,4-15 The techniques used have included measuring protein carbonyls, thiobarbiturate reactive substances, and immunohistochemical and histochemical reactivity for protein adducts formed by chemically reactive lipid peroxidation products. Although in situ studies have the clear advantage of demonstrating where lipid peroxidation products have accumulated, none of these techniques quantitatively reflects lipid peroxidation in vivo. This limitation arises from 1) the chemical reactivity of the products being measured, so that levels reflect not only production but also clearance of modified proteins; 2) the extensive metabolism of the lipid peroxidation products being measured, so that increased levels may reflect increased production or decreased metabolism; and 3) the lack of specificity of many of the assays used. 16,17
Considerable progress has been made over the last decade in developing methods to quantify lipid peroxidation in vivo. One result of this research has been the discovery of isoprostanes. Isoprostanes are products of free radical-catalyzed peroxidation of arachidonic acid and are isomeric to prostaglandins. 18 Importantly, isoprostanes are exclusive products of free radical damage and are not produced by enzymatic catalysis. Beyond specificity for lipid peroxidation, other advantages of isoprostanes are that the F-ring isoprostanes (F2-isoprostanes or F2-IsoPs) are chemically stable and are not metabolized in situ like other peroxidation products. 19 For these reasons, F2-IsoPs have been widely used as quantitative biomarkers of lipid peroxidation in vivo in many experimental settings and in humans. 19,20
Recently we have shown that F2-IsoP concentrations are increased in ventricular cerebrospinal fluid (VF) obtained post mortem from definite AD patients compared to age-matched controls. 21 We also have shown that F2-IsoPs are elevated in cerebrospinal fluid (CSF) removed from the lumbar cistern (LF) of probable AD patients early in the course of their disease. 22 The latter is an important finding because it indicates that elevation of CSF F2-IsoPs is not simply an end-stage consequence of AD. Others have extended these findings by showing that tissue levels of F-ring IsoPs are increased in diseased regions of brain from AD patients compared to controls. 23
Determining the sources of oxidative damage in AD brain is central to understanding the pathogenesis of AD and will provide the basis for a rational development of experimental therapeutics. Proposed contributors to increased oxidative damage in AD brain include Aβ peptides, activated glia in neuritic plaques (NPs), neurofibrillary tangles (NFTs), and inheritance of different alleles of the apolipoprotein E gene (APOE), among others. 1-3,24-26 Although each of these potential sources of oxidative damage has been supported by in vitro, cell culture, or animal experiments, there is little information on the relative importance of these factors to oxidative damage in the brains of AD patients. Equipped with a quantitative measure of lipid peroxidation, we have tested the hypothesis that the magnitude of brain lipid peroxidation in AD may be related to the density of NPs or NFTs, or APOE.
Materials and Methods
After appropriate consent was obtained, all 35 individuals included in this study underwent post mortem examinations as part of rapid autopsy programs at the University of Kentucky Alzheimer’s Disease Research Center. No patient had a post mortem interval greater than 4 hours. All AD patients were diagnosed with probable AD during life and were shown by neuropathological examination to meet the criteria for definite AD. 27 Controls were age- and gender-matched individuals without clinical evidence of dementia or other neurological disease. Indeed, each control individual had annual neuropsychological testing with all test scores in the normal range. Cerebral cortical atrophy was ranked by one of us (WRM) at the time of autopsy as either absent (normal apposition of gyri, n = 15), mild (slight thinning of gyri with associated enlargement of sulci, n = 8), moderate (intermediate between mild and severe, n = 8), or severe (marked thinning of gyri and widening of sulci, n = 4) in all AD patients and controls.
Neuropathological examination of controls showed only age-associated changes. Braak staging was performed on all cases. 28 NFTs and NPs, to the exclusion of diffuse plaques, were counted in Bielschowsky-stained histological sections from formalin-fixed paraffin-embedded tissue according to previously published methods. 29 NP counts were determined in five 2.35 mm 2 fields (10× objective) selected for maximum involvement. NFT counts were determined in the same way with 0.59 mm 2 fields (20× objective). Seven different brain regions were analyzed: middle frontal gyrus, inferior parietal lobule, superior and middle temporal gyri, occipital cortex areas 18 and 19, amygdala, hippocampus area CA1, and subiculum. APOE was determined post mortem in all cases. 30
Cerebrospinal fluid from the lateral ventricles (VF) was obtained and stored exactly as described previously. 21 Briefly, immediately after aspiration from the lateral ventricles, VF was sedimented at 1000 × g for 10 minutes and 1–2-ml aliquots were frozen at −80°C. No VF sample was visually contaminated by blood, nor was apolipoprotein B detected by immunoblots. Free F2-IsoP concentrations were determined exactly as described previously, using stable isotope dilution methods and gas chromatography with negative ion chemical ionization mass spectrometry. 21,31
Statistical analyses were performed using GraphPad Prism software (San Diego, CA).
