Abstract
The numbers of immune-activated brain mononuclear phagocytes (MPs) affect the progression of human immunodeficiency virus (HIV)-1-associated dementia (HAD). Such MPs originate, in measure, from a pool of circulating monocytes. To address the mechanism(s) for monocyte penetration across the blood-brain barrier (BBB), we performed cross-validating laboratory, animal model, and human brain tissue investigations into HAD pathogenesis. First, an artificial BBB was constructed in which human brain microvascular endothelial and glial cells—astrocytes, microglia, and/or monocyte-derived macrophages (MDM)—were placed on opposite sides of a matrix-coated porous membrane. Second, a SCID mouse model of HIV-1 encephalitis (HIVE) was used to determine in vivo monocyte blood-to-brain migration. Third, immunohistochemical analyses of human HIVE tissue defined the relationships between astrogliosis, activation of microglia, virus infection, monocyte brain infiltration, and β-chemokine expression. The results, taken together, showed that HIV-1-infected microglia increased monocyte migration through an artificial BBB 2 to 3.5 times more than replicate numbers of MDM. In the HIVE SCID mice, a marked accumulation of murine MDM was found in areas surrounding virus-infected human microglia but not MDM. For human HIVE, microglial activation and virus infection correlated with astrogliosis, monocyte transendothelial migration, and β-chemokine expression. Pure cultures of virus-infected and activated microglia or astrocytes exposed to microglial conditioned media produced significant quantities of β-chemokines. We conclude that microglial activation alone and/or through its interactions with astrocytes induces β-chemokine-mediated monocyte migration in HAD.
The human immunodeficiency virus (HIV)-1-associated dementia (HAD) complex is defined as cognitive, motor, and/or behavioral impairments caused by progressive viral infection and immune deterioration. Neurological disease occurs in 15 to 20% of infected individuals and is often associated with a marked depletion of CD4-positive T lymphocytes. 1-3 HIV-1 encephalitis (HIVE), a common pathological manifestation of HAD, is characterized by monocyte infiltration into brain, the formation of macrophage-derived multinucleated giant cells, microglial nodules, and myelin pallor. 4-6 Other pathological features include decreased numbers of large neurons in the neocortex and deep gray matter, alterations in neuronal dendritic and synaptic processes, and astrogliosis. 7 The principal target cells for virus are the mononuclear phagocytes (MP): microglia, perivascular brain macrophages, and multinucleated giant cells. 6 It is the number of these cells in brain that correlates best with neurological dysfunction. 8 Thus, MPs play an important role in HAD neuropathogenesis. Indeed, virus-infected immune-activated MPs can secrete a variety of neurotoxic factors that affect disease progression in HAD. Such MP secretory factors include, but are not limited to, eicosanoids (arachidonic acid and its metabolites), quinolinic acid, platelet-activating factor, tumor necrosis factor-α (TNF-α), and nitric oxide (NO). 9-13 The mechanisms that regulate MP activation and secretions may also increase macrophage brain infiltration and thus lead to HAD. 10,11,14-17
After immune activation in the central nervous system (CNS), brain macrophages, microglia, and astrocytes also secrete chemotactic cytokines (chemokines). Both MPs and astrocytes are major cellular sources of CNS β-chemokines, which specifically regulate the transendothelial migration of monocytes into brain. The β-chemokines include macrophage inflammatory protein-1α (MIP-1α) and -1β (MIP-1β); macrophage chemotactic protein (MCP)-1, MCP-2, and MCP-3; and regulated on activation normal T cell expressed and secreted (RANTES). 18 Murine microglia produce significant levels of MIP-1α after lipopolysaccharide (LPS) stimulation. 19 Similarly, human microglia activated with LPS, TNF-α, or interleukin (IL)-1β (IL-1β) secrete significant amounts of MIP-1α, MIP-1β, and MCP-1. 20 Astrocytes produce MCP-1 after treatment with TNF-α, tumor growth factor-β (TGF-β), 21 or HIV-1 Tat. 22 In multiple sclerosis (MS), demyelinating-lesion-reactive astrocytes produce MCP-1. 23 Both human and simian astrocytes treated with TNF-α, interferon γ, and/or IL-1β produce large quantities of MCP-1. 24,25 Astrocyte-derived MCP-1 affects monocyte and lymphocyte blood-brain barrier (BBB) migration. 24
Several reports have linked chemokines to the neuropathogenesis of HIVE. Schmidtmayerova and colleagues 26 showed that chemokine mRNAs are expressed in HIVE brain tissue cells with morphological features of macrophages/microglia. Both MIP-1α and MIP-1β were up-regulated in human monocyte-derived macrophages (MDM) after HIV-1 infection or treatment with TNF-α. MCP-1 was also detected in brains and cerebrospinal fluid (CSF) of patients with HAD. 22 Astrocytes and neurons principally expressed MCP-1. In simian immunodeficiency virus encephalitis, MIP-1α, MIP-1β, RANTES, and inflammatory protein (IP)-10 were found in endothelial cells and/or perivascular macrophages. 27 Most recently, Sanders et al 28 demonstrated MCP-1 in microglia/macrophages, astrocytes, and endothelium in and around microglial nodules in HIVE-affected brain tissue. These reports, taken together, demonstrated that chemokines are up-regulated in encephalitic brain tissue. The cellular sources, functional significance, and effects of chemokines in monocyte BBB migration, however, remained ill defined. Several questions about how HIV-1-infected monocytes gain entry into the brain remain unanswered. 1) What chemokines are produced by macrophages, astrocytes, and other brain cells? 2) Under what conditions are brain chemokines secreted? 3) Do microglial cells and astrocytes affect their own chemokine production and monocyte transendothelial brain migration? 4) Is there a correlation between chemokine production and immune activation in HIVE? To address these questions we used laboratory, animal model and human autopsy material to measure the cell source and function of chemokines during monocyte migration into the brain. The assays—an in vitro BBB system, an animal model of HIVE, and pathological analyses of postmortem brain tissue—were designed to cross-validate one another. The data independently showed that microglia and astrocytes are principal sources of β-chemokines and serve to control monocyte BBB migration in HAD. Conditioned media from HIV-1-infected and immune-activated microglia induced significant chemokine production from astrocytes. Importantly, in human HIVE, prominent microglial immune activation and, to a lesser extent, HIV-1 infection correlated with astrogliosis and macrophage brain infiltration. These results showed that microglial and astroglial activation in HAD are associated with monocyte transendothelial migration. These neuroimmune events are crucial components of the pathogenesis of HAD in its human host.
Materials and Methods
Microglia and Astrocytes
Fetal brain tissue (gestational age, 14–19 weeks) was obtained from elective abortions performed in full compliance with the ethical guidelines of the National Institutes of Health (NIH) and the University of Nebraska Medical Center. Microglia were isolated and characterized as previously described. 29 Adherent microglial cell preparations (>98% pure) were confirmed by CD68 and HAM-56 immunostaining. Human fetal astrocytes were prepared as previously described and were shown to be >99% pure by glial fibrillary acid protein (GFAP) immunostaining. 30
Monocytes
Peripheral blood mononuclear cells obtained from HIV- and hepatitis B-seronegative donors by leukopheresis were purified by counter-current centrifugal elutriation. 31 Cell suspensions were identified as >98% pure monocytes by Wright staining, nonspecific esterase, granular peroxidase, and CD68 immunostaining. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated pooled human serum, 10 μg/ml ciprofloxacin (Sigma), 50 μg/ml gentamicin (Sigma), and 1000 U/ml of macrophage colony-stimulating factor (a generous gift from Genetics Institute, Boston, MA). All reagents were prescreened and found negative for endotoxin (<10 pg/ml; Associates of Cape Cod, Woods Hole, MA) and mycoplasma contamination (Gen-probe II, Gen-probe, San Diego, CA).
