Abstract
An important biological feature of prostate cancer (PCa) is its marked preference for bone marrow as a metastatic site. To identify factors that may support the growth of PCa in bone marrow, expression of receptor and nonreceptor tyrosine kinases by androgen-independent PCa bone marrow metastases was assessed. Bone marrow biopsies largely replaced by PCa were analyzed using reverse transcriptase-polymerase chain reaction amplification with degenerate primers that amplified the conserved kinase domain. Sequence analyses of the cloned products demonstrated expression of multiple kinases. Expression of the receptor and nonreceptor tyrosine kinases, α platelet-derived growth factor receptor and Jak 1, respectively, was confirmed by immunohistochemistry. In contrast, the type 1 insulin-like growth factor receptor, thought to play a role in PCa development, was lost in metastatic PCa. These results implicate several specific growth factors and signaling pathways in metastatic androgen-independent PCa and indicate that loss of the type 1 insulin-like growth factor receptor contributes to PCa progression.
Bone marrow is the predominant metastatic site for prostate cancer (PCa) and bone marrow metastases account for the vast majority of PCa morbidity and mortality. This pattern of metastatic growth indicates that tumor cells home preferentially to bone marrow or that bone marrow provides particular factors required for PCa growth. Although these hypotheses are not mutually exclusive, evidence that specific homing plays a major role is limited. 1 In contrast, several studies have shown that factors in bone can stimulate the growth of PCa cell lines and some candidate growth factors have been identified. 2-8 However, biochemical efforts to identify growth factors for metastatic PCa or test the importance of candidate factors have been limited by the lack of human PCa cell lines that accurately reflect PCa dependence on the bone marrow microenvironment.
Studies from many groups have demonstrated critical roles for a series of peptide growth factors and corresponding epithelium-expressed receptor tyrosine kinases (RTKs) in normal prostate and/or in PCa. These growth factors include transforming growth factor α, 9 insulin-like growth factor 1 (IGF-1), 10 keratinocyte growth factor, 11,12 basic fibroblast growth factor, 13 hepatocyte growth factor (HGF), 7,8 platelet-derived growth factor, 14, 15 and nerve growth factors. 16 A role for HGF in advanced metastatic PCa has been suggested by immunohistochemistry showing the consistent expression of the HGF receptor (c-met) in bone marrow metastases. 7,8 However, the role of these or other novel growth factors and their corresponding receptors in metastatic androgen-independent PCa remains uncertain.
The strategy taken in this study to identify growth factor-receptor interactions that support PCa growth in bone marrow was to identify RTKs expressed by these tumors freshly isolated from human bone marrow. RTKs contain a conserved tyrosine kinase domain that can be amplified from small numbers of tumor cells by reverse transcriptase-polymerase chain reaction (RT-PCR) with degenerate oligonucleotide primers. 17 In this study, RTKs and nonreceptor tyrosine kinases (NRTKs) expressed by a series of freshly isolated human PCa bone marrow metastases and by the LNCaP PCa cell line were amplified and cloned by this method. A large number were then sequenced to determine the spectrum of expressed RTKs and NRTKs in human PCa metastatic to bone marrow. The presence or absence of particular tyrosine kinases was then confirmed by immunohistochemistry.
Materials and Methods
Sample Acquisition
A series of patients with advanced androgen-independent PCa underwent bone marrow biopsies from the posterior iliac crest, as described previously. 18,19 A portion of each biopsy was frozen in OCT and frozen sections were analyzed histologically. Biopsies in which normal marrow elements were largely or completely replaced by PCa, with minimal fibrosis, were sought and 7 such biopsies from a total of 6 patients were identified. Adjacent 6-μm frozen sections (n-4 or 5) were then used for RNA extraction. LNCaP, a human androgen receptor expressing PCa cell line, 20 was maintained in Dulbecco’s modified Edgle’s medium with 10% fetal calf serum.
Tyrosine Kinase Domain Amplification
RNA was extracted with RNAzol B (Tel-Test, Friendswood, TX) and cDNA was synthesized using an oligo-dT primer, as described previously. 18 The oligo-dT was then removed by spin dialysis with a Microcon-100 filter (Amicon, Beverly, MA). cDNA was amplified in 50-μl reactions containing 500 ng of each degenerate primer (see below), Taq polymerase, bovine serum albumin at 0.1 mg/ml, and standard buffer containing 1.5 mmol/L MgCl2 as supplied by the manufacturer (Promega, Madison, WI). The cycles were 94° for 20 seconds, 48° for 45 seconds and 72° for 30 seconds for 35 cycles. Secondary PCR amplifications were performed using the same conditions.
Protein kinases have a conserved catalytic domain that can be divided into 11 subdomains. 17,21 This study used a series of degenerate sense primers in subdomain VI and anti-sense primers in subdomains VIII and IX (Table 1) ▶ . Primer sequences were chosen to preferentially amplify tyrosine kinases rather than serine/threonine kinases. The TK1 primer in domain VI was further chosen to bias against src family intracellular tyrosine kinases and in favor of RTKs and non-src family intracellular tyrosine kinases. However, the other domain VI primers would be predicted to amplify both src and other tyrosine kinases equally.
Table 1.
