Abstract
Kaposi’s sarcoma is a vascular tumor commonly associated with human immunodeficiency virus (HIV)-1 and human herpesvirus (HHV-8) also known as Kaposi’s sarcoma-associated herpesvirus. The principal features of this tumor are abnormal proliferation of vascular structures lined with spindle-shaped endothelial cells. HHV-8 may transform a subpopulation of endothelial cells in vitro via viral and cellular gene expression. We hypothesized that among the cellular genes, vascular endothelial growth factors (VEGFs) and their cognate receptors may be involved in viral-mediated transformation. We have shown that HHV-8-transformed endothelial cells (EC-HHV-8) express higher levels of VEGF, VEGF-C, VEGF-D, and PlGF in addition to VEGF receptors-1, -2, and -3. Furthermore, antibodies to VEGF receptor-2 inhibited cell proliferation and viability. Similarly, inhibition of VEGF gene expression with antisense oligonucleotides inhibited EC-HHV-8 cell proliferation/viability. The growth and viability of primary endothelial cells and a fibroblast cell line however were unaffected by either the VEGF receptor-2 antibody or the VEGF antisense oligodeoxynucleotides. VEGF and VEGF receptors are thus induced in EC-HHV-8 and participate in the transformation. Inhibitors of VEGF may thus modulate the disease process during development and progression.
Kaposi’s sarcoma (KS) is the most common tumor associated with human immunodeficiency virus (HIV)-1 infection. 1-4 Two features of AIDS-KS tumors include aberrant proliferation of vascular structures, proliferation of endothelial and spindle (tumor) cells, and enhanced vascular permeability. Endothelial cell (EC) growth factors are thus likely to play a central role in the development and progression of KS. 5-8 KS cells have previously been shown to produce several growth factors that have autocrine growth activity; these include basic fibroblast growth factor (bFGF), interleukin (IL)-1, IL-6, IL-8, and oncostatin-M. These factors are also shown to be expressed in the primary tumor tissue. 7-13
Vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) is an angiogenic factor that induces EC proliferation, angiogenesis, and enhances vascular permeability. 14-16 VEGF/VPF receptors are localized primarily to the ECs. KS cells however also express VEGF and VEGF receptors (VEGFR) and use VEGF as an autocrine growth factor. 17 The role of VEGF in the pathogenesis of KS may thus be significant. Several VEGF-related proteins have been isolated by homology search and include VEGF-B, VEGF-C, VEGF-D, and PlGF (placental growth factor). 18-21 VEGF-C and VEGF-D are expressed primarily in lymphatic endothelium and bind to VEGFR-3 as homodimers and to VEGFR-2 and VEGFR-3 as heterodimers with VEGF. VEGF-C and VEGFR-3 expression in KS cells have also been shown. 22 These results are consistent with the consideration that KS may be derived from lymphatic endothelium.
Isolation of Kaposi’s sarcoma-associated herpesvirus/HHV-8 from KS tumor tissue and evidence for latent infection of KS spindle cells supports its role in KS pathogenesis. 23,24 Transformation of ECs with HHV-8 may suggest the role of this virus in the development of KS. 25 In this study, we examined the VEGFs in HHV-8-transformed cells. We show that expression of VEGF, VEGF-C, VEGF-D, PlGF, and their receptors is higher in transformed cells than in primary ECs. Furthermore, inhibition of VEGF binding to the cognate receptors or inhibition of VEGF expression reduces the proliferation and viability of HHV-8-transformed ECs. These studies indicate that induction of VEGF may be one of the ways that HHV-8 plays a role in KS pathogenesis.