HIV-1 Infection of Microglia and Monocytes
Adherent monocytes and microglia were cultured in 96-well plates at a density of 10 5 cells/well for 7 days before infection with HIV-1ADA at a multiplicity of infection of 0.1. Monocytes and microglia in suspension were cultured in Teflon flasks at a density of 10 6 cells/ml for 7 days before viral infection. The cell-free viral inoculum used for each experiment was standardized for all experiments by reverse-transcriptase activity (2 × 10 5 cpm/10 6 cells) as described previously. 31
Construction of the Three-Dimensional BBB Model
The BBB model was constructed on inserts with collagen-coated polycarbonate in Transwell membrane (pore diameter, 3 μm; Corning-Costar Corp., Cambridge, MA) as described. 30 Brain microvascular endothelial cells and human fetal astrocytes were placed, respectively, on the upper and lower surfaces of the membrane. In the constructs, the lower surface was coated with human fibronectin and seeded with 10 5 astrocytes in an inverted position. After 2 hours to allow cell adherence, the construct was placed upright, and brain microvascular endothelial cells were subsequently inoculated (200 μl of 10 5 cells) into the upper chamber. These models were used for a minimum of 5 days after cell seeding, when they acquired high electrical resistance and negligible permeability for [3H] inulin.
Human MDM were cultured in Teflon flasks. The MDM (7 × 105) (HIV-1ADA-infected or uninfected controls) were seeded on glass coverslips (Corning Costar, Cambridge, MA) and then placed on the bottoms of wells in 24-well Costar plates. In a similar fashion, 10 5 microglial cells (HIV-1ADA-infected or uninfected controls) on coverslips were placed in the lower chambers of 24-well plates. The addition of the MDM or microglia to the BBB model permitted analysis of the ability of each macrophage cell type to affect monocyte transendothelial migration.
Transendothelial Migration of Monocytes in the BBB Model
To investigate penetration of fresh-blood-derived monocytes (from virus-negative donors) through the artificial BBB, 10 5 monocytes were placed in 100 μl of medium in the upper chambers of a 24-well tissue culture insert. At 48 hours, numbers of migrated monocytes were counted on the lower chamber coverslips. Because monocytes express high levels of peroxidase and low levels of acid phosphatase and have distinct morphology, 32 monocytes were easily differentiated from MDM or microglia. Cells were counterstained with hematoxylin. A minimum of 20 random fields (objective ×20) of each coverslip was analyzed for migrated cells.
Enzyme-Linked Immunosorbent Assays (ELISA) for TNF-α and Chemokines
TNF-α and the CC chemokines MIP-1α, MIP-1β, MCP-1, and RANTES were assayed by using the Quantikine ELISA kits (R&D Systems, Minneapolis, MN) and following the manufacturer’s instructions. Cells were stimulated with LPS (1 μg/ml), obtained from Escherichia coli, serotype 0111:B4 (Sigma), for 2 hours and were washed three times with media. Conditioned media from unstimulated or LPS-stimulated cells were collected at 24 hours, and chemokine levels were detected by ELISA. The levels of chemokines were normalized to cell numbers by measuring cell viability by the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. 33 The normalized values of chemokines per 10 5 cells were determined. These were analyzed statistically with the two-tailed Student’s t-test. Experiments were repeated four times with cells derived from four different donors for both MDM and microglia. LPS-treated cells were used to mimic the immune activation of macrophages that occur in HAD. 13 Astrocytes were cultured for 7 days as adherent monolayers on 96-well plates. Cells were plated at a density of 5 × 10 5 cells/well. Cultured fluids were obtained from control (uninfected) and HIV-1-infected MDM or microglia after LPS activation and were placed onto astrocytes for 2 hours. The cells were subsequently washed three times with medium. Conditioned medium was collected from the astrocytes 24 hours later, and chemokine levels were detected by ELISA.
SCID Mouse Model of HIVE
Male C.B-17/IcrCrl-SCID mice, 3 to 4 weeks old, were purchased from Charles River Laboratories (Wilmington, MA). Intracerebral injections of HIV-1ADA-infected MDM (eight mice) or uninfected MDM (eight mice), uninfected microglia (six mice), or HIV-1ADA-infected microglia (six mice) were performed with cells derived from two different donors. 34 Each animal was inoculated with 15 μl of suspension containing 1.5 × 10 5 cells or 15 μl of culture medium (sham operated, four control mice). At 7 days postinoculation, all mice were sacrificed, and the whole brain was collected for neuropathological analysis.
Histopathology and Immunohistochemistry
Mouse brain tissue was fixed in 4% phosphate-buffered paraformaldehyde and was paraffin-embedded. Immunohistochemistry was performed on 5-μm paraffin tissue sections. Human MDM/microglia were identified with anti-CD68 KP-1 (1:100; Dako) or antivimentin (1:50; Boehringer Mannheim, Indianapolis, IN) monoclonal antibodies (Abs). Mouse astrocytes were recognized with polyclonal Abs against GFAP (Dako; 1:1000 dilution). Mouse microglia/macrophages were identified with biotinylated Griffonia simplifolica Lectin-Isolectin B4 (Vector Laboratories, Burlingame, CA; 1:100 dilution). Anti-human leukocyte antigen clone CR3/43 (Boehringer Mannheim) and clone LN3 (Accurate Chemicals, New York, NY) at 1:25 and 1:40 dilutions, respectively, and HIV-1 p24 monoclonal Abs (Dako) at a 1:10 dilution were used to detect cellular activation and viral gene products. To detect primary Abs, avidin-biotin immunoperoxidase staining with a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used with 3,3′-diaminobenzidine as the chromogen. The sections were counterstained with Mayer’s hematoxylin.
Brain Autopsy Materials
Brain tissue from 14 HIV-1-infected and 5 control cases (who were HIV-1 seronegative) were used for neuropathological and immunohistological assessments. A concise description of clinical history pertaining to each of the brain samples is shown in Table 1 ▶ . Immunohistochemical evaluation of macrophage infiltration, activation, level of infection, astrogliosis, and expression of chemokines was performed using monoclonal Abs to CD68, HAM-56, HLA-DR (CR3/43 and LN-3), GFAP, and HIV-1 p24 on paraffin-embedded tissue sections with the avidin-biotin immunoperoxidase Vectastain Elite ABC kit (Vector Laboratories). 30,34 Double immunostainings were performed for chemokines MIP-1α, MIP-1β, MCP-1, and RANTES (LeukoSite Inc., Cambridge, MA) at a 1:50 to 1:100 dilution, and HAM-56 (macrophages/microglia) or GFAP (astrocytes) on frozen tissue sections by indirect immunofluorescence as previously described. 29,30
Table 1.
Human Brain Tissues: Clinical and Pathological Descriptions
| Case no. | Age | AIDS* | HIVE† | Clinical and neuropathological diagnosis |
|---|---|---|---|---|
| 1 | 37 | + | +++‡ | HIVE/progressive dementia |
| 2 | 40 | + | +++ | HIVE/progressive dementia |
| 3 | 42 | + | +++ | HIVE/encephalopathy |
| 4 | 38 | + | +++ | HIVE/encephalopathy |
| 5 | 45 | + | ++ | HIVE/encephalopathy |
| 6 | 37 | ++ | HIVE/large cell lymphoma | |
| 7 | 8 | + | ++ | HIVE/encephalopathy |
| 8 | 12 | + | ++ | HIVE |
| 9 | 8 | + | + | HIVE, disseminated Mycobacterium avium intracellulaire and Candida infection |
| 10 | 4 | + | − | HIV-1 infection |
| 11 | 39 | + | − | Cirrhosis, hepatitis C |
| 12 | 34 | + | − | Brain abscess (Nocardia asteroides) |
| 13 | 51 | + | − | Medullar infarction |
| 14 | 36 | + | − | Cytomegalovirus encephalitis |
| 15 | 58 | − | − | Chronic lymphoblastic leukemia |
| 16 | 71 | − | − | Squamous cell carcinoma of lung |
| 17 | 65 | − | − | Chronic obstructive pulmonary disease |
| 18 | 79 | − | − | Coronary artery disease |
| 19 | 51 | − | − | Hepatocellular carcinoma/altered mental status |
*AIDS (Centers for Disease Control and Prevention definition of HIV-seropositive subject with CD4+ T lymphocytes < 200 mm3).