Results
The study group consisted of 23 patients with definite AD and 12 control individuals shown by pathological examination to have only age-related changes in brain. Clinical and pathological data as well as average F2-IsoP concentrations in cerebrospinal fluid from the lateral ventricles (VF) of AD patients and controls are presented in Table 1 ▶ . Only brain weight and F2-IsoP concentrations were significantly different between AD patients and controls. Scatter plots of VF F2-IsoP concentrations and brain weights in AD patients and controls are presented in Figure 1 ▶ . Although statistically significantly different, there was overlap between AD patients’ and controls’ brain weight and VF F2-IsoP levels; only one-third of AD patients had values outside of the control range. This distribution of VF F2-IsoP concentrations is identical to the distribution of F2-IsoPs in cerebrospinal fluid from the lumbar cistern of probable AD patients that we observed previously. 22
Table 1.
Clinical and Pathological Data and Average F2-IsoP Concentrations in Cerebrospinal Fluid from the Lateral Ventricals of AD Patients and Controls
| N | Age (years) | F:M | PMI (hours) | Brain weight (g) | Alleles as ɛ4 of APOE | F2-IsoP (pg/ml) | |
|---|---|---|---|---|---|---|---|
| AD | 23 | 78.2 ± 1.5 | 1.3:1 | 2.6 ± 0.1 | 1110 ± 28 | 57% | 64.1 ± 5.5 |
| Control | 12 | 80.2 ± 2.2 | 1.4:1 | 2.8 ± 0.2 | 1212 ± 34* | 17% | 37.8 ± 5.2† |
*p < 0.05 (t-test).
†p < 0.01 (Mann-Whitney test).
Figure 1.
Scatterplot of VF F2-IsoP concentrations (A) and brain weight (B) for the 23 AD patients and 13 controls. Horizontal lines are means. VF F2-IsoP levels were significantly higher in AD patients than controls (p < 0.01, Mann-Whitney test). Brain weights were significantly lower in AD patients than controls (p < 0.05, t-test).
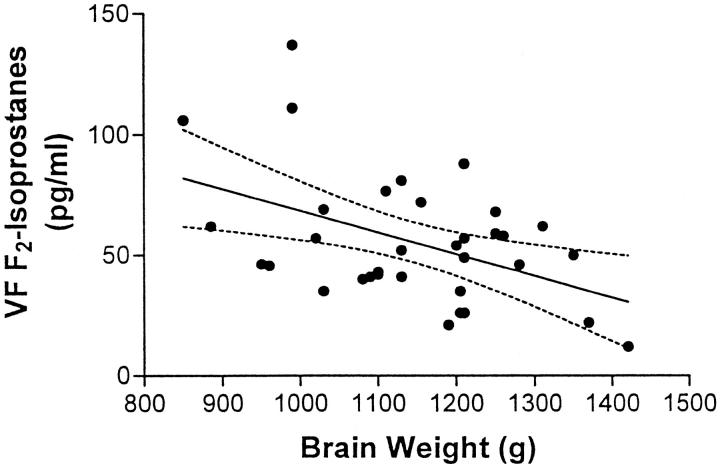
Previously, in a smaller series of patients, we observed that VF F2-IsoP levels have a negative linear relationship with brain weight. 21 Here we confirm that finding with more than twice as many AD patients than in the previous study (p < 0.01, Figure 2 ▶ ). Moreover, when analysis was restricted to AD patients alone (n = 23), VF F2-IsoP levels also were significantly correlated with brain weight (p < 0.05). Although reduction in brain weight is a feature of AD, there are limitations to extrapolating from brain weight to the extent of neurodegeneration. Therefore, we compared directly F2-IsoP concentrations with the degree of cortical atrophy as assessed by gross examination of the brain (Figure 3) ▶ . Spearman’s ranked correlation demonstrated a highly significant positive relationship between the degree of cerebral cortical atrophy and F2-IsoP concentration (p < 0.01) for all 35 individuals. Analysis restricted to AD patients only also was statistically significant (n = 23, p < 0.05).