Primers Used for PCR Amplification of Tyrosine Kinases
| Domain | Amino acids | Nucleotide sequence | |
|---|---|---|---|
| TK1 | VI | HRDLA(A/T)RN | CA(T/C)(A/C)GNGA(C/T)(C/T)TNGCN(G/A)CN(A/C)GNAA |
| TK2 | VI | (I/V)HRDL | CGGG(G/A)T(G/C)CAC(A/C)GNGA(C/T)(C/T)T |
| TK3 | VI | IHRDL | CGGGATGCCAC(A/C)GNGA(C/T)(C/T)T |
| TK4 | VIII | WTA(P/L)E | GGAATTC(TC)TCN(AG)(AG)NGCNGTCCA |
| TK5 | VIII | WMA(L/W/P)E | GGAATTC(TC)TCN(AGC)(AG)NGCCATCCA |
| TK6 | VIII | WMPPE | GGAATTC(TC)TCNGGNGGCATCCA |
| TK7 | IX | D(V/M)W(S/A)(F/Y)G(I/V) | A(C/T)NCC(G/A)(T/A)AN(G/C)(T/A/C)CCANA(C/T)(G/A)TC |
| TK8 | IX | DVWS(F/Y)G | GGAATTCCA(T/A)AGGACCA(G/C)AC(G/A)TC |
Nucleotides added to generate BamH1 or EcoR1 restriction sites are underlined.
In one series of experiments, tyrosine kinases were amplified with subdomain VI and IX primers followed by a seminested amplification with subdomain VI and VIII primers. The primary amplifications in these experiments used the degenerate primers TK1 and TK7. This was followed by a secondary amplification using 5 to 10% of the primary amplification, the TK1 primer, and a mix of the TK4, -5, and -6 subdomain VIII primers. These secondary amplifications were for 20 to 25 cycles. The resulting PCR products were gel-purified and ligated into either pBluescript (Stratagene, La Jolla, CA) or pCRII (Invitrogen, Carlsbad, CA), in some cases using an EcoR1 site added to the subdomain VIII primers.
A second series of amplifications used a different pair of subdomain VI and IX primers. The primary amplifications were carried out with primers TK2 and TK8 in subdomains VI and IX, respectively. Secondary amplifications for 12 to 15 cycles were then carried out using 25% of the primary reactions and replacing primer TK2 with the nearly identical primer TK3. This latter step served to incorporate a nondegenerate BamH1 site at the 5′ end. The PCR products were then digested with BamH1 and EcoR1 and ligated into pBluescript.
Tyrosine Kinase Analysis
In the initial analyses, bacterial colonies containing tyrosine kinases were identified by PCR amplification with flanking primers in the vector and subsequent agarose gel electrophoresis to identify clones containing the correctly sized insert. In later screens, these PCR-amplified inserts were also dot blotted with oligonucleotide probes specific for tyrosine kinases frequently isolated in the initial screens. The sequences of these probes were as follows: Jak 1, ACCAAAGCAATTGAAACCGAT; FER, ATCTTCTGGCTTAAAGCA; α platelet-derived growth factor receptor (αPDGF-R), GATTCGAACTATGTGTCG; Lyn, GTCTCCGAGTCACTAATGTGC. Clones that hybridized strongly (consistent with identity) were counted toward the total for each receptor. A fraction of these strongly hybridizing clones was chosen at random and sequenced, which in all cases confirmed their identity. The total number of confirmed tyrosine kinase isolates (based upon hybridization and/or sequencing) from metastatic PCa analyzed in this series of experiments was 448.
To analyze a larger sampling of these libraries for particular tyrosine kinases, PCR products generated from the libraries by the domain VI and IX primers (primers TK2 and TK8, respectively) were dot blotted and hybridized with specific oligonucleotide probes. In addition to the probes above, probes for the type 1 insulin-like growth factor receptor (type 1 IGF-R) (GTAGCCGAAGATTTCACAGTC), FGF receptor 2 (FGF-R2) (GATATCAACAATATAGACTAT), and a probe for both the FGF-R1 and FGF-R3 (GACATTCACCACATCGACTAC) were used.
Immunohistochemistry
B5-fixed, decalcified bone marrow biopsies and formalin-fixed prostate and transurethral resection specimens were embedded in paraffin and sectioned for αPDGF-R, Jak 1, and prostate-specific antigen (PSA) immunohistochemistry. Sections were pretreated by microwaving in citrate buffer (10 mmol/L, pH 6.0) twice for 5 minutes each at 600W. The sections were incubated with polyclonal rabbit antibodies to PSA (Dako, Copenhagen, Denmark; dilution 1:2000), αPDGF-R (antibody C-20 generated against amino acids 1065–1084, Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:40), or Jak 1 (antibody Q-19, against amino acids 1122–1141, Santa Cruz Biotechnology; dilution 1:60). The primary antibody incubations for 1 hour were followed by incubation with a biotinylated goat anti-rabbit Ig secondary and then alkaline phosphatase-conjugated streptavidin (Biogenex, San Ramon, CA). The reaction product was visualized by new fuchsin (Dako) as a chromogen. Staining intensities were graded semiquantitatively as 0, no staining; +, weak staining; ++, moderate staining; and +++, strong staining. Staining for PSA was strong in all tumor samples and was not graded.
Nonspecific reactivity was assessed by omission of the primary antibody. The specificity of staining for the αPDRG-R and Jak 1 was also confirmed in bone marrow sections as well as in control tissues by preabsorption of the antisera with blocking peptides supplied by the manufacturer. Equimolar concentrations of antibody and blocking peptide were incubated for 1 hour at room temperature.
Expression of the type 1 IGF-R was examined in frozen and paraffin sections. Prostate and bone marrow biopsies frozen in OCT were sectioned at 6 μm and postfixed in methanol. The primary was a mouse anti-human type 1 IGF-R mAb (mAb 391, R&D Systems, Minneapolis, MN), used at 0.5 μg/ml. The secondary was a biotinylated goat anti-mouse Ig followed by streptavidin-HRP, as above. The samples were counterstained with hematoxylin. Alternatively, prostate core biopsies were taken from fresh prostatectomy specimens with a 14 gauge biopsy needle and processed identically to bone marrow biopsies. Fixation was in 95% ethanol:37% formaldehyde at 4:1, followed by overnight decalcification in EDTA. After deparaffinization, sections were microwaved in 1 mmol/L EDTA, pH 8.0, twice for 6 minutes each at 600W, followed by cooling for 20 minutes. Blocking of endogenous biotin was done using an avidin-biotin blocking kit (Vector Labs, Burlingame, CA) for 15 minutes. The primary mouse anti-human type 1 IGF-R mAb (Lab Vision Corp., Fremont, CA) was added for 45 minutes. Biotinylated horse anti-mouse IgG (1:200) in a solution containing glucose (50 mg/ml) and glucose oxidase (7 U, Sigma, St. Louis, MO) was used as a secondary for 30 minutes followed by Vectastain Elite reagent (Vector). Visualization was done with Vector peroxidase kit (peroxidase substrate kit, Vector VIP, SK-4600) for 10 minutes and counterstaining by acid hemalaun.