†HIVE, HIV-1 encephalitis.
‡+++, Severe HIVE, defined as expression of HLA-DR on 80–90% of microglia, HIV-1 infection of ramified microglia, formation of 1–2 microglial nodules and 25–50 infiltrating macrophages per five ×10 power fields, and abundant multinucleated cells; ++, moderate HIVE, defined as expression of HLA-DR on 20–70% of microglia, frequent multinucleated giant cells, 5–25 infiltrating macrophages per five ×10 power fields, and formation of 1–2 microglial nodules per 10–15 ×10 power fields; +, mild HIVE, defined as expression of HLA-DR on 10–15% of microglia, rare multinucleated cells, 3–5 CD68-positive infiltrating macrophages (per five ×10 power fields), and one microglial nodule (per 20 ×10 power fields).
Computer Image and Statistical Analyses
Intensity of reactive astrocytosis was quantified as area occupied by GFAP-positive astrocytes on serial coronal paraffin sections of brains injected with HIV-1-infected MDM (six animals) or microglia (six animals) for 1 week. Image analysis was performed as previously described 34 with a cooled closed-circuit digital (CCD) camera (Photometrics, Tucson, AZ) mounted on a Nikon Microphot-FXA. Digital images were analyzed with the Oncor Image V1.6 (Oncor Inc., Gaithersburg, MD) computer image system. The scanned zone covered 1000 μm medially and laterally from needle track on coronal sections. GFAP-immunostained areas were expressed as a percentage of the total brain area assayed. Differences between means were analyzed with a two-tailed Student’s t-test.
Results
Microglia and MDM Differentially Affect Monocyte Migration through the BBB
Because the degree of HAD parallels the numbers of macrophages, 8 we studied the conditions that affect monocyte entry into the brain. To this end we constructed an artificial BBB to evaluate monocyte transendothelial migration. 30 The transendothelial passage of monocytes (purified from peripheral blood leukocytes at the day of each experiment) was assessed after application of HIV-1-infected/uninfected MDM or microglia to the astrocyte (brain) side of the BBB model. Monocyte migration was measured at 48 hours after cell placement in two independent experiments. Twenty random fields (objective ×20) in each coverslip (experiments performed in duplicate) were assayed for peroxidase-positive monocytes. Microglia placed in the astrocyte compartment induced 2- to 3.5-fold greater monocyte migration across the BBB model (P < 0.004) than replicate numbers of MDM (Figure 1) ▶ . HIV-1-infected microglia cells elicited a significant increase in monocyte migration compared to uninfected microglia (P < 0.013).
Figure 1.
Effect of microglia/MDM on monocyte migration across a model of the BBB. HIV-1ADA-infected/control uninfected MDM (7 × 105) or virus-infected/control microglia cells (105) were placed in the lower chamber. Fresh blood monocytes were applied to the upper chamber of the BBB model (105). Numbers reflect migrated monocytes attached to coverslips in the lower chamber at 48 hours. Twenty random fields (objective ×20) of each coverslip were assayed. Experiments were performed in duplicate. Data presented (mean ± SE) are from one of two independent experiments.
Comparison of Microglial, MDM, and Astrocyte-Secreted Chemokines and Cytokines
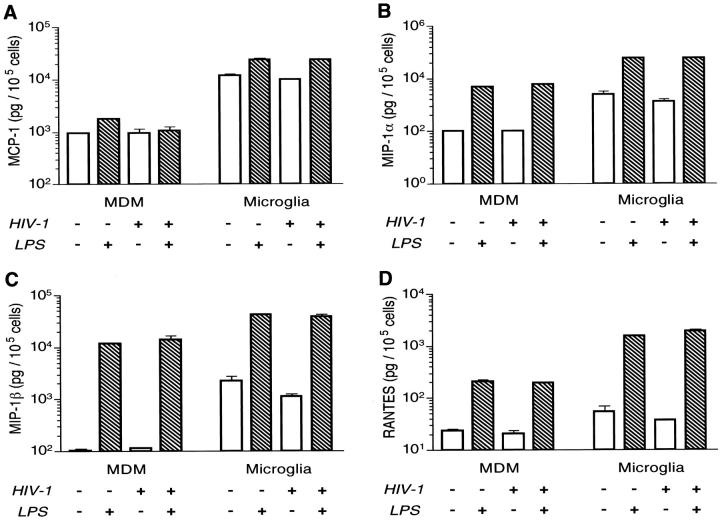
These initial results with our BBB model indicated that microglia are a major source of secretory factors affecting monocyte migration. To uncover what these factors might be, we measured chemokine secreted by MDM or microglia after HIV-1 infection and activation (LPS stimulation). Culture media from control (unstimulated) or activated (LPS-stimulated) cells were collected at 24 hours, and chemokine levels were measured in culture fluids by ELISA. In both cell types (MDM and microglia), uninfected and HIV-infected cells activated with LPS led to the highest levels of chemokines and TNF-α.
TNF-α was not observed in MDM conditioned media. Unstimulated microglia (with or without HIV-1 infection) produced low levels of TNF-α (<20 pg/10 5 cells). Cell activation significantly increased TNF-α secretion by both cell types. However, activated microglia produced 20-fold more TNF-α than did replicate numbers of MDM (data not shown). High levels of MCP-1 were found in infected or uninfected microglia (Figure 2A) ▶ . Activated uninfected or uninfected microglia produced nearly 13-fold higher levels of MCP-1 than did MDM (Figure 2A) ▶ . Moreover, microglia produced 10- to 20-fold higher levels of MIP-1α and MIP-1β (Figure 2, B and C) ▶ than did replicate virus-infected or uninfected MDM. LPS activation significantly increased MIP-1α and MIP-1β in both MDM and microglia (24- to 60-fold) with microglia producing 2- to 12-fold more than MDM. Unstimulated MDM and microglia produced 24 and 54 pg/10 5 cells of RANTES, respectively (Figure 2D) ▶ , the lowest levels of all chemokines studied. A 7- to 50-fold increase in RANTES was observed after LPS activation. In all cases, microglia produced more chemokines on a per-cell basis than did MDM (range of P values, <0.0001–0.0068).
Figure 2.
Production of chemokines by microglia and MDM. Adherent MDM and microglia were cultured on 96-well plates at a density of 10 5 and 5 × 10 4 cells/well, respectively, for 7 days before infection with HIV-1ADA at multiplicity of infection of 0.1. Replicate microglia and monocytes were stimulated with LPS (1 g/ml), and culture fluids from infected or uninfected cells were collected 24 hours later. The MCP-1 (A), MIP-1α (B), MIP-1β (C), and RANTES (D) were assayed using the Quantikine ELISA kits (R&D Systems). The levels of chemokines were normalized to cell numbers by measuring cell viability at the end of the sample collection by the MTT assay. The normalized values of chemokines per 10 5 cells were used to perform statistical analysis by the two-tailed Student’s t-test. Data presented in a log scale (mean ± SE) are from one of two independent experiments. Comparison of microglia- versus MDM-treated similarly yielded statistically significant differences (P < 0.0001).
