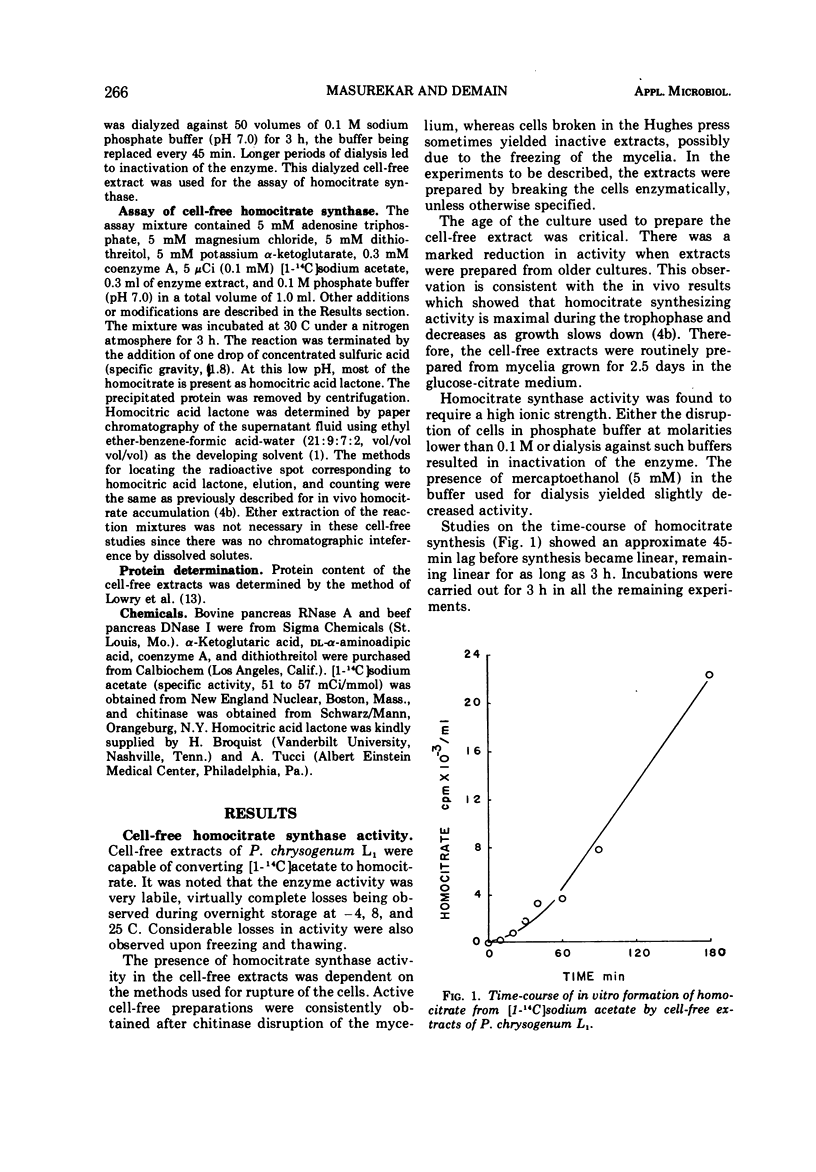
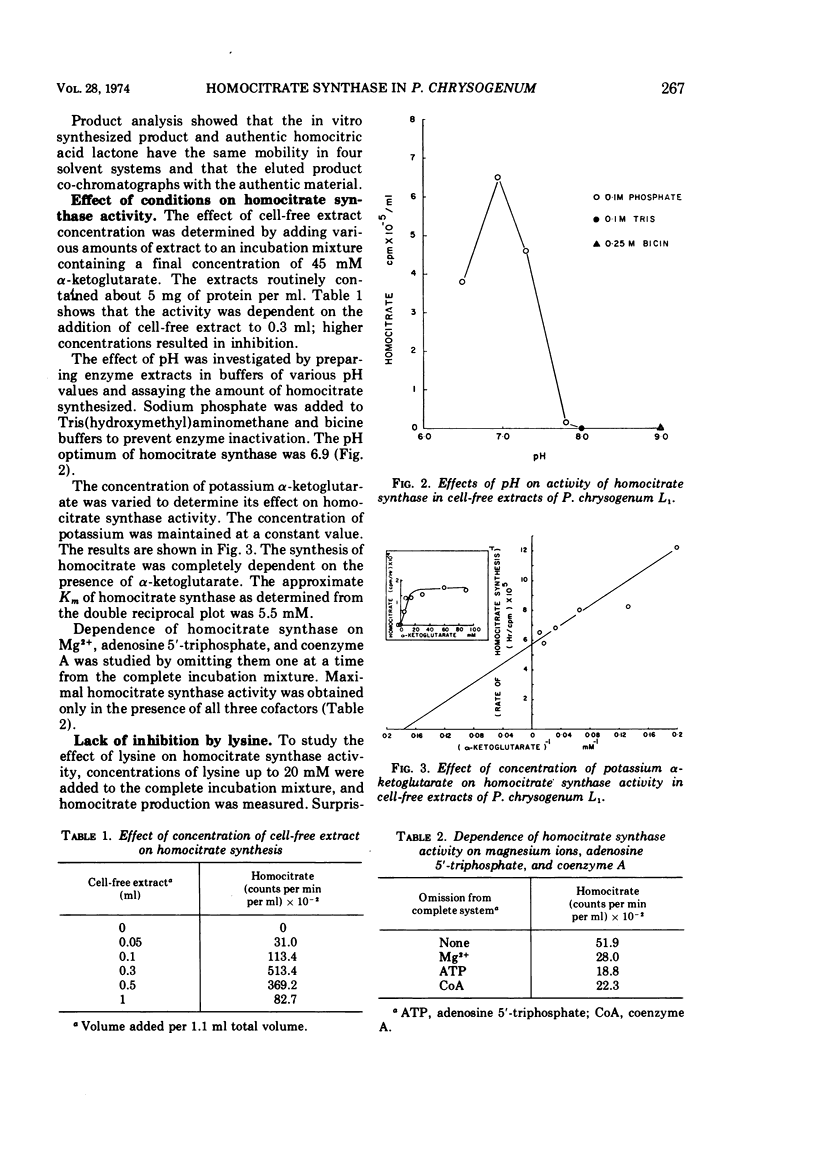
Abstract
We previously reported that lysine inhibits in vivo homocitrate synthesis in the lysine bradytroph, Penicillium chrysogenum L1, and that such feedback inhibition could explain the known lysine inhibition of penicillin formation. In the present study, it was found that dialyzed cell-free extracts of mutant L1 converted [1-14C]acetate to homocitrate. This homocitrate synthase activity was extremely labile but could be stabilized by high salt concentrations. The pH optimum of the reaction was 6.9, and the Km was 5.5 mM with respect to α-ketoglutarate. The reaction was also dependent upon the presence of Mg2+, adenosine 5′-triphosphate, and coenzyme A. Surprisingly, the activity in these crude extracts was not inhibited by lysine. Benzylpenicillin at a high concentration (20 mM) partially inhibited the enzyme, an effect that was enhanced by lysine. Casein hydrolysate also partially inhibited the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee J. K., Strassman M. Accumulation of tricarboxylic acids related to lysine biosynthesis in a yeast mutant. J Biol Chem. 1967 May 25;242(10):2542–2546. [PubMed] [Google Scholar]
- DEMAIN A. L. Inhibition of penicillin formation by lysine. Arch Biochem Biophys. 1957 Mar;67(1):244–246. doi: 10.1016/0003-9861(57)90265-5. [DOI] [PubMed] [Google Scholar]
- Datta P. Homoserine dehydrogenase of Rhodospirillum rubrum. Conformational changes in the presence of substrates and modifiers. Biochemistry. 1971 Feb 2;10(3):402–408. doi: 10.1021/bi00779a007. [DOI] [PubMed] [Google Scholar]