Results
Tyrosine Kinase Expression by the LNCaP Cell Line
To determine whether the degenerate PCR primers would be useful for amplification of diverse tyrosine kinases from prostate cancer, the LNCaP cell line was examined initially. LNCaP was derived from a human PCa metastatic to lymph node. 20 Its growth is stimulated by androgen and it produces PSA. Although it expresses a mutant androgen receptor, the mutation is identical to one found in some patients with androgen-independent prostate cancer. 19,22 LNCaP does not form bone marrow metastases when transplanted into immunodeficient mice, 23 but has nonetheless been the most commonly used human model for PCa.
Tyrosine kinase expression by LNCaP cells was examined by RT-PCR using the primers in subdomains VI and IX (primers TK1 and TK7, respectively). This was followed by a secondary amplification with subdomain VI and VIII primers (TK1 and a pool of TK4, -5, and -6). A total of 38 clones containing inserts of the correct size to be kinase domains were identified. Sequencing revealed that there were 7 different kinases represented (Table 2) ▶ . Among the kinases isolated, the type 1 IGF-R and Bmx have been reported previously in these cells. 24-27 Although it is clearly the case that LNCaP expresses additional tyrosine kinases, these initial results indicated that diverse tyrosine kinases could be amplified under these conditions.
Table 2.
Receptor and Nonreceptor Kinases Identified by RT-PCR in LNCaP Cells
| Receptor tyrosine kinases | Nonreceptor tyrosine kinases |
|---|---|
| Type 1 IGF-R | FAK |
| eph1 | FER |
| ror1 | abl |
| Bmx |
Kinases are listed in descending order based upon the frequency with which they were isolated.
Tyrosine Kinase Expression in Vivo by PCa Bone Marrow Metastases
A series of 7 androgen-independent PCa bone marrow metastases from 6 patients were next examined by RT-PCR for tyrosine kinase expression. These particular samples were selected based upon their high proportion of tumor cells and absence of normal bone marrow elements. The cDNA libraries were amplified in an initial series of experiments with the primers described above for LNCaP. To maximize the number of different kinases, a second series of amplifications was carried out with a distinct set of primers in subdomains VI and IX (see Material and Methods).
The results from both sets of primers were combined, yielding a total of 448 clones containing kinase domains from the 7 biopsies. The vast majority could be identified as previously described receptor or nonreceptor tyrosine kinases (Table 3) ▶ . Although the kinases are listed in Table 3 ▶ based upon the frequency with which they were isolated, this does not necessarily reflect abundance of the respective transcripts and more likely reflects the efficiency with which they were amplified by the degenerate primers. Two novel kinases were also identified, each in single biopsies. Although these are indicated as RTKs in Table 3 ▶ , it has not yet been determined whether they are receptor or nonreceptor kinases.
Table 3.
Receptor and Nonreceptor Tyrosine Kinases Identified by RT-PCR in Metastatic Androgen-Independent PCa Biopsies
| Receptor tyrosine kinases | Nonreceptor tyrosine kinases |
|---|---|
| αPDGF-R | Jak 1 |
| KDR | FER |
| eph1 | Lyn |
| DDR2 (TKT, Tyro 10) | Bmx |
| Tie-1 | brk |
| Tie-2* (angiopoietin 1 receptor, tek) | Yes |
| βPDGF-R | Jak 2 |
| EGF-R | abl |
| Novel kinase 1† | |
| Novel kinase 2† |
Kinases are listed in descending order based upon the frequency with which they were isolated.
*Differs from murine Tie-2 by 2 amino acids and is presumed to be the human homologue.
†Do not have homology with known human kinases or kinases from other species and may be receptor or nonreceptor kinases.
Immunohistochemical Analysis of αPDGF-R and Jak 1 Expression in Bone Marrow Metastases
The PCa biopsies examined contained predominantly tumor cells, but nontumor cells were also certainly present and could have been the source for one or more of the kinases identified in Table 3 ▶ . Therefore, immunohistochemistry was used to further assess tumor expression of particular kinases. The αPDGF-R and Jak 1 proteins were examined, as these receptor and nonreceptor tyrosine kinases, respectively, were identified in the majority of the PCa samples.
An analysis of these proteins in normal bone marrow was carried out initially to determine whether there were normal cells in the biopsies that might be the origin of these transcripts. In normal bone marrow, moderate to strong staining for αPDGF-R was found consistently in megakaryocytes and osteoblasts and occasionally in plasma cells, endothelial cells, and fibroblasts (Figure 1A) ▶ . However, the vast majority of myeloid and bone marrow stromal elements did not stain. Strong staining for Jak 1 in these sections of normal bone marrow was detected only in macrophages (Figure 1B) ▶ . Weak Jak 1 staining was seen on scattered mononuclear cells, which appeared to be myeloid precursors, whereas all other cells were negative.
Figure 1.