It is interesting that these data were not concordant with the increased BBB monocyte migration shown with HIV-1-infected microglial cells in our BBB model. Such results likely reflect the ability of other cells of BBB (eg, astrocytes) to produce chemokines elicited by secretory products of MPs. Indeed, astrocytes treated with culture fluids from HIV-1-infected, LPS-activated MDM produced significantly more MIP-1α than did supernatants from uninfected cells similarly activated (Tables 2 and 3) ▶ ▶ . Only conditioned media from LPS-stimulated HIV-1-infected microglia were able to up-regulate MIP-1β in astrocytes (twofold). Although RANTES was undetectable in astrocyte supernatants under most conditions, this chemokine was secreted by astrocytes treated with conditioned media derived from activated and HIV-1-infected MDM and microglia (Tables 2 and 3) ▶ ▶ . Astrocytes produced MCP-1 in much higher amounts when compared with the other chemokines. As in microglia, MCP-1 was constitutively produced by astrocytes. Application of conditioned media from immune-stimulated MDM (with or without HIV-1 infection) up-regulated production of MCP-1 by astrocytes (1.4- and 1.5-fold, respectively; P < 0.016) when compared with unstimulated MDM. The most significant response was induced by supernatants of immune-activated HIV-1-infected microglia (1.9-fold, P = 0.005) as compared with uninfected microglia cells. Astrocytes treated by microglia supernatants produced similar or higher levels of chemokines compared with replicated numbers of MDM. Taken together, these results suggest complex intercellular interactions between microglia/MDM and astrocytes for chemokine production. Both immune activation and HIV-1 infection of MP appear to contribute to significantly enhanced chemokine secretion and monocyte BBB transendothelial migration.
Table 2.
β-Chemokines Secreted by Human Astrocytes after Treatment with MDM-Conditioned Media
| Astrocytes treated with MCM* | Chemokines secreted (pg/105 cells) | ||||
|---|---|---|---|---|---|
| HIV | LPS | MCP-1 | MIP-1α | MIP-1β | RANTES |
| − | − | 16,196 ± 652 | 230 ± 12 | 129 ± 2 | ND† |
| − | + | 24,424 ± 794 | 262 ± 8 | 208 ± 25 | ND |
| + | − | 15,463 ± 190 | 211 ± 5 | 121 ± 0.4 | ND |
| + | + | 23,077 ± 379 | 489 ± 23‡ | 163 ± 15 | 3± 1 |
| Control (untreated) | 13,604 ± 310 | 228 ± 1 | 128 ± 0.5 | ND |
Astrocytes, seeded at a density of 5 × 105 cells/well in 96-well plates, were treated with supernatants derived from MDM (2 × 106 cells/well) for 2 hours. New media were placed onto cells and collected 24 hours later. Results are the mean and SEM given in pg/105 cells.
*MCM, Monocyte-derived macrophage conditioned medium.
†ND, Not detectable.
‡Statistically significant difference as compared with similarly treated uninfected cells (P = 0.011).
Table 3.
β-Chemokines Secreted by Human Astrocytes after Treatment with Microglial-Conditioned Media
| Astrocytes treated with μCM* | Chemokines secreted (pg/105 cells) | ||||
|---|---|---|---|---|---|
| HIV | LPS | MCP-1 | MIP-1α | MIP-1β | RANTES |
| − | − | 15,139 ± 950 | 247 ± 10 | 139 ± 6 | ND† |
| − | + | 20,264 ± 1866 | 239 ± 12 | 134 ± 7 | ND |
| + | − | 17,887 ± 134 | 213 ± 5 | 129 ± 3 | ND |
| + | + | 29,011 ± 293‡ | 500 ± 49‡ | 297 ± 29‡ | 21± 11 |
| Control (untreated) | 13,604 ± 310 | 228 ± 1 | 128 ± 0.5 | ND |
Astrocytes seeded at a density of 5 × 105 cells/well in 96-well plates were treated with supernatants derived from microglia (0.3 × 106 cells/well) for 2 hours. New media were placed onto cells and collected 24 hours later. Results are the mean and SEM given in pg/105 cells.
*μCM, Microglial-conditioned medium.
†ND, Not detectable.
‡Statistically significant difference as compared with similarly treated uninfected cells (P value range, from 0.032 to 0.044).
Monocyte Transendothelial Migration into Brains of SCID Mice with HIVE
To determine the possible proinflammatory and transendothelial migratory effects of resident brain macrophages in our SCID mouse model for HIV-1 encephalitis, 34 human MDM and microglia (infected or uninfected) were stereotactically placed into SCID mouse brains. SCID mice received 15 μl of suspension containing 1.5 × 10 5 HIV-1-infected or replicate uninfected microglia or MDM into the basal ganglia (the region of brain tissue most affected in humans). Mice inoculated with 15 μl of monocyte culture media served as controls. At 7 days after MDM or microglial inoculation, neuropathological analyses were performed. Equal numbers of HIV-infected microglia and monocytes (15 to 30 cells/5-μm section) were observed in the putamen (Figure 3, A and C) ▶ . Nearly 80% of the MDM or microglia expressed HIV-1 p24 antigen, and up to one-third of the cells were multinucleated (Figure 3, C and D) ▶ . CD68-positive cells were found in the cortex and basal ganglia around the site of injection. The majority of the microglia preserved their oval shape, resembling the activated ameboid cells commonly observed in HIVE. The injected microglia and the MDM were observed around microvessels, mimicking their distribution in HIVE. Because Griffonia simplicifolica lectin-isolectin B4 detects mouse MDM or microglia, we could assess the numbers of migrating mouse macrophages into brain. An area of 200 μm around the placement of human cells was examined to determine numbers of migrating murine monocytes. A pronounced accumulation of mouse monocytes and microglia was found in and around the location of virus-infected human microglia (Figure 3F) ▶ . Here, 45.2 ± 3.2 or 28.7 ± 2.5 mouse MDM were found per power field (×20) in and around injection sites containing HIV-1-infected or uninfected microglia cells, respectively. This was infrequently observed in mouse brains with HIV-1-infected MDM (Figure 3E) ▶ . Significantly lower numbers of mouse MDM were identified by lectin staining as per replicate brain areas around human virus-infected MDM (10.7 ± 1.1, P < 0.03 as compared with infected microglia) or uninfected MDM (5.3 ± 0.6, P < 0.04 as compared with uninfected microglia).
Figure 3.

Microglia-mediated changes in SCID mice with HIV-1 encephalitis. Equal numbers of HIV-1-infected MDM (A) and microglia (B) were stereotactically inoculated into basal ganglia. Both MDM (C) and microglia (D) express high levels of HIV-1 p24 antigen in their cytoplasm. Pronounced accumulation of mouse macrophages and microglia is found in and around the location of virus-infected human microglia (F). This is infrequently observed in mouse brains with HIV-1-infected MDM (E). More pronounced astrogliosis (GFAP immunostaining) is detected in areas contained microglia (H) as compared with MDM (G). A–D, G and H and G and B, D, and H present serial coronal sections immunostained with anti-CD68 (A and B), HIV-1 p24 (C and D), and GFAP antibodies (G and H). E and F are stained with biotinylated Griffonia simplicifolica lectin-isolectin B4. Primary Abs or lectin-stained cells are detected by Vectastain Elite Kit with DAB as a substrate. Tissue sections were counterstained with Mayer’s hematoxylin. Original magnifications, ×200 (A-F) and ×100 (G and H).
A prominent astrogliosis followed 7 days of microglia and MDM inoculation. The extent and intensity of the astrocyte reaction were more prevalent in mouse brains with HIV-infected microglia as compared with MDM. This was confirmed by computer-directed image analyses (Figure 3, G and H) ▶ . GFAP immunoreactivity (indicating reactive astrogliosis) measured in the area of the HIV-infected microglia was significantly higher in the putamen (16.9 ± 0.86%) as compared with areas around human MDM (11.80 ± 0.53%, P < 0.02). The features of the reactive astrogliosis (due to introduction of virus-infected microglia/MDM) included astrocyte accumulation/proliferation and hypertrophy with prominence of long and thick cytoplasmic processes. There was no accumulation of murine macrophages in the brains of control (media-inoculated) animals, and only a moderate increase of GFAP immunostaining was detected in astrocytes along the needle track (data not shown).