Figure 2.
x-y plot and linear regression of VF F2-IsoP concentrations and brain weight for the 23 AD patients and 13 controls. Drawn are the best-fit linear regression line (——) and 95% confidence intervals (– – –) (p < 0.01 for linear regression). Regression analysis limited to AD patients alone also was statistically significant (p < 0.05).
Figure 3.
Average VF F2-IsoP concentrations (± SEM) were plotted against cortical atrophy graded as absent (degree 0, n = 15), mild (degree 1, n = 8), moderate (degree 2, n = 8), or severe (degree 3, n = 4) in all AD patients and controls. Spearman’s ranked correlation gave p < 0.01. Analysis restricted to AD patients only also was statistically significant (n = 23, p < 0.05).
Next, we sought to correlate VF F2-IsoP concentrations with histopathological markers of AD, NFTs, and NPs that have been proposed to contribute to oxidative stress in AD brain. The density of NFTs or NPs in any of the brain regions examined did not correlate with F2-IsoP levels, either for the whole group or when divided into AD patients and controls. The correlation coefficients for VF F2-IsoP levels and NFT density in the different brain regions ranged from 0.02 to 0.13. The correlation coefficients for VF F2-IsoP levels and NP density in the different brain regions ranged from 0.00 to 0.05. None of these correlations approached statistical significance.
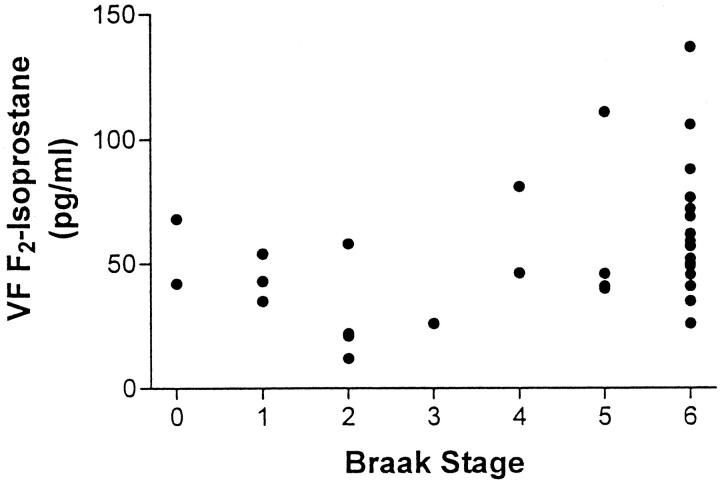
In addition to testing for a correlation to tissue NFT density, we also tested for a relationship between F2-IsoP concentration and the brain distribution of NFTs, using the staging system of Braak and Braak. AD patients had Braak scores of 4–6, and controls had Braak scores of 0–4. Spearman’s ranked test of VF F2-IsoP concentrations with Braak score showed a positive correlation between these two variables (p = 0.02) for all 35 individuals (Figure 4) ▶ . Braak score tended to increase with increasing F2-IsoP concentrations when the analysis was limited to AD patients alone; however, this was not statistically significant.
Figure 4.
VF F2-IsoP concentrations were plotted against the Braak stage of AD patients (n = 23) and controls (n = 12). Spearman’s ranked correlation gave p = 0.02.
Finally, APOE genotype was correlated with VF F2-IsoP levels. Individuals were grouped by the number of ε4 alleles of APOE (no ε4 allele, n = 11; 1 ε4 allele, n = 16; or 2 ε4 alleles, n = 8). There was no relationship between VF F2-IsoP levels and the number of APOE4 alleles for all 35 individuals (Figure 5) ▶ or when analysis was restricted to AD patients (n = 23) or controls (n = 12).
Figure 5.
Average VF F2-IsoP concentrations (± SEM) were plotted against the number of ε4 alleles of APOE for the 23 AD patients and 12 controls (no ε4 allele, n = 11; 1 ε4 allele, n = 16; or 2 ε4 alleles, n = 8). Spearman’s ranked correlation was not statistically significant for the group of 35 individuals or when analysis was restricted to AD patients alone.