Immunohistochemical analyses of αPDGF-R and Jak 1 in metastatic PCa in bone marrow. A: Normal bone marrow stained with αPDGF-R Ab showing strong staining by megakaryocytes (arrow). B: Normal bone marrow stained with Jak 1 Ab showing strong staining in scattered macrophages (arrow). C–E: PCa bone marrow metastases stained with anti-PSA, anti-αPDGF-R, and anti-Jak 1 antibodies, respectively. F: PCa biopsy stained with αPDGF-R antibody after preincubation with the immunizing peptide. Original magnifacation, ×200.
A series of 11 formalin-fixed bone marrow biopsies containing metastatic PCa, obtained from eight patients, were next examined by immunohistochemistry. Tumor cells in all of these biopsies stained strongly for PSA, confirming that they were PCa (Figure 1C) ▶ . In each of the biopsies, all of the carcinoma cell complexes displayed unequivocal staining for the αPDGF-R (Figure 1D) ▶ . The majority of the samples showed staining of constant intensity, ranging from weak (+) to strong (+++) (Table 4) ▶ , although in some cases single carcinoma cells stained stronger than those nearby. No αPDGF-R staining was seen in the stromal elements surrounding the tumor cells (Figure 1D) ▶ .
Table 4.
Immunohistochemical Analysis of αPDGF-R and Jak 1 Expression in PCa Bone Marrow Metastases
| Patient | αPDGF-R | Jak 1 | αPDGF-R (primary) |
|---|---|---|---|
| 1a | +++ | nd | ++/+++ |
| 1b | +++ | ++ | |
| 2a | +++ | ++ | ++/+++* |
| 2b | ++ | + | |
| 3a | ++ | ++ | +++ |
| 3b | + | ++ | |
| 4a | + | nd | na |
| 4b | ++ | + | |
| 5 | ++ | nd | na |
| 6 | + | + | na |
| 7 | + | + | na |
| 8 | ++ | nd | ++ |
*Moderate to strong staining was observed in the more differentiated areas, whereas many poorly differentiated areas were negative.
na, not available; nd, not done.
Metastatic PCa expression of Jak 1 was examined by immunohistochemistry in eight bone marrow biopsies. Weak to moderate tumor cell staining was detected in all of the samples (Figure 1E ▶ and Table 4 ▶ ). The pattern observed was similar to αPDGF-R, with uniform staining in most carcinoma cell complexes. No staining was observed in the stroma. Staining for αPDGF-R and Jak 1 was completely abolished by preincubation with the appropriate peptides (Figure 1F ▶ and data not shown). Therefore, these immunohistochemical analyses in conjunction with the RT-PCR results confirmed that αPDGF-R and Jak 1 were expressed by PCa bone marrow metastases.
Immunohistochemical Analysis of αPDGF-R and Jak 1 Expression in Primary PCa and Nonneoplastic Prostate
Archival primary PCa samples from needle biopsies or radical prostatectomies were available from four of the patients examined here. Tumor cell staining for αPDGF-R was observed in each of these four samples (Figure 2A ▶ and Table 4 ▶ ). In three, uniform tumor staining similar to that seen in the corresponding metastatic tumors was observed. In the fourth case (patient 2), moderate to strong staining was observed in the more differentiated areas, whereas many poorly differentiated areas were negative. Jak 1 protein expression was examined in three additional primary prostate cancers. In each case there was uniform moderate to strong staining for Jak 1 (Figure 2B) ▶ .
Figure 2.
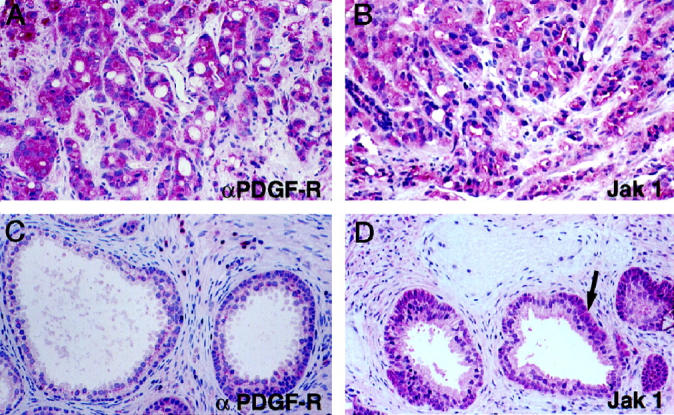
Immunohistochemical analyses of αPDGF-R and Jak 1 in primary PCa. A and B: Primary PCa stained with αPDGF-R and Jak 1 antibodies, respectively. C and D: Nonneoplastic prostate stained with αPDGF-R and Jak 1 antibodies, respectively. Arrow in D shows basal cell staining. Original magnification ×200.
Expression of αPDGF-R and Jak 1 in transurethral resection specimens from five patients showing variable degrees of hyperplasia were also examined. Staining intensity relative to the tumor samples was assessed. Luminal epithelial cells showed absent or very weak staining for αPDGF-R in the vast majority of glands (Figure 2C) ▶ . Moderate reactivity (++) was observed rarely and was restricted to single glands. Smooth muscle cells of the fibromuscular stroma stained very weakly, whereas macrophages and plasma cells exhibited moderate to strong staining.
Weak immunoreactivity of prostate luminal epithelial was found for Jak 1. In some cases basal cells stained more strongly than the overlying luminal epithelial cells (Figure 2D) ▶ . Occasional smooth muscle cell bundles showed moderate staining but most stained weakly or were negative. Strong Jak 1 staining was observed only in macrophages.
Comparative Analysis of Tyrosine Kinases Expressed in Normal Prostate versus Metastatic Androgen-Independent PCa
It was noteworthy that several RTKs believed to be important in normal and neoplastic prostate growth, including the type 1 IGF-R and the receptors for KGF (also termed FGF7) and bFGF, were not identified in this analysis of metastatic androgen-independent PCa. With respect to the type 1 IGF-R, this did not appear to be an unintended primer bias, as this receptor was readily amplified from the LNCaP cell line (Table 1) ▶ . To determine whether this might simply reflect inadequate sampling, expression of transcripts for the type 1 IGF-R and FGF-R1, -R2, and -R3 was specifically examined by RT-PCR and dot blot hybridization.