Materials and Methods
Cell Lines
Human umbilical vein ECs and ECs transformed with HHV-8 (EC-HHV-8) were maintained as described. 25 The production of EC-HHV-8 has been described previously. 25 Briefly, purified HHV-8 virus particles were isolated from an EBV-negative primary effusion lymphoma cell line (BC-3). Human umbilical vein ECs were infected with 5 to 10 genome equivalents/cell and cultured in the presence of VEGF. These cultures have been continuously maintained for more than 4 years. ECs were grown on gelatin (1%)-coated flasks in Iscove-modified Dulbecco’s media and F-12 Nutrient Mixture (Ham) (1:1) media supplemented with 15% fetal calf serum, 2 mmol/L glutamine, 30 μg/ml EC growth supplement (Boehringer Mannheim, Indianapolis, IN), 2 U/ml heparin, 100 U/ml penicillin, and 100 μg/ml streptomycin. No supplemental VEGF was added. T1 fibroblast cultures were obtained from Dr. Peter Jones, USC/Norris Comprehensive Cancer Center, and were grown in Dulbecco’s minimal essential medium containing 10% fetal calf serum, penicillin, and streptomycin. KSC-10 is a long-term spindle cell isolate established from KS lesions of an AIDS-KS patient as previously described. 26 It has been maintained in RPMI 1640 medium supplemented with 15% fetal calf serum, 2 mmol/L glutamine, 0.5% essential amino acids, 0.5% nonessential amino acids, 1 mmol/L sodium pyruvate, and 1% Nutridoma HU (Boehringer Mannheim) in the absence of conditioned medium from transformed T cell lines.
Materials
Neutralizing antibody to VEGFR-2 and polyclonal antibodies to VEGFR-1 and VEGFR-3 were obtained from R&D Systems (Minneapolis, MN). Phosphorothioate-modified oligonucleotides were synthesized and purified by Operon Technologies, Inc. (Alameda, CA). VEGF antisense (AS) oligonucleotides of the human VEGF-coding region shown previously to inhibit VEGF were used. 17 The sequence and location of AS-1 and AS-3 oligonucleotide are: AS-1, 5′-AGA CAG CAG AAA GTT CAT GGT-3′ (−3 to +18); AS-3, 5′-TGG CTT GAA GAT GTA CTC GAT-3′ (+261 to +281). An oligonucleotide consisting of a scrambled AS-3 sequence (S) 5′-TAC GTA GTA TGG TGT ACG ATC-3′ was used as a negative control. rhVEGF and VEGF enzyme-linked immunosorbent assay kits were purchased from R&D Systems.
Cell Viability Assay
Cells (EC, EC-HHV-8) were seeded at a density of 1 × 10 4 per well in 24-well gelatin-coated plates on day 0. For experiments with antibodies, the cells were treated on day 1 at concentrations ranging from 10 to 1000 ng/ml and the cell viability was measured on day 3 by MTT assay. For experiments with oligonucleotides, cells were treated on days 1 and 3 at concentrations ranging from 1 to 10 μmol/L on, and the cell viability was measured on day 5 by MTT assay. The assays were performed in triplicate.
Amplification of Human VEGF/VEGFR mRNA Using Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Cells were plated as described, harvested, and total RNA was extracted. cDNAs were synthesized using a Superscript II kit (Life Technologies, Inc., Gaithersburg, MD) by standard protocols. Two μl of the cDNA reaction were amplified by RT-PCR for the VEGF family members, and as described earlier. 17 Amplification of the receptors was from 4 μl of cDNA. Primers for the amplification of VEGF, VEGF-B, VEGF-C, VEGF-D, PlGF, and the receptors VEGFR-1, VEGFR-2, and VEGFR-3 are shown in Table 1 ▶ . Each PCR cycle consisted of denaturation at 94°C for 1 minute, primer annealing at the temperatures indicated in Table 1 ▶ for 2 minutes, and extension at 72°C for 3 minutes. The samples were amplified for 30 cycles, 10-μl aliquots of PCR reaction mixtures were resolved by 1.5% agarose gel electrophoresis. The integrity and quantity of RNA was confirmed by RT-PCR for β-actin. RT-PCR reactions for the receptors were modified by increasing the primer concentration to 100 pmol.
Table 1.