- Demain A. L. Biochemistry of penicillin and cephalosporin fermentations. Lloydia. 1974 Jun;37(2):147–167. [PubMed] [Google Scholar]
- Demain A. L., Masurekar P. S. Lysine inhibition of in vivo homocitrate synthesis in Penicillium chrysogenum. J Gen Microbiol. 1974 May;82(1):143–151. doi: 10.1099/00221287-82-1-143. [DOI] [PubMed] [Google Scholar]
- Glatzer L., Eakin E., Wagner R. P. Acetohydroxy acid synthetase with a pH optimum of 7.5 from Neurospora crassa mitochondria: characterization and partial purification. J Bacteriol. 1972 Oct;112(1):453–464. doi: 10.1128/jb.112.1.453-464.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordee E. Z., Day L. E. Effect of exogenous penicillin on penicillin biosynthesis. Antimicrob Agents Chemother. 1972 Apr;1(4):315–322. doi: 10.1128/aac.1.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden S. A., Chattaway F. W. End-product control of acetohydroxyacid synthetase by valine in Penicillium chrysogenum Q 176 and a high penicillin-yielding mutant. J Gen Microbiol. 1969 Nov;59(1):111–118. doi: 10.1099/00221287-59-1-111. [DOI] [PubMed] [Google Scholar]
- Görisch H., Lingens F. 3-D-eoxy-D-arabino-heptulosonate-7-phosphate synthase of Streptomyces aureofaciens Tü 24. II. Repression and inhibition by tryptophan and tryptophan analogues. Biochim Biophys Acta. 1971 Sep 22;242(3):630–636. doi: 10.1016/0005-2744(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Hogg R. W., Broquist H. P. Homocitrate formation in Neurospora crassa. Relation to lysine biosynthesis. J Biol Chem. 1968 Apr 25;243(8):1839–1845. [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. The regulation of methionine synthesis and the nature of cystathionine gamma-synthase in Neurospora. J Biol Chem. 1970 Apr 10;245(7):1842–1855. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Light R. J. The biosynthesis of 6-methylsalicylic acid. Crude enzyme systems from early and late producing strains of Penicillium patulum. J Biol Chem. 1967 Apr 25;242(8):1880–1886. [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- Maragoudakis M. E., Holmes H., Strassman M. Control of lysine biosynthesis in yeast by a feedback mechanism. J Bacteriol. 1967 May;93(5):1677–1680. doi: 10.1128/jb.93.5.1677-1680.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurekar P. S., Demain A. L. Lysine control of penicillin biosynthesis. Can J Microbiol. 1972 Jul;18(7):1045–1048. doi: 10.1139/m72-162. [DOI] [PubMed] [Google Scholar]
- Miyajima R., Shiio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. VI. Effects of isoleucine and valine on threonine dehydratase activity and its formation. J Biochem. 1972 Jun;71(6):951–960. doi: 10.1093/oxfordjournals.jbchem.a129866. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Tucci A. F., Ceci L. N. Homocitrate synthase from yeast. Arch Biochem Biophys. 1972 Dec;153(2):742–750. doi: 10.1016/0003-9861(72)90393-1. [DOI] [PubMed] [Google Scholar]
- Wu T. W., Scrimgeour K. G. Properties of inosine-5'-phosphate dehydrogenase from Bacillus subtilis. Can J Biochem. 1971 Apr;49(4):473–475. doi: 10.1139/o71-070. [DOI] [PubMed] [Google Scholar]
- Yamamoto L. A., Segel I. H. The inorganic sulfate transport system of Penicillium chrysogenum. Arch Biochem Biophys. 1966 Jun;114(3):523–538. doi: 10.1016/0003-9861(66)90376-6. [DOI] [PubMed] [Google Scholar]


