Abstract
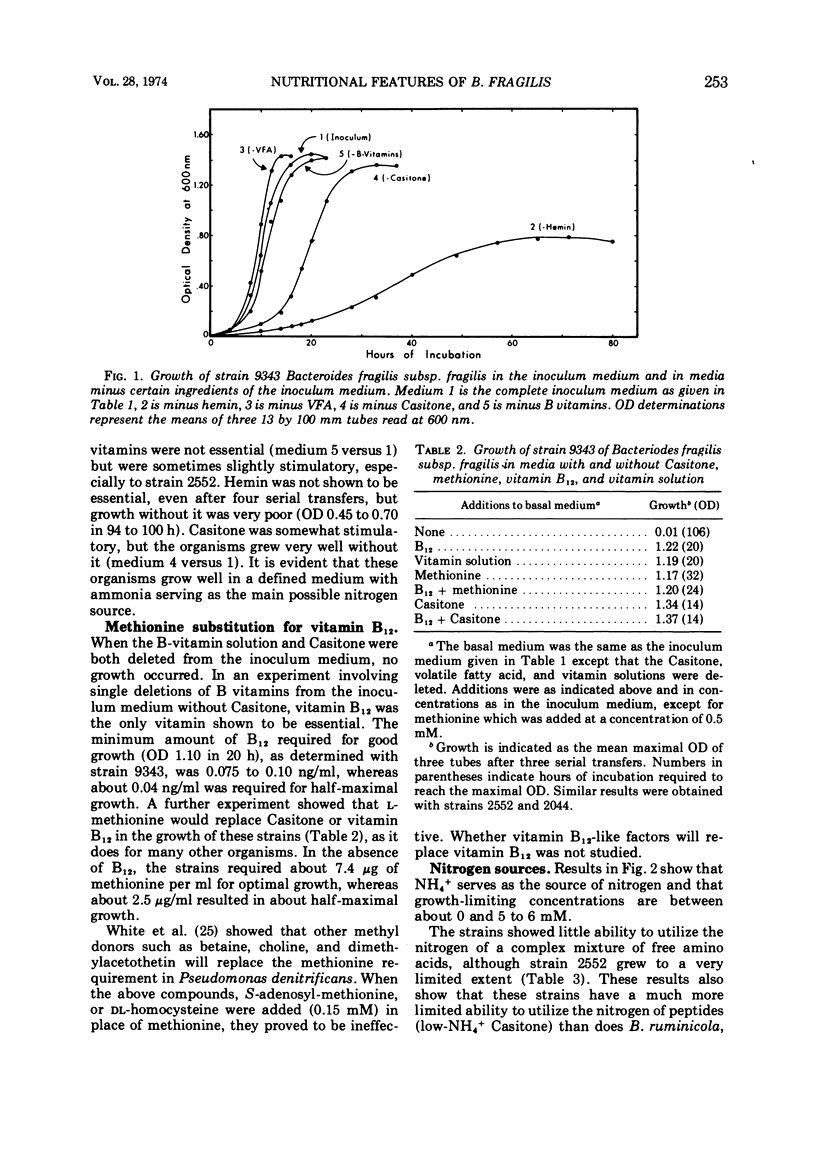
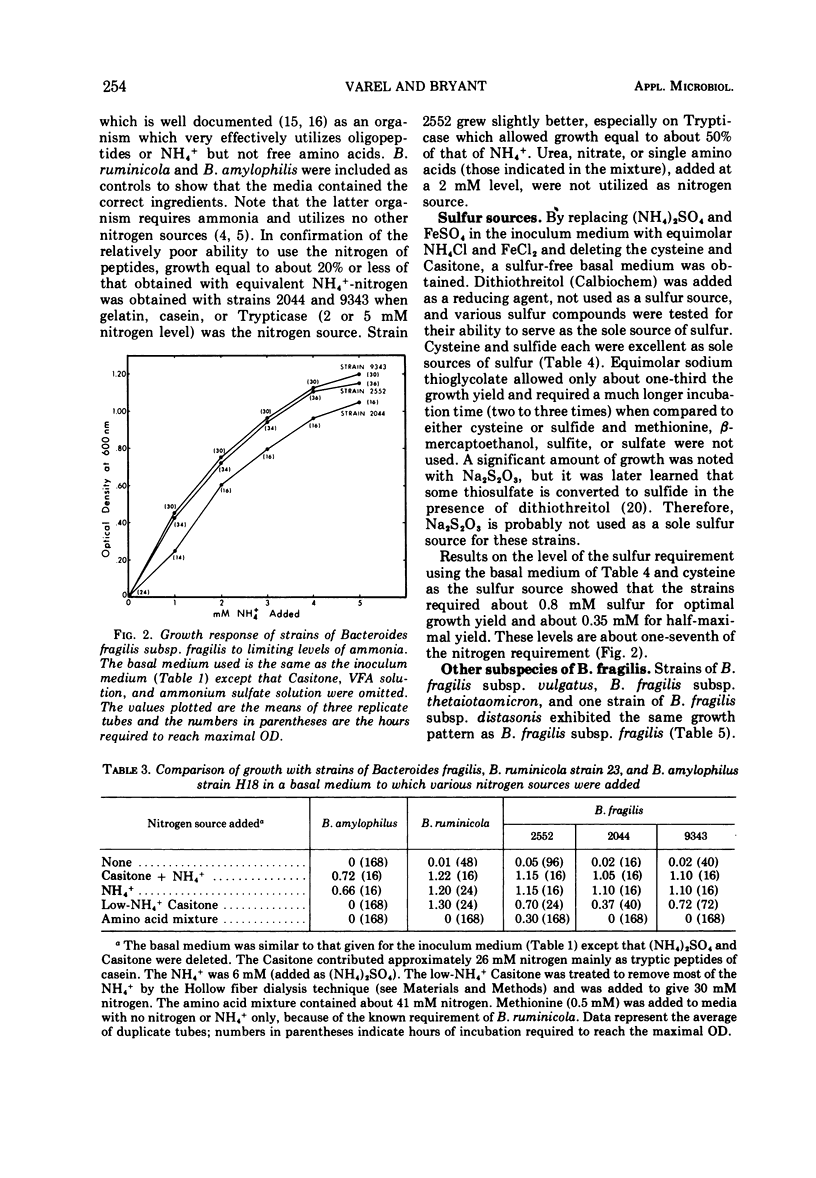
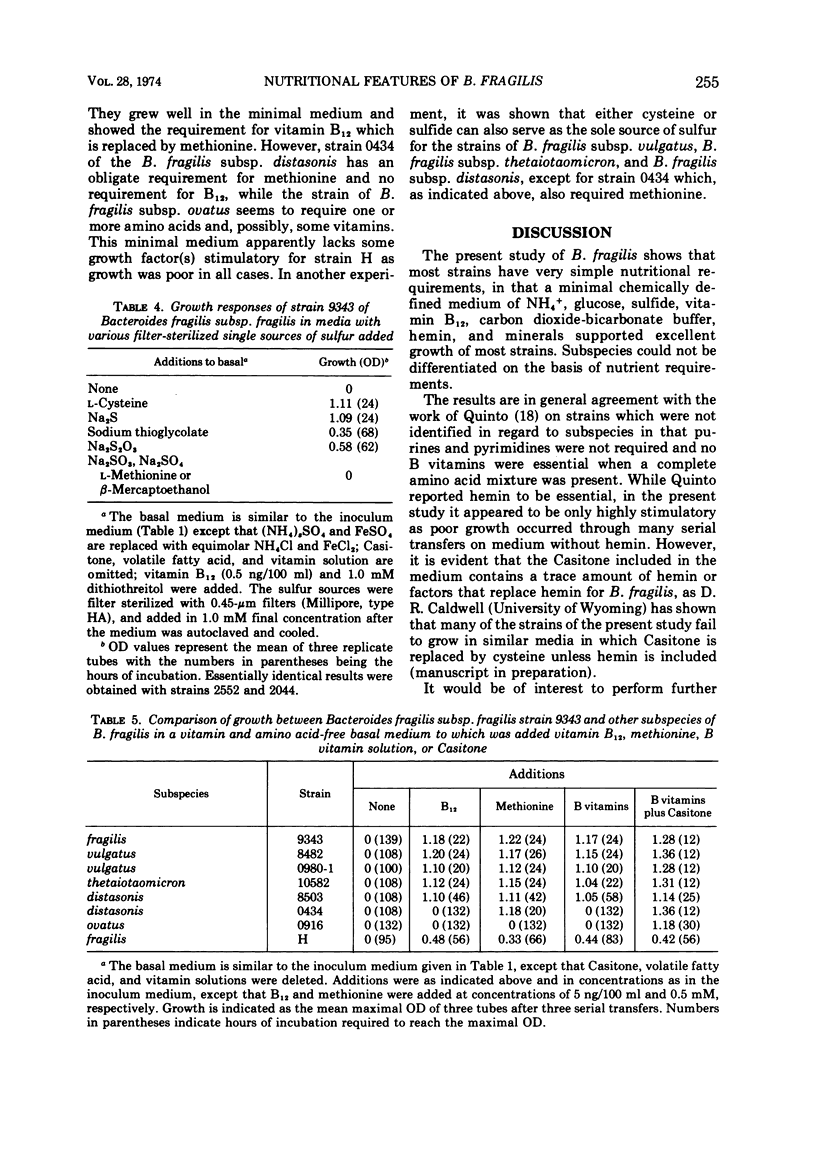
Studies of three reference strains of Bacteroides fragilis subsp. fragilis showed that they grow well in a minimal defined medium containing glucose, hemin, vitamin B12, minerals, bicarbonate-carbon dioxide buffer, NH4Cl, and sulfide. The vitamin B12 requirement of 0.1 ng/ml was replaced with 7.5 μg of methionine. Cysteine or sulfide was an excellent source of sulfur, thioglycolate was a poor source, and thiosulfate, methionine, β-mercaptoethanol, dithiothreitol, sulfate, or sulfite did not serve as sole sources of sulfur. Neither single amino acids, nitrate, urea, nor a complex mixture of L-amino acids or peptides effectively replaced ammonia as the nitrogen source. Comparative studies with a few strains of other subspecies of B. fragilis including B. fragilis subsp. vulgatus, B. fragilis subsp. thetaiotaomicron, and B. fragilis subsp. distasonis indicate that they exhibit similar growth responses in the minimal medium. A single strain of B. fragilis subsp. ovatus required other materials. The results indicate the great biosynthetic ability of these organisms and suggest that, in their ecological niche within the large intestine, many nutrients such as amino acids are in very low supply, whereas materials such as ammonia, heme, and vitamin B12, or related compounds, must be available during much of the time.