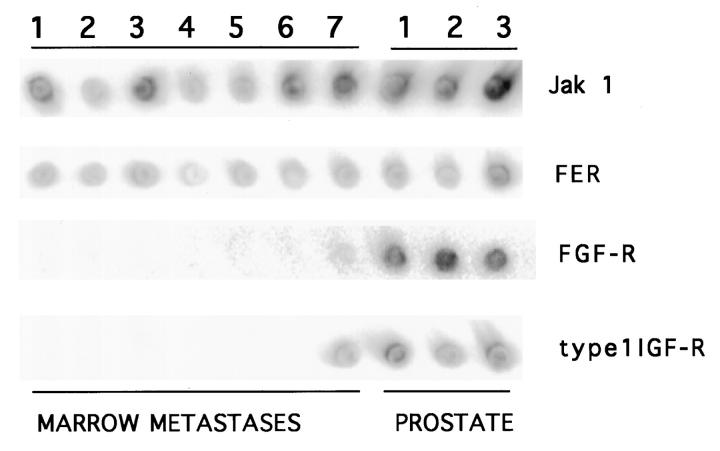
The tyrosine kinase libraries from the seven metastatic prostate cancers and from three sections of nonneoplastic prostate generated identically were amplified by PCR. Equivalent amounts of PCR product, based upon ethidium bromide staining, were then dot blotted and hybridized with a series of kinase-specific probes. There were no marked difference in Jak 1 transcript levels between the metastatic tumors and the normal prostate samples (Figure 3) ▶ . Expression of FER, which has not been examined previously in prostate, was also similar in all samples. Consistent with previous reports, this analysis detected FGF receptors and the type 1 IGF-R in normal prostate. 10-13 In contrast, in six of the seven metastatic PCa samples there was weak or absent hybridization with the pool of FGF-R1, -R2, and -R3 probes. It should be noted that both the PCR primers and probes used for this FGF-R analysis were in the kinase domain and would recognize the alternatively spliced form of the FGF-R2 found in the Dunning rat PCa model and recently in human PCa. 28,29 Finally, the type 1 IGF-R was detected in only one of the metastatic PCa samples. Therefore, these results were consistent with the failure to identify FGF-Rs or the type 1 IGF-R among the 448 tyrosine kinases analyzed.
Figure 3.
Dot blot analysis of tyrosine kinase expression. Replicate dot blots of amplified kinase domains from bone marrow metastases or normal prostate were hybridized with the indicated probes.
Immunohistochemical Analysis of Type 1 IGF-R Expression
Immunohistochemistry was used to confirm loss of type 1 IGF-R expression in metastatic androgen-independent PCa. The first experiments used frozen sections, because none of the initially tested type 1 IGF-R antibodies worked in formalin-fixed and paraffin-embedded tissues. Frozen sections from nonneoplastic prostate showed expression confined to the epithelium, with moderate staining of most glands (Figure 4A ▶ , at higher power in Figure 4B ▶ ). Primary PCa also expressed the type 1 IGF-R at levels that were comparable to normal epithelium, based upon staining intensity on identically processed sections (Figure 4C ▶ , at higher power in Figure 4D ▶ ). However, there was heterogeneity with most tumors containing a minority of cells that were negative for type 1 IGF-R staining. In contrast, no type 1 IGF-R expression was detectable in frozen sections from a series (n = 7) of androgen-independent bone marrow metastases (Figure 4, E and F) ▶ .
Figure 4.
Type 1 IGF-R immunohistochemistry on frozen sections of PCa in prostate and bone marrow metastases. Frozen sections were stained with a monoclonal mouse anti-human type 1 IGF-R. A and B: Low and high power of nonneoplastic prostate. C and D, Low and high power of primary PCa. E and F: metastatic androgen-independent PCa in bone marrow biopsies from two patients. Original magnifications, A, ×100; B, ×200; C, ×200; D–E, ×400.
The analysis of type 1 IGF-R expression was subsequently extended to formalin-fixed tissue using another antibody. Staining was similarly confined to the epithelium in nonneoplastic prostate, with moderate expression by luminal epithelium and stronger expression by basal cells in most glands (Figure 5A ▶ , at higher power in Figure 5B ▶ ). The strongest expression by both luminal epithelium and basal cells was seen in atrophic glands (Figure 5A ▶ , at right margin). Neoplastic glands showed moderate type 1 IGF-R expression (Figure 5C ▶ , at higher power in Figure 5D ▶ ). However, the expression was heterogeneous, with areas in some tumors being completely negative. Figure 5E ▶ is another area from the same biopsy as in Figure 5C ▶ , showing type 1 IGF-R negative tumor cells surrounding a positive non-neoplastic gland. Finally, formalin-fixed bone marrow metastases from four additional patients were examined. In two cases there were no positive cells and in the other two there was staining by a few scattered cells (<10% overall). Figure 5F ▶ shows an area in one section in which scattered positive cells can be readily seen.
Figure 5.