Relationship between Inflammatory Markers and Neuropathology in HIVE
To analyze the role of microglia in HIV-1 neuropathogenesis, we evaluated postmortem brain tissue from nine patients with HIVE of different intensity, five HIV-1-seropositive patients without evidence of HIVE, and five seronegative individuals (Table 1) ▶ . Neuropathological features of severe HIVE (cases 1 to 4) included 1) a pronounced infiltration of CD68-positive MDM into the brain parenchyma (25 to 50 cells/five ×10 power fields) (Figure 4A) ▶ , 2) formation of 1 to 2 microglial nodules composed of ramified and ameboid microglia cells per five 10× power fields (Figure 4A) ▶ , 3) signs of microglia activation (increased amount of cytoplasm and few short processes), and 4) diffuse astrogliosis (see below). Microglia cells with thin long processes were HIV-1 p24-positive (HIVE cases 1 to 4; Figure 4B ▶ ). Most microglia cells (80 to 90% within nodules or directly outside of them) showed strong positive immunostaining for HLA-DR in white matter, suggesting diffuse immune activation in cases of significant HIVE (Figure 4C) ▶ . Activated microglia cells with few thick processes and round or elongated bodies were found in gray matter in severe HIVE. Up to 30% of them expressed HLA-DR. In cases of severe HIVE, HIV-1 p24-positive multinucleated cells had long cell processes, which suggested that part of fused giant macrophages had arisen from microglia. They were present around microvessels and within neuropil in the central white matter of the cerebral hemispheres, thalamus, and basal ganglia (Figure 4E) ▶ . These multinucleated cells were immune activated (HLA-DR positive), and their localization corresponded to the areas of most intensive monocyte infiltration and microglial nodule formation (Figure 4F) ▶ . Moderate HIVE (cases 5 to 8) was characterized by expression of HLA-DR on 20 to 70% of microglia in white matter and <5% in gray matter; frequent multinucleated giant cells; 5 to 25 infiltrating macrophages per five (×10 power) fields, and formation of 1 to 2 microglial nodules/10 to 15 (×10 power) fields.
Figure 4.

HIV-1 infection and immune activation of microglia in HIVE. Significant accumulation of monocytes (CD68-positive) around microvessels in the subcortical white matter (A) coincides with HIV-1 infection of microglia (HIV-1 p24 positive) (B) and its immune activation (as detected by HLA-DR expression) (C). Same areas of the brain tissue are characterized by prominent astrogliosis as identified by GFAP immunoreactivity (D). HIV-1-infected multinucleated giant cells (E) express markers of immune activation (HLA-DR-positive, F) and are especially prominent in zones of significant monocyte infiltration. A-D and E-F present serial sections of brain tissue from cases 1 and 2, respectively. Sections are immunostained with anti-CD68 (A), HIV-1 p24 (B and E), HLA-DR (C and F), and GFAP antibodies (D). Primary Abs are detected by Vectastain Elite Kit with DAB as a substrate. Tissue sections were counterstained with Mayer’s hematoxylin. Original magnification, ×200.
HIVE characterized by minimal macrophage brain infiltration (no more than 3 to 5 CD68-positive MDM per five ×10 power fields) and rare microglial modules (1 per 20 ×10 power fields) was observed in case 9. This is referred to as mild encephalitis. Occasional multinucleated giant cells were present around microvessels and were HIV-1 p24 positive (data not shown). Accumulation of microglia was less prominent in the brain tissue with mild HIVE, and only rare microglial cells (10 to 15%) expressed HLA-DR in white matter only. In addition, ramified microglia cells did not contain virus antigen. Diffuse astrogliosis (identified as accumulation and proliferation of GFAP-positive hypertrophied astrocytes) was evident in different areas but especially prominent in zones most affected by MDM infiltration and microglial nodule formation in severe HIVE (Figure 4D) ▶ , whereas astrocyte reaction was less obvious in mild HIVE.
Brain tissue derived from HIV-1-seropositive subjects without HIVE showed limited pathology (anoxic damage and/or hemorrhage). Case 14 showed signs of CMV encephalitis characterized by amphophilic intranuclear inclusions (case 14). Macrophage infiltration, p24 antigen positivity, or multinucleated giant cells were absent. There were no signs or minimal evidence of immune activation in microglial cells (apart from focal lesions). Less than 10% of microglial cells expressed HLA-DR in white matter. Brain tissue derived from control HIV-negative patients (Table 1) ▶ showed nonspecific changes with minimal expression of HLA-DR in <10% of microglia located in white matter.
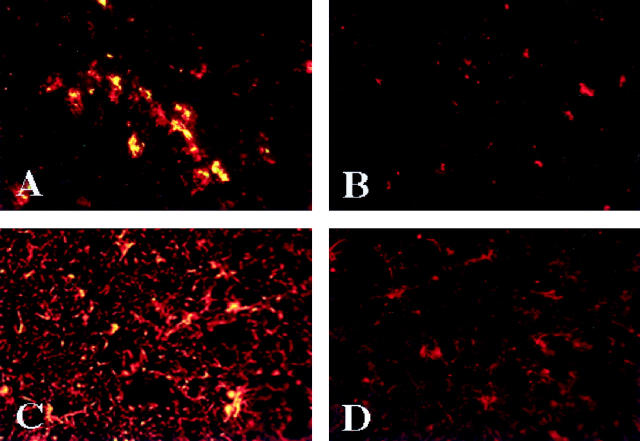
Evidence for increased chemokine expression in HIVE was shown by double immunostaining for chemokines and cellular markers in severe and, to a lesser degree, moderate HIVE. MCP-1 was identified in the cytoplasm of macrophages and microglial cells in microglial nodules and individual microglial cells in the neuropil (Figure 5A) ▶ . Astrocytes expressed MCP-1 in the areas of most prominent macrophage infiltration and microglial nodule formation (Figure 5C) ▶ . Vascular end feet of astrocytes surrounding microvessels expressed MCP-1 in HIVE. MIP-1α and, to a lesser extent, RANTES were expressed in microglia and astrocytes throughout the white matter and were associated with microglial nodules in HIVE. Moderate MIP-1β immunostaining was associated with cytoplasm of astrocytes around microglial nodules. Brain tissue from HIV-positive patients without HIVE showed occasional microglial and astrocytic cells expressing MCP-1 and MIP-1α. Chemokines were not detected in control (HIV-1 negative) brains (without HIV-1 infection) (Figure 5B,D) ▶ . In toto, analysis of human brain tissue showed that HIVE was associated with viral infection, microglial activation, MDM brain infiltration, astrogliosis, and β-chemokine expression.
Figure 5.
Microglia and reactive astrocytes express MCP-1 in HIVE. In severe HIVE (case 3), microglial cells (red) expressing MCP-1 (green) are stained yellow in microglial nodules (A) whereas control brain tissue (case 16) without HIV-1 infection shows no MCP-1 in microglia (red; B). Part of reactive astrocytes (red) in severe HIVE (case 3) express MCP-1 (green) resulting in yellow cytoplasmic staining, whereas astrocytes (red) are MCP-1-negative in controls (case 16). MCP-1 and microglia are identified by double immunolabeling on frozen sections with antibodies to MCP-1 (fluoroscein isothiocyanate) and HAM-56 (TRITC) (A and B), respectively. MCP-1 expression in astrocytes was detected with antibodies to MCP-1 (fluoroscein isothiocyanate) and GFAP (rhodamine) (C and D). Antigen expression was detected by indirect immunofluorescence. Original magnification, ×200.