Sequences of Oligonucleotide Primers Used for RT-PCR
| Gene | Primer sequences (forward and reverse 5′ to 3′) | Position | Size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| VEGF-A | 5′-CGA AGT GGT GAA GTT CAT GGA TG-3′ | +170/+192 | 607, 535, 403 | 60 |
| 5′-TTC TGT ATC AGT CTT TCC TGG TGA G-3′ | +681/+705 | |||
| VEGF-B | 5′-TGG CCA AAC AGC TGG TGC-3′ | +191/+208 | 411 | 55 |
| 5′-GAG GAA GCT GCG GCG TCG-3′ | +585/+602 | |||
| VEGF-C | 5′-GAT CTG GAG GAG CAG TTA CGG TC-3′ | +263/+285 | 294 | 60 |
| 5′-TTA AG AAG CTG TTT GTC GCG ACT-3′ | +535/+557 | |||
| VEGF-D | 5′-TTG TAC GTC CAG CTG GTG CAG′ | +40/+60 | 320 | 60 |
| 5′-CTC CAC GCA CGT TTC TCT AGG-3′ | +337/+357 | |||
| PlGF | 5′-ATG AGG CTG TCC CCT TGC TTC-3′ | +10/+30 | 388 | 60 |
| 5′-AGA GGC CGG CAT TCG CAG CGA A-3′ | +326/+398 | |||
| VEGFR-1 | 5′-CAA GTG GCC AGA GGC ATG GAG TT-3′ | +3262/+3284 | 498 | 62 |
| 5′-GAT GTA GTC TTT ACC ATC CTG TTG-3′ | +3736/+3759 | |||
| VEGFR-2 | 5′-GAG GGC CTC TCA TGG TGA TTG T-3′ | +2954/+2975 | 709 | 62 |
| 5′-TGC CAG CAG TCC AGC ATG GTC TG-3′ | +3640/+3662 | |||
| VEGFR-3 | 5′-GTG ACA GCC TGT CCA TCT CCT-3′ | +131/+151 | 320 | 60 |
| 5′-GGT TGA CCA CGT TGA GGT G-3′ | +431/+451 | |||
| β-actin | 5′-GTG GGG CGC CCC AGG CAC CA-3′ | 546 | 55 | |
| 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′ |
Immunocytochemistry for VEGF Receptors
Cells were collected onto glass slides using a Cytospin centrifuge (Shandon, Astmoor, UK) and fixed in acetone for 5 minutes. Slides were incubated with the primary rabbit antibodies against either VEGFR-1 or VEGFR-2 (1:100) at 4°C overnight. Isotype-specific rabbit IgG was used as control. The immunoreactivity for these receptors was revealed using an avidin-biotin kit from Vector Laboratories (Burlingame, CA). Peroxidase activity was revealed by the diaminobenzidine (Sigma-Aldrich, St. Louis, MO) cytochemical reaction. The slides were then counterstained with 0.12% methylene blue or hematoxylin and eosin.
Results
HHV-8-Transformed ECs Express VEGF, VEGF-C, VEGF-D, and PlGF
Supernatants from equal numbers of EC-HHV-8, ECs, KS primary isolate (KSC-10), and fibroblast (T1) (1 × 10 6 cells per six-well plate) were cultured for 24 hours in the absence of VEGF or other growth factors and the supernatant VEGF levels were measured by enzyme-linked immunosorbent assay. The levels of VEGF protein were substantially higher in EC-HHV-8 cells than in ECs or the T1 fibroblast cell line but were comparable to a KS isolate, KSC-10 (Figure 1A) ▶ . We then examined the gene expression of various VEGF family members by RT-PCR (Figure 1B) ▶ . VEGF, VEGF-C, VEGF-D, and PlGF were expressed in EC-HHV-8 but not seen in ECs. VEGF-B expression was not observed in either cell type (Figure 1B) ▶ . Low input in this cDNA may explain the discrepancy between these results and our earlier findings of VEGF expression detected by RT-PCR in ECs. 17 However, induction of most of the VEGF family member gene expression is observed in the presence of HHV-8. These results suggest that HHV-8 induces the expression of VEGF, VEGF-C, and VEGF-D and PlGF in ECs. We have previously shown that HHV-8 viral G-protein coupled receptor induces VEGF expression in fibroblasts. 27 However, these data cannot rule out the role of latency associated gene regulation of VEGFs.
Figure 1.