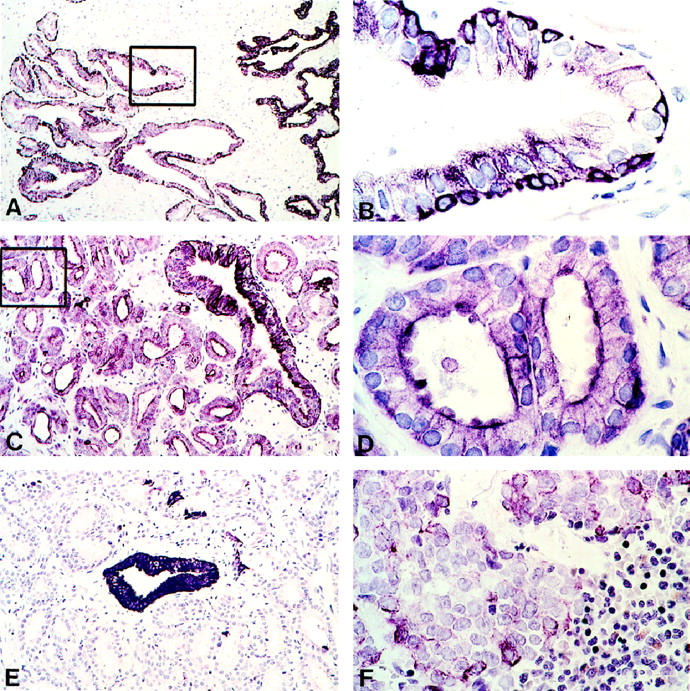
Type 1 IGF-R immunohistochemistry on paraffin sections of PCa in prostate and bone marrow metastases. Sections were stained with mouse anti-human type 1 IGF-R mAb, after antigen retrieval. A and B: Low and high power (boxed area) of nonneoplastic prostate; atrophic glands are also present on the right margin of A. C and D: Low and high power (boxed area) of primary PCa. E: Another area of PCa from the same biopsy as in C and D. F: Bone marrow biopsy containing PCa (with some normal marrow elements in lower right corner). Original magnifications, A, ×90; B, D, ×660; C, E, ×140; F, ×420.
Discussion
The growth of normal prostate epithelium and primary PCa appears to be influenced by a number of growth factors, many of which function through binding to RTKs. The selective metastatic growth of PCa in bone marrow indicates that this microenvironment may similarly provide crucial growth factors. To identify candidate growth factor-receptor interactions mediating PCa growth in bone marrow, RTK expression by a series of freshly isolated bone marrow metastases was assessed. A total of 8 RTKs were identified by RT-PCR in one or more of the tumor samples.
Expression of αPDGF-R protein by metastatic PCa was confirmed by immunohistochemistry in a series of bone marrow metastases. Further immunohistochemical analyses showed moderate to strong αPDGF-R expression in primary prostate cancers, but weak or absent expression in nonneoplastic prostate epithelium and stroma. Previous studies similarly showed αPDGF-R expression in primary PCa and in prostatic intraepithelial neoplasia (PIN), a potential precursor lesion to PCa, but not in normal prostate or benign prostatic hypertrophy. 14,15 Interestingly, the former study of primary PCa suggested a correlation between αPDGF-R expression and more differentiated tumors. Although a similar observation was made in one of the primary tumors examined here (Table 4 ▶ , patient 2), the consistent finding of αPDGF-R in metastatic tumors demonstrated that αPDGF-R expression was not a marker of more benign disease.
A series of NRTKs were also identified and Jak 1 protein expression was assessed further as it was isolated from each of the biopsies. Immunohistochemical studies indicated that Jak 1 protein was weakly expressed in normal prostate luminal epithelial cells and suggested increased expression in primary and metastatic tumor cells. Jak 1 functions to transmit activation signals from multiple cytokine receptors. 30,31 These observations indicate that Jak 1-linked cell surface receptors, possibly the IL-6 receptor or other receptors yet to be identified, function in normal prostate epithelial cells and may contribute to neoplastic growth.
An analysis of tyrosine kinase expression by the LNCaP cell line was also carried out, although it was less extensive then that of the patient tumor samples and certainly identified only a fraction of the kinases expressed by LNCaP. Nonetheless, a number of kinases were found in both the patient samples and in LNCaP. These included eph1, FER, and Bmx. Eph1 and Bmx were also identified previously in a tyrosine kinase screen of the CWR22 prostate cancer cell line, 32 suggesting a consistent role for these kinases in PCa. Bmx was recently reported to be an effector of IL-6 and phosphatidylinositol 3 kinase (PI3-kinase) in LNCaP cells. 27 Preliminary immunohistochemical studies have confirmed eph1 expression by prostate epithelial cells (T. Li and S. Balk, unpublished findings), but further studies are clearly necessary to confirm the cellular origin of other kinases identified in this screen. This is particularly the case for KDR, Tie-1, and Tie-2, which are generally expressed on endothelial cells.
One significant difference between LNCaP and the metastatic PCa biopsies was cloning of the type 1 IGF-R from LNCaP, and the failure to amplify this transcript from the PCa bone marrow biopsies. Loss of the type 1 IGF-R in metastatic androgen-independent PCa was further confirmed by immunohistochemistry. This loss was surprising as signal transduction by the type 1 IGF-R, through PI3-kinase and Akt, provides critical survival signals for many cells, 33 and recent studies have linked increased IGF-1 levels to PCa. 34-36 The importance of this pathway in prostate cancer is further supported by the frequent loss in PCa of PTEN, a downstream negative regulator of PI3-kinase. 37-42 Antisense RNA to the type 1 IGF-R has also been shown to directly suppress the growth and metastatic potential of prostate cancer cells. 43-44
Nonetheless, a previous in situ hybridization and immunohistochemical study that quantitated type 1 IGF-R expression found a moderate decrease in primary PCa relative to normal epithelium. 45 Decreased type 1 IGF-R expression was also reported in more tumorigenic SV40 T antigen-transformed human prostate epithelial cells, 46 and restoring expression in these cells to normal levels by retroviral infection led to decreased tumorigenicity and enhanced apoptosis. 47,48 In the mouse SV40 T antigen-induced PCa model, decreased type 1 IGF-R expression was recently reported in metastatic and androgen-independent tumors. 49 Although it is not yet clear to what extent these latter observations in SV40 T antigen-transformed tumors reflect SV40 T antigen versus PCa biology, they support the conclusion that loss of type 1 IGF-R receptor contributes to human metastatic androgen-independent PCa.