Discussion
We used an artificial BBB system, primary cell cultures (MDM, microglia, astrocytes), an animal model of HIVE, and human autopsy tissue to investigate the mechanisms for monocyte brain transendothelial migration during HAD. HIV-infected microglia elicited the greatest monocyte migratory response in our BBB model. In cell cultures, activated microglia produced significantly more chemokines than did similar numbers of MDM. HIV-1 infection did not change chemokine secretory activities in immune-stimulated MDM/microglia. Placement of infected macrophage fluids onto primary human fetal astrocytes significantly up-regulated astroglia chemokines, principally MCP-1. Indeed, astrocytes (treated with supernatants from infected/activated microglia) were a rich source of β-chemokines. A marked accumulation of mouse MDM with concomitant astrogliosis was seen in areas surrounding virus-infected human microglia in SCID mice with HIVE. In human HIVE, microglial activation and virus infection was associated with prominent astrogliosis, macrophage infiltration, and β-chemokine expression in affected brain tissue. Cross-validating results obtained in laboratory and animal model systems, together with the supporting data obtained from human brain tissue analyses, strongly support a role of microglial activation in the neuropathogenesis of HAD. Such results support a role for intercellular glial interactions in the neuropathogenesis of HAD. 9,12 Nonetheless, alternative interpretations of the data set need also be considered. For example, developmental factors could account for some of the observed differences between our microglia and MDM cultures. Currently we are testing adult microglia in replicate assays as shown in this report. If any age-related effects will be demonstrated for microglial infection and activation, this could provide at least one explanation for the differential susceptibility of the developing brain for HIV-1 infection and its associated neural injury.
Despite more than a decade of work exploring how HIV-1 affects the human brain, the specific role of microglia in HAD remains uncertain. In other neurodegenerative disorders such as Alzheimer’s disease, 35,36 MS, 37 stroke, 38 and Parkinson’s disease, the importance of microglial activation in the pathogenesis appears more obvious. 39 Microglia activation (demonstrated by HLA-DR immunoreactivity) 40-42 and the concomitant secretion of cytokines and chemokines could be crucial events in HIV-1 neuropathogenesis. Microglial nodules are detected perivascularly, and in HIVE are associated with perivascular cuffs of macrophages, suggesting local production of chemokines. Neuropathological analysis of HIVE brain tissue showed a direct relationship between microglial HLA-DR expression, the intensity of macrophage infiltration, and microglial nodule formation (markers of HIVE severity). The levels of virus infection of ramified microglia correlated with HLA-DR expression, transition of microglia from ramified to ameboid morphology, and the formation of microglial nodules. The severity of HIVE (characterized by HIV-1 infection and immune activation of microglia) correlated with β-chemokine expression and macrophage infiltration. Recently, Sanders et al 28 reported an association between HLA-DR and chemokine antigen expression. In this report, a prominent astrogliosis was observed in HIVE, which followed the distribution of microglial nodules and the numbers of infiltrated macrophages. Such interrelationships between tissue pathology and macrophage activation support the importance of glial interplay in HAD pathogenesis. The significance of macrophage-astrocyte interactions was also confirmed in our experiments in which fluids from immune-stimulated MDM/microglia elicited a significant β-chemokine secretion in astrocytes (Tables 2 and 3) ▶ ▶ . The significance of microglial activation in HIVE was underscored by its demonstrated relationship to neurological impairments (Table 1) ▶ .
These works explore the regulation of chemokines by immune-stimulated and virus-infected microglia. Moreover, important secretory differences in MP function were demonstrated between microglia and MDM. For example, microglial cells secreted significantly more TNF-α and β-chemokines (MIP-1α, MIP-1β, MCP-1, and RANTES) than equal numbers of MDM. Immune activation further enhanced chemokine production. Furthermore, supernatants from HIV-1-infected, immune-stimulated microglia elicited significant levels of MCP-1 in astrocytes. The augmented production of MCP-1 by microglia and astrocytes, as compared with other chemokines, may help to explain the selective migration of monocytes into brain in HIVE. The functional importance of microglial and/or astrocyte MCP-1 was confirmed both in the BBB and SCID mouse models of HIVE. Human microglia (particularly those HIV-1 infected) enhanced monocyte migration in both model systems. Moreover, the accumulation of mouse monocytes in brains of SCID mice injected with virus-infected microglia was accompanied by a marked astrogliosis reflecting a relationship between monocyte infiltration and brain injury.
In previous works, attempts to correlate virus-infected microglia with disease progression were inconclusive. 8,43-45 MPs were grouped together, as a homogenous cell unit. The production of neurotoxins by microglia and brain macrophages was suggested as a major but indirect pathogenetic factor in HAD. 3 Our previous works, however, suggested that both cell types (microglia and macrophages) have unique functions. In this regard, diffuse microglia activation in HAD may explain a paradox: how relatively small numbers of infected perivascular macrophages can produce widespread neurological dysfunction. For example, activation of microglia and diffuse microgliosis were previously shown to correlate with ventricular expansion and neuropathological changes in HAD. 15,46,47
The discordance, in select patients, between the level of pathology and the clinical course of disease may be related to several factors. These include genetic susceptibilities to dementia in patient subpopulations, peripheral immune activation, and limitations of autopsy tissue analyses. The examination of brain tissue at one point in time may not reflect dynamic events that occur continuously over time. Increased permeability of the BBB and enhanced access of neurotoxins into the CNS could explain the discordance between limited brain pathology and the clinical course in some HAD patients. Indeed, in a patient with severe HAD, levels of macrophage-secreted neurotoxins (quinolinic acid, TNF-α, and NO) in the peripheral blood were higher than in CSF. 48 How activated microglia and reactive astrocytes can affect functional tightness of BBB is currently under investigation in our laboratories.
It is clear that microglia play an important role in the inflammatory responses associated with nervous system dysfunction during progressive HIV-1 infection and in other neurodegenerative disorders. 49 Microglia are the primary cell type to respond to injury in the CNS. Microglial activation in response to a stimulus includes proliferation, recruitment, and differentiation into phagocytic cells. Activated microglia express major histocompatibility complex class I and II antigens and adhesion molecules, and they secrete cytokines, numerous immune-modulatory molecules, and reactive oxygen intermediates. It is these abilities that permit the microglial cell to play a unique role in brain injury and inflammatory responses as well as in the regulation of normal CNS homeostasis. 50-54 Understanding the means by which microglial cells produce neurotoxin or neurotrophic activities may certainly prove to be critical for deciphering the neuropathogenic mechanisms in a broad range of neurodegenerative disorders.
MCP-1 was the principal microglia- and astrocyte-secreted chemokine demonstrated in these experimental works. The contribution of MCP-1 to monocyte migration into brain is supported by a number of studies including animal models for brain injury (brain trauma, neuronal damage induced by kainic acid, and cerebral ischemia). 37,38 Expression of MCP-1 and other β-chemokines was found in experimental autoimmune encephalomyelitis, a model for MS. 55-57 Furthermore, in MCP-1-transgenic mice, mononuclear cell infiltrates are found in brain parenchyma only at times of maximal chemokine expression. 58 MCP-1 is the most potent chemoattractant for monocytes, 59 and it is readily seen in the CSF of patients with HAD. 22 Thus, the likely importance of MCP-1 production is obvious in HAD pathogenesis.