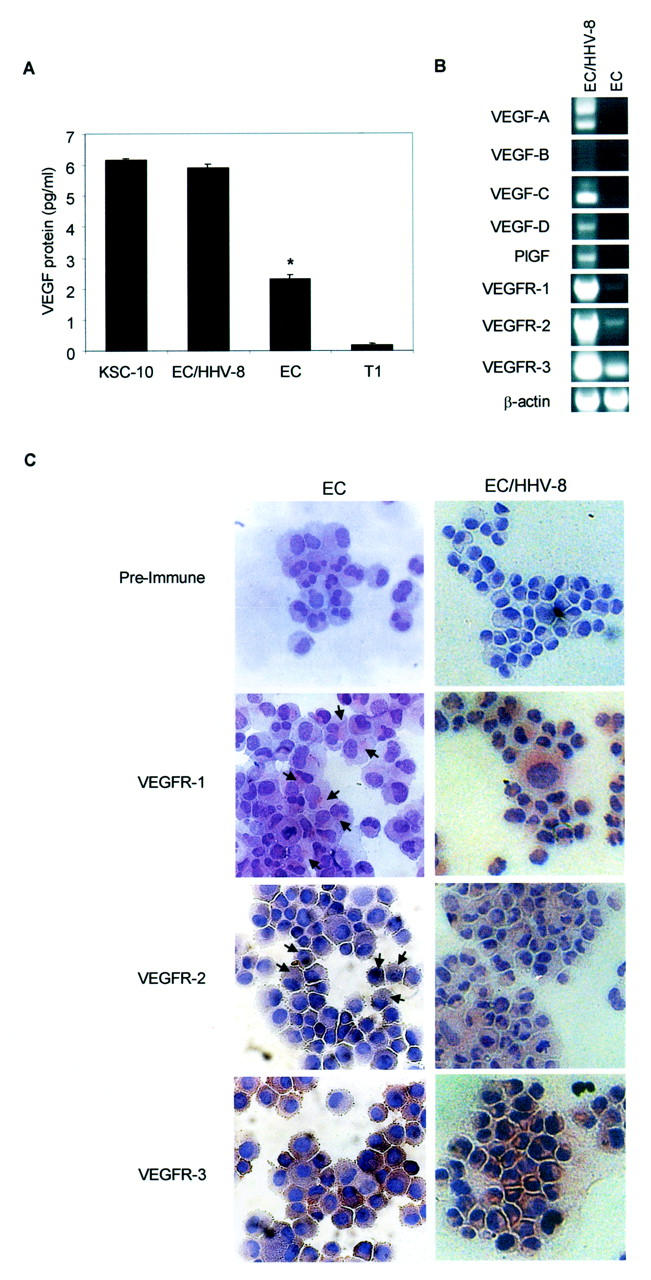
Expression of VEGF family proteins and their receptors in EC-HHV-8. A: Twenty-four hours of VEGF secretion by 1 × 10 6 cells of a KS cell isolate (KSC-10), EC/HHV-8, ECs, and T1 fibroblasts. Cells were cultured in the absence of growth factors, including VEGF. Results shown are mean ± SE of an experiment performed in quadruplicate. The asterisk indicates a significant difference between VEGF production in EC/HHV-8 and ECs of P = 0.0015 by Student’s t-test. There was no significant difference in VEGF production by KSC-10 and EC/HHV-8 (P = 0.155). B: RT-PCR of EC/HHV-8 and ECs for VEGF family member genes. An equal amount of total RNA from each cell line was reverse-transcribed to generate cDNA. Gene-specific primers as shown in Table 1 ▶ were used to amplify all VEGF family members (VEGF-A, -B, -C, and -D, PlGF). VEGFR-1, VEGFR-2, and VEGFR-3 were also amplified using paired primers (see Table 1 ▶ ) from the same cDNA. All bands detected were confirmed to be the correct size. Integrity and amount of cDNA added to each reaction was confirmed by a parallel amplification of β-actin. C: Immunocytochemical analysis for VEGFR-1, VEGFR-2, and VEGFR-3 in primary ECs and EC/HHV-8 cells was performed using standard techniques described in Material and Methods. Positive stain is indicated by brown color. Strong stain is seen for all receptors in EC/HHV8 and for VEGFR3 in primary ECs. Arrows indicate representative positive stain for VEGFR-1 and -2 in the weakly positive ECs. Control (preimmune serum) showed no color development.