The molecular basis for selective pressure against type 1 IGF-R expression in metastatic androgen-independent PCa has not been determined. It is also unclear to what extent type 1 IGF-R loss is associated with androgen independence versus metastatic growth. We propose that loss of type 1 IGF-R may be linked to PTEN loss during PCa progression. This hypothesis implies that overstimulation of the type 1 IGF-R signal transduction pathway in cells that have lost PTEN, a negative regulator of this pathway, may be deleterious. It should be noted that this requirement for type 1 IGF-R loss cannot be absolute, as the LNCaP cell line has lost PTEN and still expresses the type 1 IGF-R. In any case, these results indicate that IGF-1, although possibly playing a role in prostate cancer development, does not contribute to the growth of advanced metastatic androgen-independent PCa. The results similarly suggest that fibroblast growth factors, which may contribute to PCa development, do not stimulate the growth of advanced metastatic androgen-independent PCa.
The identification of tyrosine kinases that regulate PCa growth in bone marrow is significant as bone marrow metastases account for the vast majority of PCa morbidity and mortality. Moreover, specific tyrosine kinase antagonists are being developed that could be useful therapeutically. This study identified a series of tyrosine kinases that may regulate the growth of advanced androgen-independent PCa. Unfortunately, model systems that directly test the importance of these kinases in PCa metastatic to bone marrow are not readily available, and the development of such systems clearly represents a major future challenge. Nonetheless, based upon the evidence presented here, we have initiated clinical trials of a PDGF-R antagonist in metastatic androgen-independent PCa that may help to address the role of this particular receptor. Finally, if signaling through particular RTKs can inhibit tumor growth or stimulate apoptosis, then activation of these pathways may represent another novel therapeutic approach in PCa.
Footnotes
Address reprint requests to Steven P. Balk, Hematology-Oncology Division, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215. E-mail: sbalk@caregroup.harvard.edu.
Supported by National Institutes of Health grants CA-65647 to S. P. B. and CA-70297 to Z. S., by an American Cancer Society grant to G. J. B. (EDT-112), and by the Hershey Family Prostate Cancer Research Fund. D. M. was supported by an NIH Hematology Career Training Program grant (HL07516).
Equally important contributions to this work were made by the first two authors.
Current address of Z. Sun: Department of Surgery and Genetics, Stanford University School of Medicine, Stanford, CA.
References
- 1.Haq M, Goltzman D, Tremblay G, Brodt P: Rat prostate adenocarcinoma cells disseminate to bone and adhere preferentially to bone marrow-derived endothelial cells. Cancer Res 1996, 52:4613-4619 [PubMed] [Google Scholar]
- 2.Chackal-Roy M, Niemeyer C, Moore M, Zetter BR: Stimulation of human prostatic carcinoma cell growth by factors present in human bone marrow. J Clin Invest 1989, 84:43-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW: Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res 1991, 51:3753-3761 [PubMed] [Google Scholar]
- 4.Gleave ME, Hsieh JT, von Eschenbach AC, Chung LW: Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol 1992, 147:1151-1159 [DOI] [PubMed] [Google Scholar]
- 5.Rossi MC, Zetter BR: Selective stimulation of prostatic carcinoma cell proliferation by transferrin. Proc Natl Acad Sci USA 1992, 89:6197-6201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh JT, Wu HC, Gleave ME, von Eschenbach AC, Chung LW: Autocrine regulation of prostate-specific antigen gene expression in a human prostatic cancer (LNCaP) subline. Cancer Res 1992, 53:2852-2857 [PubMed] [Google Scholar]
- 7.Pisters LL, Troncoso P, Zhau HE, Li W, von Eschenbach AC, Chung LW: c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol 1995, 154:293-298 [PubMed] [Google Scholar]
- 8.Humphrey PA, Zhu X, Zarnegar R, Swanson PE, Ratliff TL, Vollmer RT, Day ML: Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol 1995, 147:386-396 [PMC free article] [PubMed] [Google Scholar]
- 9.Sherwood ER, Lee C: Epidermal growth factor-related peptides and the epidermal growth factor receptor in normal and malignant prostate. World J Urol 1995, 13:290-296 [DOI] [PubMed] [Google Scholar]
- 10.Peehl DM, Cohen P, Rosenfeld RG: The insulin-like growth factor system in the prostate. World J Urol 1995, 13:306-311 [DOI] [PubMed] [Google Scholar]
- 11.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL: Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol 1992, 6:2123-2128 [DOI] [PubMed] [Google Scholar]
- 12.Peehl DM, Rubin JS: Keratinocyte growth factor: an androgen-regulated mediator of stromal-epithelial interactions in the prostate. World J Urol 1995, 13:312-317 [DOI] [PubMed] [Google Scholar]
- 13.Story MT: Regulation of prostate growth by fibroblast growth factors. World J Urol 1995, 13:297-305 [DOI] [PubMed] [Google Scholar]
- 14.Fudge K, Wang CY, Stearns ME: Immunohistochemistry analysis of platelet-derived growth factor A and B chains and platelet-derived growth factor alpha and beta receptor expression in benign prostatic hyperplasias and Gleason-graded human prostate adenocarcinomas. Mod Pathol 1994, 7:549-554 [PubMed] [Google Scholar]
- 15.Fudge K, Bostwick DG, Stearns ME: Platelet-derived growth factor A and B chains and the alpha and beta receptors in prostatic intraepithelial neoplasia. Prostate 1996, 29:282-286 [DOI] [PubMed] [Google Scholar]
- 16.Pflug BR, Dionne C, Kaplan DR, Lynch J, Djakiew D: Expression of a Trk high affinity nerve growth factor receptor in the human prostate. Endocrinology 1995, 136:262-268 [DOI] [PubMed] [Google Scholar]
- 17.Wilks AF: Cloning members of protein-tyrosine kinase family using polymerase chain reaction. Methods Enzymol 1991, 200:533-546 [DOI] [PubMed] [Google Scholar]