In recent years, divergent studies on the neuropathogenesis of HAD have begun to tie together, suggesting a common immune mechanism for this apparent metabolic encephalopathy. In a patient with severe HAD, highly active antiretroviral therapy combined with anti-inflammatory treatment significantly ameliorated the clinical neurological deficit while markedly diminishing viral load and macrophage-secreted neurotoxins (quinolinic acid, TNF-α, and NO) in the peripheral blood and CSF. 48 Recently, expression of the chemokine receptor CXCR4 (a coreceptor for lymphotropic HIV-1 strains) has been found on a number of brain cells, including neurons, astrocytes, and microglia. 60-62 Astrocyte production of SDF-1 (the ligand for CXCR4) also attests to the importance of glial activation and astrocytosis in the HAD pathogenesis. 63 Binding of viral proteins (gp120) and SDF-1 to CXCR4 expressed on neurons could be a pathway for the neuronal apoptosis, suggesting an additional mechanism for neurodegeneration developed in HAD and HIVE. 63,64 These observations, taken together with those in this report, strongly support the idea that HAD is a metabolic encephalopathy fueled by viral replication and immune activation of macrophages and astrocytes. The heterogeneity in MP secretory functions and their interaction with other glial cells certainly play important roles in the pathogenesis of HAD and its associated encephalitis. Certainly, the mechanisms mediating the recruitment of monocytes into the brain, as demonstrated in this work, underscore the importance of MP transendothelial migration for expanding the viral reservoir and for regulating MP neurotoxic activities in HAD.
Acknowledgments
We thank Ms. Julie Ditter and Robin Taylor for excellent editorial support. We thank Dr. Charles Mackay (LeukoSite, Inc.) for the beta chemokine antibodies. Special thanks go to Drs. Leroy Sharer and Rodney McComb for neuropathological tissue and helpful discussions.
Footnotes
Address correspondence and reprint requests to Yuri Persidsky, M.D., Ph.D., Center for Neurovirology and Neurodegenerative Disorders, 985215 Nebraska Medical Center, Omaha, Nebraska 68198-5215. E-mail: ypersids\@unmc.edu.
Supported in part by National Institutes of Health grants R29 AI42404–01R29 (to Y. P.), K08 MH01552–01A1 (to J. L.), R01HL61951 (to K. S. K.), P01NS31492–01, R01NS34239–01, R01NS34239–02, R01NS36126–01, and P01MH57556–01, and the University of Nebraska Biotechnology Start Up Funds (to H. E. G.). A. G. and M. S. are Pediatric AIDS Foundation Scholars.
References
- 1.Navia BA, Jordan BD, Price RW: The AIDS dementia complex. I. Clinical features. Ann Neurol 1986, 19:517-524 [DOI] [PubMed] [Google Scholar]
- 2.Price R, Brew B, Sidtis J, Rosenblum M, Scheck A, Cleary P: The brain and AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 1988, 239:586-592 [DOI] [PubMed] [Google Scholar]
- 3.Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R: The neuropathogenesis of HIV-1 dementia. AIDS 1997, 11(suppl. A):S35-S45 [PubMed] [Google Scholar]
- 4.Sharer LR, Cho E, Epstein LG: Multinucleated giant cells and HTLV-III in AIDS encephalopathy. Hum Pathol 1985, 16:170-176 [DOI] [PubMed] [Google Scholar]
- 5.Budka H: Multinucleated giant cells in the brain: a hallmark of the acquired immunodeficiency syndrome (AIDS). Acta Neuropathol (Berl) 1986, 69:253-256 [DOI] [PubMed] [Google Scholar]
- 6.Koening S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GM, Yungbluth M, Janotta F, Aksamit A, Marti MA, Fauci AS: Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 1986, 233:1089-1093 [DOI] [PubMed] [Google Scholar]
- 7.Masliah E, Ge N, Morey M, De Teresa R, Terry RD, Wiley CA: Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Invest 1992, 66:285-291 [PubMed] [Google Scholar]
- 8.Glass JD, Fedor H, Wesselingh SL, McArthur JC: Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 1995, 38:755-762 [DOI] [PubMed] [Google Scholar]
- 9.Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, Epstein LG, Gendelman HE: Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med 1992, 176:1703-1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelbard HA, Nottet HSLM, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi Y-B, Zhang D, Lipton SA, Tourtellotte WW, Epstein LG, Gendelman HE: Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol 1994, 68:4628-4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukrinsky M, Nottet HSLM, Schmidtmayerova H, Dubrovsky L, Flanagan CR, Mullins ME, Lipton SA, Gendelman HE: Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med 1995, 181:735-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nottet HSLM, Jett M, Flanagan CR, Zhai Q-H, Persidsky Y, Rizzino A, Bernton EW, Genis P, Baldwin T, Schwartz J, LaBenz CJ, Gendelman HE: A regulatory role for astrocytes in HIV-1 encephalitis: an overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-α by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol 1995, 154:3567-3581 [PubMed] [Google Scholar]
- 13.Nottet HSLM, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai Q-H, Sharer LR, McComb R, Swindells S, Soderland C, Gendelman HE: Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol 1996, 156:1284-1295 [PubMed] [Google Scholar]
- 14.Heyes MP, Rubinow D, Lane C, Markey SP: Cerebrospinal fluid quinolinic acid correlations are increased in acquired immune deficiency syndrome. Ann Neurol 1989, 26:275-277 [DOI] [PubMed] [Google Scholar]
- 15.Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE: Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol 1992, 31:349-360 [DOI] [PubMed] [Google Scholar]
- 16.Griffin DE, Wesselingh SL, McArthur JC: Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol 1994, 35:592-597 [DOI] [PubMed] [Google Scholar]
- 17.Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE: Intracerebral cytokine mRNA expression in AIDS. Ann Neurol 1993, 33:576-582 [DOI] [PubMed] [Google Scholar]
- 18.Luster AD: Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME: Production and function of monocyte chemoattractant protein-1 and β-chemokines in murine glial cells. J Neuroimmunol 1995, 60:43-48 [DOI] [PubMed] [Google Scholar]
- 20.McManus CM, Brosnan CF, Berman JW: Cytokine induction of MIP-1α and MIP-1β in human fetal microglia. J Immunol 1998, 161:1449-1455 [PubMed] [Google Scholar]
- 21.Hurwitz AA, Lyman WD, Berman JW: Tumor necrosis factor a and transforming growth factor b upregulate astrocyte expression of monocyte chemoattractant protein-1. J Neuroimmunol 1995, 57:193-198 [DOI] [PubMed] [Google Scholar]
- 22.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO: Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA 1998, 95:3117-3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Der Voorn P, Tekstra J, Beelen RHJ, Tenesen CP, Van Der Valk P, De Groot JA: Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis. Am J Pathol 1999, 154:45-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss JM, Downie SS, Lyman WD, Berman JW: Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leucocytes across a model of the human blood-brain barrier. J Immunol 1998, 161:6898-6903 [PubMed] [Google Scholar]
- 25.Croitoru J, Guillemin G, Boussin FD, Lebel-Binay S, LeGrand R, Gras G, Dormont D: Chemotactic cytokines and their receptor expression in adult Simian astrocyte cultures. Abstracts of 12th World AIDS Conference (Geneva). 1998, p 257 (Abstr.)