Discussion
Regional oxidative damage to brain, especially lipid peroxidation and its products, has been proposed to contribute to the pathogenesis of AD. 1 The sources of oxidative stress in AD are not fully resolved, but tissue culture and animal experiments have suggested Aβ peptide aggregation and deposition or glial activation in NPs, posttranslational modifications of NFTs, APOE genotype, and others. 1,2,24,26 However, there are few data available to discern which among these potential sources of oxidative stress contributes significantly to regional brain oxidative damage in AD patients. This is an important issue because it may guide the development of therapeutic strategies designed to limit brain oxidative damage in AD patients. Recently we have demonstrated the utility of a quantitative biomarker, F2-IsoP, of lipid peroxidation in CSF from AD patients. 21,22 Here we have performed a correlative study between F2-IsoP concentrations in cerebrospinal fluid from the lateral ventricles (VF) and pathological and genetic features of AD to begin to understand what aspects of AD may be most closely related to brain oxidative damage in this disease.
One important outcome of our experiments is that the relative distribution of F2-IsoPs in AD patients and age-matched controls is very similar in three different tissue sources: diseased brain studied post mortem, 23 VF studied post mortem, and LF studied intra vitam early in the course of disease. 22 Moreover, the absolute concentrations of F-ring isoprostanes from each of these tissue sources follow the expected gradient for molecules derived from cerebrum: brain tissue > VF > LF. Using a slightly different technique, others have demonstrated F-ring IsoPs in the frontal pole of AD patients ranging up to 2 ng/g tissue, 23 a result we have corroborated by measuring 2.1 and 2.8 ng F2-IsoP/g hippocampus from two AD patients (unpublished data). In our model, F2-IsoPs are liberated from cells and their processes into the extracellular space, where they are diluted into VF; VF F2-IsoP concentrations in AD patients range from 30 to 150 pg/ml, or are about 20-fold less concentrated than in brain tissue. LF F2-IsoP levels are about one-half of VF levels. 22 This may be related to two variables. First, CSF metabolites derived from brain demonstrate a concentration gradient along the neuraxis, with the highest levels in the lateral ventricles and significantly lower levels in the lumbar cistern. 32 The magnitude of the concentration gradient between CSF from the lateral ventricles and the lumbar cistern varies with different molecules but commonly attains a factor of 2, as observed with the F2-IsoPs. Another possible variable that must be considered is that the LF F2-IsoPs were measured early in the course of disease, 22 whereas VF F2-IsoPs were measured in patients with more advanced AD.
The major advantage of comparing the entire group of 35 individuals is the opportunity to observe the apparent continuum of normal aging, pathological aging, and overt AD. 33 To guard against possible covariance, comparisons of the entire group of individuals were always followed by separate comparisons to AD patients and controls. There was remarkable consistency among the features of AD that correlated with F2-IsoP levels: decreasing brain weight, increasing cortical atrophy, and increasing histopathological stage of disease. In addition, two of these, brain weight and degree of cortical atrophy, but not Braak stage, were significantly correlated with VF F2-IsoP levels in AD patients alone. In aggregate, these findings suggest that VF F2-IsoP levels are closely related to the extent of degeneration in AD brain. It is interesting that F2-isoprostane levels correlated with advancing Braak stage but not with increasing tissue density of NPs or NFTs, even though NP and NFT densities increase with increasing Braak stage in AD patients and in nondemented aged individuals. 34 These results may suggest that it is the distribution, rather than the total burden, of disease that is more closely related to the magnitude of lipid peroxidation.
Oxidative damage to brain in AD patients likely represents a disturbed balance between oxidative stress and antioxidant defenses. Results from several experimental studies have suggested that NFTs, NPs, or APOE genotype may significantly contribute to oxidative damage, either by increasing oxidative stress or reducing antioxidant capacity. 1-3 Our data did not support the hypotheses that the magnitude of brain lipid peroxidation was significantly influenced by the density of NPs or NFTs, or by the number of ε4 alleles of APOE. This result is consistent with our earlier immunohistochemical studies that suggested the overall amount of protein adducts from the lipid peroxidation product 4-hydroxy-2-nonenal in AD brain does not vary with APOE. 6,8 In contrast, these earlier immunohistochemical studies suggested that the cellular distribution of 4-hydroxy-2-nonenal protein adducts in AD brain may vary with APOE; 6,8 however, this result has not been observed by others. 10 It must be stressed that these results do not dismiss NPs, NFTs, and APOE as unimportant in brain oxidative damage. For example, others have shown that the different human apolipoprotein E isoforms may significantly influence the response to injury in the brain. 35,36 Thus it may be possible to modify the functional outcome of brain lipid peroxidation without altering the magnitude of brain lipid peroxidation.