- 18.Taplin ME, Bubley JG, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP: Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med 1995, 332:1393-1398 [DOI] [PubMed] [Google Scholar]
- 19.Taplin ME, Bubley GJ, Ko Y-J, Small EJ, Upton M, Rajeshkumar B, Balk SP: Selection for androgen receptor mutations in prostate cancers treated with antiandrogen. Cancer Res 1999, 59:2511-2515 [PubMed] [Google Scholar]
- 20.Horoszewisz JS, Leong SS, Kawanski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP: LNCaP model of prostatic carcinoma. Cancer Res 1983, 43:1809-1818 [PubMed] [Google Scholar]
- 21.Hanks SK, Quinn AM, Hunter T: The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 1988, 241:42-52 [DOI] [PubMed] [Google Scholar]
- 22.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E: The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol 1992, 41:665-669 [DOI] [PubMed] [Google Scholar]
- 23.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW: Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res 1994, 54:2577-2581 [PubMed] [Google Scholar]
- 24.Iwamura M, Sluss PM, Casamento JB, Cockett AT: Insulin-like growth factor I: action and receptor characterization in human prostate cancer cell lines. Prostate 1993, 22:243-252 [DOI] [PubMed] [Google Scholar]
- 25.Pietrzkowski Z, Mulholland G, Gomella L, Jameson BA, Wernicke D, Baserga R: Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Res 1993, 53:1102-1106 [PubMed] [Google Scholar]
- 26.Kimura G, Kasuya J, Giannini S, Giannini S, Honda Y, Mohan S, Kawachi M, Akimoto M, Fujita-Yamaguchi Y: Insulin-like growth factor (IGF) system components in human prostatic cancer cell-lines: LNCaP, DU145, and PC-3 cells. Int J Urol 1996, 3:39-46 [DOI] [PubMed] [Google Scholar]
- 27.Qiu Y, Robinson D, Pretlow TG, Kung HJ: Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA 1998, 95:3644-3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL: Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol 1993, 13:4513-4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carstens RP, Eaton JV, Krigman HR, Walther PJ, Garcia-Blanco MA: Alternative splicing of fibroblast growth factor receptor 2 (FGF-R2) in human prostate cancer. Oncogene 1997, 15:3059-3065 [DOI] [PubMed] [Google Scholar]
- 30.Briscoe J, Guschin D, Muller M: Signal transduction: just another signalling pathway. Curr Biol 1994, 4:1033-1035 [DOI] [PubMed] [Google Scholar]
- 31.Schindler C, Darnell JE, Jr: Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 1995, 64:621-651 [DOI] [PubMed] [Google Scholar]
- 32.Robinson D, He F, Pretlow T, Kung HJ: A tyrosine kinase profile of prostate carcinoma. Proc Natl Acad Sci USA 1996, 93:5958-5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantley LC, Neel BG: New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 1999, 96:4240-4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantzoros CS, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami HO: Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br J Cancer 1997, 76:1115-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M: Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 1998, 279:563-566 [DOI] [PubMed] [Google Scholar]
- 36.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D: Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst 1998, 90:911-915 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R: PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275:1943-1947 [DOI] [PubMed] [Google Scholar]
- 38.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV: Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997, 15:356-362 [DOI] [PubMed] [Google Scholar]
- 39.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D: Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 1997, 57:4997-5000 [PubMed] [Google Scholar]
- 40.Pesche S, Latil A, Muzeau F, Cussenot O, Fournier G, Longy M, Eng C, Lidereau R: PTEN/MMAC1/TEP1 involvement in primary prostate cancers. Oncogene 1998, 16:2879-2883 [DOI] [PubMed] [Google Scholar]
- 41.Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL: Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA 1998, 95:5246-5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J: Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res 1998, 58:2720-2723 [PubMed] [Google Scholar]
- 43.Long L, Rubin R, Baserga R, Brodt P: Loss of the metastatic phenotype in murine carcinoma cells expressing an antisense RNA to the insulin-like growth factor receptor. Cancer Res 1995, 55:1006-1009 [PubMed] [Google Scholar]
- 44.Burfeind P, Chernicky CL, Rininsland F, Ilan J: Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci USA 1996, 93:7263-7268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tennant MK, Thrasher JB, Twomey PA, Drivdahl RH, Birnbaum RS, Plymate SR: Protein and messenger ribonucleic acid (mRNA) for the type 1 insulin-like growth factor (IGF) receptor is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to benign prostate epithelium. J Clin Endocrinol Metab 1996, 81:3774-3782 [DOI] [PubMed] [Google Scholar]
- 46.Plymate SR, Tennant M, Birnbaum RS, Thrasher JB, Chatta G, Ware JL: The effect on the insulin-like growth factor system in human prostate epithelial cells of immortalization and transformation by simian virus-40 T antigen. J Clin Endocrinol Metab 1996, 81:3709-3716 [DOI] [PubMed] [Google Scholar]
- 47.Plymate SR, Bae VL, Maddison L, Quinn LS, Ware JL: Reexpression of the type 1 insulin-like growth factor receptor inhibits the malignant phenotype of simian virus 40 T antigen immortalized human prostate epithelial cells. Endocrinology 1997, 138:1728-1735 [DOI] [PubMed] [Google Scholar]
- 48.Plymate SS, Bae VL, Maddison L, Quinn LS, Ware JL: Type-1 insulin-like growth factor receptor reexpression in the malignant phenotype of SV40-T-immortalized human prostate epithelial cells enhances apoptosis. Endocrine 1997, 7:119-124 [DOI] [PubMed] [Google Scholar]
- 49.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM: The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res 1999, 59:2203-2209 [PubMed] [Google Scholar]