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDSON R. M., Jr NORMAL BACTERIAL POPULATIONS OF THE INTESTINE AND THEIR RELATION TO INTESTINAL FUNCTION. N Engl J Med. 1964 May 7;270:994–CONTD. doi: 10.1056/NEJM196405072701907. [DOI] [PubMed] [Google Scholar]
- LOESCHE W. J., SOCRANSKY S. S., GIBBONS R. J. BACTEROIDES ORALIS, PROPOSED NEW SPECIES ISOLATED FROM THE ORAL CAVITY OF MAN. J Bacteriol. 1964 Nov;88:1329–1337. doi: 10.1128/jb.88.5.1329-1337.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Keudell K. C., Milford A. F. Succinate as a growth factor for Bacteroides melaninogenicus. J Bacteriol. 1971 Oct;108(1):175–178. doi: 10.1128/jb.108.1.175-178.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBRINK K. J. A modified Conway unit for microdiffusion analysis. Biochem J. 1955 Jan;59(1):134–136. doi: 10.1042/bj0590134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTMAN K. A., BRYANT M. P. PEPTIDES AND OTHER NITROGEN SOURCES FOR GROWTH OF BACTEROIDES RUMINICOLA. J Bacteriol. 1964 Aug;88:401–410. doi: 10.1128/jb.88.2.401-410.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman K. A., Lakshmanan S., Bryant M. P. Oligopeptide uptake by Bacteroides ruminicola. J Bacteriol. 1967 May;93(5):1499–1508. doi: 10.1128/jb.93.5.1499-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUINTO G. Nutrition of five Bacteroides strains. J Bacteriol. 