- 26.Schmidtmayerova H, Nottet HSLM, Nuovo G, Raabe T, Flanagan CR, Dubrovsky L, Gendelman HE, Cerami A, Bukrinsky M, Sherry B: Human immunodeficiency virus type 1 infection alters chemokine β peptide expression in human monocytes: implication for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA 1996, 93:700-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield D, Ringler DJ, Lackner AA: Chemokine expression on simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol 1996, 149:1459-1467 [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders VJ, Pittman CA, White MG, Wiley CA, Achim CL: Chemokines and receptors in HIV encephalitis. AIDS 1998, 12:1021-1026 [PubMed] [Google Scholar]
- 29.Ghorpade A, Nukuna A, Che MH, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman HE: HIV neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol 1998, 72:3340-3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M: A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol 1997, 158:3499-3510 [PubMed] [Google Scholar]
- 31.Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra M, Phipps T, Wahl L, Lane HC, Fauci AS, Burke DS, Skillman D, Meltzer MS: Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med 1988, 167:1428-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gendelman HE, Narayan O, Kennedy-Stotskopf S, Kennedy PGE, Ghotbi Z, Clements JE, Stanley J, Pezeshkpour G: Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol 1986, 58:67-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manthrope M, Fagnani R, Skaper SD, Varon S: An automated colorimetric microassay for neuronotrophic factors. Dev Brain Res 1986, 25:191-198 [DOI] [PubMed] [Google Scholar]
- 34.Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor TW, Patil A, Nottet HSLM, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE: Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol 1996, 149:1027-1053 [PMC free article] [PubMed] [Google Scholar]
- 35.Tooyama I, Kimura H, Akiama H, McGeer PL: Reactive microglia express class I and II major histocompatibility complex antigens in Alzheimer’s disease. Brain Res 1990, 523:273-280 [DOI] [PubMed] [Google Scholar]
- 36.McGeer PL, McGeer EG: The inflammation response system of brain implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev 1995, 95:195-218 [DOI] [PubMed] [Google Scholar]
- 37.Ransohoff RM, Tani M: Do chemokines mediate leukocyte recruitment in post-traumatic CNS inflammation? Trends Neurosci 1998, 21:154-159 [DOI] [PubMed] [Google Scholar]
- 38.Ransohoff RM: Chemokines in neurological disease models: correlation between chemokine expression patterns and inflammatory pathology. J Leuk Biol 1998, 62:645-652 [DOI] [PubMed] [Google Scholar]
- 39.Cotter RL, Burke WJ, Thomas VS, Potter JF, Zheng J, Swartz J, Sheppard R, Smeds M, Grace M, Gendelman HE: New insights into the neurodegenerative process of Alzheimer’s disease: role of microglia-associated inflammation and neurotoxicity. J Leuk Biol 1999, 65:416-427 [DOI] [PubMed] [Google Scholar]
- 40.Achim CL, Morey MK, Wiley CA: Expression of major histocompatibility complex and HIV antigens within the brains of AIDS patients. AIDS 1991, 5:535-541 [DOI] [PubMed] [Google Scholar]
- 41.Dickson DW: Macrophages in HIV CNS disease: microglia as reservoirs and perpetrators of HIV CNS disease. Gendelman HE Lipton S Epstein L Swindells S eds. Neurological and Neuropsychiatric Manifestations of HIV-1 Infection. 1997, :pp 97-116 Chapman & Hall, New York [Google Scholar]
- 42.Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C: Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia 1993, 7:75-83 [DOI] [PubMed] [Google Scholar]
- 43.Glass JD, Wesselingh SL, Selnes OA, McArthur JC: Clinical-neuropathological correlation in HIV-1 associated dementia. Neurology 1993, 43:2230-2237 [DOI] [PubMed] [Google Scholar]
- 44.Brew BJ, Rosenblum M, Cronin K, Price RW: AIDS dementia complex and HIV-1 brain infection: clinical-virological correlations. Ann Neurol 1995, 38:563-570 [DOI] [PubMed] [Google Scholar]
- 45.Johnson RT, Glass DJ, McArthur JC, Chesebro BW: Quantitation of human immunodeficiency virus in brain of demented and nondemented patients with acquired immunodeficiency syndrome. Ann Neurol 1995, 39:392-395 [DOI] [PubMed] [Google Scholar]
- 46.Weis S, Neuhaus S, Mehraein P: Activation of microglia is not dependent on the presence of HIV-1 antigens. Neuroreport 1994, 5:1514-1521 [DOI] [PubMed] [Google Scholar]
- 47.Gelman BB: Diffuse microgliosis associated with cerebral atrophy in the acquired immunodeficiency syndrome. Ann Neurol 1992, 34:65-70 [DOI] [PubMed] [Google Scholar]
- 48.Gendelman HE, Zheng J, Coulter CL, Ghorpade A, Che M, Thylin M, Rubocki R, Persidsky Y, Hahn F, Reinhard J, Swindells S: Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in HIV-associated dementia. J Infect Dis 1998, 178:1000-1007 [DOI] [PubMed] [Google Scholar]
- 49.Giulian D, Haverkamp LJ, Kashir WL, Yu J, Tom D, Li X, Kirpatrick JB: Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem Int 1995, 27:119-137 [DOI] [PubMed] [Google Scholar]
- 50.Gehrmann J, Matsumoto Y, Kreutzberg GW: Microglia: intrinsic immune effector cell of the brain. Brain Res Rev 1995, 20:269-287 [DOI] [PubMed] [Google Scholar]
- 51.Ling EA, Wong DC: The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia 1993, 7:9-18 [DOI] [PubMed] [Google Scholar]
- 52.Mauerhoff T, Pujol-Borrell R, Mirakian R, Battazo GF: Differential expression and regulation of major histocompatibility complex (MHC) products in neural and glial cells of the human fetal brain. J Neuroimmunol 1988, 18:271-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry VH, Lawson LJ, Reid DM: Biology of the mononuclear phagocyte system of the central nervous system and HIV infection. J Leukoc Biol 1994, 56:399-406 [DOI] [PubMed] [Google Scholar]
- 54.Sedgewick JD, Schwender S, Gregersen R, Dorries R, ter Meulen V: Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex positive. J Exp Med 1993, 177:1145-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tani M, Glabinski AR, Tuohy VK, Stoler MH, Esters MI, Ransohoff RM: In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental encephalomyelitis. Am J Pathol 1996, 148:889-896 [PMC free article] [PubMed] [Google Scholar]
- 56.Glabinski A, Tani M, Basingam V, Yong VW, Ransohoff RM: Chemokine monocyte chemoattractant protein-1 (MCP-1) is expressed after mechanical injury to the brain. J Immunol 1996, 156:4363-4368 [PubMed] [Google Scholar]
- 57.Glabinski A, Tani M, Strieter R, Tuohy V, Ransohoff R: Synchronous synthesis of a- and β-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of experimental autoimmune encephalomyelitis. Am J Pathol 1997, 150:617-630 [PMC free article] [PubMed] [Google Scholar]
- 58.Fuentes ME, Durham SK, Swerdeel MR, Lewin AC, Barton DS, Megill JR, Bravo L, Lira SA: Controlled recruitment of monocytes/macrophages to specific organs through transgenic expression of MCP-1. J Immunol 1995, 155:5769-5776 [PubMed] [Google Scholar]
- 59.Bell MD, Taub DD, Perry VH: Overriding brain’s intrinsic resistance to leucocyte recruitment with intraparenchymal injection of recombinant chemokines. Neuroscience 1996, 74:283-292 [DOI] [PubMed] [Google Scholar]
- 60.Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper C: Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol 1997, 158:2882-2896 [PubMed] [Google Scholar]
- 61.Lavi E, Strizki JM, Ulrich AM, Zang W, Fu L, Wang Q, O’Connor M, Hoxie JA, Gonzales-Scarano F: CXCR-4 (fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol 1997, 151:1035-1042 [PMC free article] [PubMed] [Google Scholar]
- 62.He J, Chen YM, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang Y, Hoffman W, Newman W, McKay RC, Sodroski J, Gabuzda D: CCR3, and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 1997, 385:645-649 [DOI] [PubMed] [Google Scholar]
- 63.Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Coter R, Nieman D, Che MH, Zeng Y-C, Gelbard HA, Shepard RS, Swartz JM, Gendelman HE: Intracellular CXCR4 signaling, neuronal apoptosis, and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmmunol 1999, 98:185-200 [DOI] [PubMed] [Google Scholar]
- 64.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson J, Horuk R: Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α mediated by the chemokine receptor CXCR4. Curr Biol 1998, 8:595-598 [DOI] [PubMed] [Google Scholar]