In summary, we have shown that VF F2-IsoP concentrations significantly correlated with three different measures of brain degeneration in AD. In combination with our previous study measuring LF F2-IsoP levels early in the course of AD, 22 these data strongly support the hypothesis that brain lipid peroxidation is closely linked with the progression of AD. The sources of increased brain lipid peroxidation in AD were not determined, but did not appear to include NPs, NFTs, or the number of ε4 alleles of APOE.
Acknowledgments
We thank Dr. David Wekstein and Mr. Cecil Runyons for their invaluable assistance.
Footnotes
Address reprint requests to Dr. Thomas J. Montine, Department of Pathology, C3321-A Medical Center North, Vanderbilt University Medical Center, Nashville, TN 37232. E-mail: tom.montine@mcmail.venderbilt.edu.
Supported by National Institutes of Health grants AG16835, AG00774, AG05144, CA77839, DK48831, DK26657, GM42056, and GM15431 and grants from the Alzheimer’s Disease and Related Disorders Association, the American Foundation for Aging Research, and the Abercrombie Foundation.
References
- 1.Markesbery WR, Carney JM: Oxidative alterations in Alzheimer’s disease. Brain Pathol 1999, 9:133-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith M, Sayre L, Monnier V, Perry G: Radical AGEing in Alzheimer’s disease. Trends Neurosci 1995, 18:172-176 [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Sayre L, Perry G: Is Alzheimer’s a disease of oxidative stress? Alzheimer’s Dis Rev 1996, 1:63-67 [Google Scholar]
- 4.Lovell M, Ehmann W, Butler S, Markesbery W: Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 1995, 45:1594-1601 [DOI] [PubMed] [Google Scholar]
- 5.Markesbery WR, Lovell MA: 4-Hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging 1998, 19:33-36 [DOI] [PubMed] [Google Scholar]
- 6.Montine K, Olson S, Amarnath V, Whetsell W, Graham D, Montine T: Immunochemical detection of 4-hydroxynonenal adducts in Alzheimer’s disease is associated with APOE4. Am J Pathol 1997, 150:437-443 [PMC free article] [PubMed] [Google Scholar]
- 7.Montine KS, Kim PJ, Olson SJ, Markesbery WR, Montine TJ: 4-Hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J Neuropathol Exp Neurol 1997, 56:866-871 [DOI] [PubMed] [Google Scholar]
- 8.Montine K, Reich E, Olson SJ, Markesbery WR, Montine T: Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer’s disease is associated with APOE genotype. J Neuropathol Exp Neurol 1998, 57:415-425 [DOI] [PubMed] [Google Scholar]
- 9.Pappolla MA, Omar RA, Kim KS, Robakis NK: Immunohistochemical evidence of antioxidant stress in Alzheimer’s disease. Am J Pathol 1992, 140:621-628 [PMC free article] [PubMed] [Google Scholar]
- 10.Sayre LM, Zelasko DA, Harris PLR, Perry G, Salomon RG, Smith MA: 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer disease. J Neurochem 1997, 2092–2097 [DOI] [PubMed]
- 11.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR: Excess brain protein oxidation and enzyme dysfunction in normal aging and Alzheimer disease. Proc Natl Acad Sci USA 1991, 88:10540-10543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MA, Kutty RK, Richey PL, Yan SD, Stern D, Chader GJ, Wiggert B, Petersen RB, Perry G: Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer’s disease. Am J Pathol 1994, 145:42-47 [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N: Oxidative damage in Alzheimer’s. Nature 1996, 382:120-121 [DOI] [PubMed] [Google Scholar]
- 14.Smith MA, Sayre LM, Monnier VM, Perry G: Oxidative posttranslational modifications in Alzheimer disease. A possible pathogenic role in the formation of senile plaques and neurofibrillary tangles. Mol Chem Neuropathol 1996, 28:41-48 [DOI] [PubMed] [Google Scholar]
- 15.Smith MA, Sayre LM, Anderson VE, Harris PL, Beal MF, Kowall N, Perry G: Cytochemical demonstration of oxidative damage in Alzheimer’s disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J Histochem Cytochem 1998, 46:731-735 [DOI] [PubMed] [Google Scholar]