1962 Sep;84:559–562. doi: 10.1128/jb.84.3.559-562.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUINTO G., SEBALD II. IDENTIFICATION OF THREE HEMIN-REQUIRING BACTEROIDES STRAINS. Am J Med Technol. 1964 Nov-Dec;30:381–384. [PubMed] [Google Scholar]
- Quinto G. Amino Acid and vitamin requirements of several bacteroides strains. Appl Microbiol. 1966 Nov;14(6):1022–1026. doi: 10.1128/am.14.6.1022-1026.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMIMI H. A., HILTBRAND W., LOERCHER H. Some growth requirements of Bacteroides fragilis. J Bacteriol. 1960 Oct;80:472–476. doi: 10.1128/jb.80.4.472-476.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo V., Korzeniowski M., Spaulding E. H. Tryptic soy bile-kanamycin test for the indentification of Bacteroides fragilis. Appl Microbiol. 1974 Mar;27(3):480–483. doi: 10.1128/am.27.3.480-483.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visek W. J. Effects of urea hydrolysis on cell life-span and metabolism. Fed Proc. 1972 May-Jun;31(3):1178–1193. [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. F., Kaplan L., Birnbaum J. Betaine-homocysteine transmethylase in Pseudomonas denitrificans, a vitamin B 12 overproducer. J Bacteriol. 1973 Jan;113(1):218–223. doi: 10.1128/jb.113.1.218-223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