- 16.Gutteridge JMC, Halliwell B: The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci 1990, 15:129-135 [DOI] [PubMed] [Google Scholar]
- 17.Esterbauer H, Schaur RJ, Zollner H: Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic Biol Med 1991, 11:81-128 [DOI] [PubMed] [Google Scholar]
- 18.Morrow J, Hill K, Burk R, Nammour T, Badr K, Roberts L: A series of prostaglandin-like compounds produced in vivo in humans by a non-cyclooxygenase, free radical catalyzed mechanism. Proc Natl Sci USA 1990, 87:9383-9387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrow JD, Roberts LJ: The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res 1997, 36:1-21 [DOI] [PubMed] [Google Scholar]
- 20.Pratico D, Barry OP, Lawson JA, Adiyaman M, Hwang SW, Khanapure SP, Iuliano L, Rokach J, Fitzgerald GA: IPF2-α-I—an index of lipid peroxidation in humans. Proc Natl Acad Sci USA 1998, 95:3449-3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ: Cerebrospinal fluid F2-isoprostanes are increased in Alzheimer’s disease. Ann Neurol 1998, 44:410-413 [DOI] [PubMed] [Google Scholar]
- 22.Montine TJ, Beal MF, Cudkowicz ME, Brown RH, O’Donnell H, Margolin RA, McFarland L, Bachrach AF, Zackert WE, Roberts LJ, Morrow JD: Increased cerebrospinal fluid F2-isoprostane concentration in probable Alzheimer’s disease. Neurology 1999, 52:562-565 [DOI] [PubMed] [Google Scholar]
- 23.Pratico D, Lee VM, Trojanowski JQ, Rokach J, Fitzgerald GA: Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J 1998, 12:1777-1784 [DOI] [PubMed] [Google Scholar]
- 24.Coyle JT, Puttfarcken P: Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262:689-695 [DOI] [PubMed] [Google Scholar]
- 25.Beal MF: Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol 1995, 38:357-366 [DOI] [PubMed] [Google Scholar]
- 26.Bowling AC, Beal MF: Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci 1994, 56:1151-1171 [DOI] [PubMed] [Google Scholar]
- 27.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41:479-486 [DOI] [PubMed] [Google Scholar]
- 28.Braak H, Braak E: Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991, 82:239-259 [DOI] [PubMed] [Google Scholar]
- 29.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenova M, Gabbita SP, Wu JF, Lovell M, Markesbery WR, Butterfield DA: Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem 1995, 65:2146-2156 [DOI] [PubMed] [Google Scholar]
- 30.Saunders AM, Schmader K, Breitner JC, Benson MD, Brown WT, Goldfarb L, Goldgaber D, Manwaring MG, Szymanski MH, McCown N, Roses A: Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet 1993, 342:710-711 [DOI] [PubMed] [Google Scholar]
- 31.Morrow JD, Roberts LJ: Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical catalyzed mechanism. Methods Enzymol 1994, 233:163-174 [DOI] [PubMed] [Google Scholar]
- 32.Kjeldsberg CR, Krieg AF: Cerebrospinal fluid and other body fluids. ed 17 Henry JB eds. Clinical Diagnosis and Management by Laboratory Methods, 1984, :pp 459-492 W B Saunders Co, Philadelphia [Google Scholar]
- 33.Dickson DW: Aging in the central nervous system. Markesbery WR eds. Neuropathology of Dementing Disorders. 1998, :pp 56-88 Arnold, New York [Google Scholar]
- 34.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR: Alzhiemer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 1999, 58:376-388 [DOI] [PubMed] [Google Scholar]
- 35.Mahley R, Nathan B, Bellosta S, Pitas R: Apolipoprotein E: impact of cytoskeletal stability in neurons and the relationship to Alzheimer’s disease. Curr Opin Lipidol 1995, 6:86-91 [DOI] [PubMed] [Google Scholar]
- 36.Pitas RE, Ji ZS, Weisgraber KH, Mahley RW: Role of apolipoprotein E in modulating neurite outgrowth: potential effect of intracelular apolipoprotein E. Biochem Soc Trans 1998, 26:257-262 [DOI] [PubMed] [Google Scholar]