Expression of VEGF Receptors Is Increased in EC-HHV-8
Because the EC-HHV-8 cells express most VEGF family members, and ECs express the VEGF receptors, we considered the possibility that VEGF is an autocrine growth factor for EC-HHV-8 cells. The expression of VEGF receptors was examined. Because receptors are typically low-copy number mRNAs, twice the input cDNA was used in these PCR reactions than was used for the VEGF ligand molecules. By RT-PCR the receptor tyrosine kinases VEGFR-1, VEGFR-2, and VEGFR-3 were strongly expressed in EC-HHV-8 cells. Although the gene expression for all three receptors was detected in ECs, the level of expression was lower in all cases (Figure 1B) ▶ . The integrity of the mRNA was confirmed by the amplification of β-actin. Expression of VEGF receptors was also evaluated by immunocytochemistry. Expression of all three receptors was detected in the primary ECs, however, only VEGFR-3 expression was apparent in the majority of ECs with very low signal for VEGFR-1 and VEGFR-2 (Figure 1C ▶ , left-hand column). Arrows point to representative staining for VEGFR-1 and VEGFR-2 in the primary ECs. Note that although not quantitative, the RT-PCR results indicating mRNA levels agree with the relative levels of expression of the receptor proteins obtained by immunocytochemistry. For the EC-HHV-8 cells, in contrast, strong staining for all three VEGF receptors was evident (Figure 1C ▶ , right-hand column). Both RT-PCR and immunocytochemistry confirm the robust expression of VEGF receptors in HHV-8-transformed ECs. It should be noted that HHV-8 was present in only 1 to 5% of the total cell population. 25
VEGF Is an Autocrine Growth Factor for EC-HHV-8
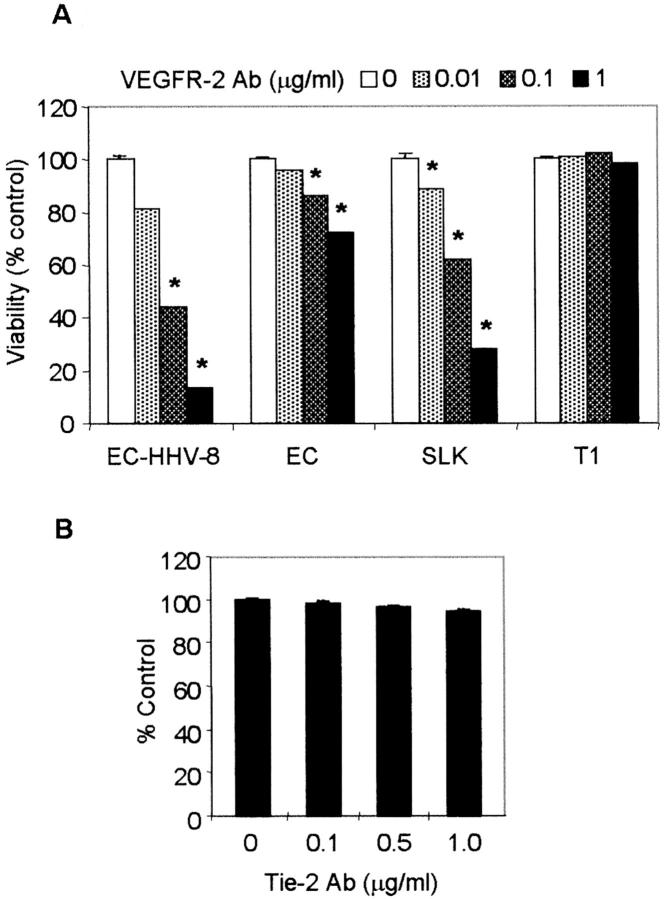
We next wished to determine whether the endogenous production of VEGF can induce proliferation of HHV-8-transformed ECs through VEGFR-2. A dose-dependent inhibition of EC-HHV-8 cell proliferation was observed in response to treatment with neutralizing antibody to VEGFR-2 (Figure 2A) ▶ . Similarly, a KS cell line (KS-SLK) showed significant inhibition of cell proliferation consistent with autocrine growth factor activity of VEGF in KS. However, the effect of the antibody was minimal in T1 fibroblasts and modest in primary ECs (Figure 2A) ▶ . Antibody to another EC-specific receptor tyrosine kinase, tie-2, had no effect on HHV-8-transformed ECs (Figure 2B) ▶ . These results strongly support the consideration that induction of VEGF in EC-HHV-8 cell cultures plays an important role in cell proliferation and viability.
Figure 2.
Effect of VEGFR-2-neutralizing antibody on cell viability. A: EC, HHV-8-transformed EC (EC-HHV-8), KS (SLK), and fibroblast (T1) cell lines (1 × 10 4 cells/well) were seeded into gelatin-coated 24-well plates and treated with 0, 0.01, 0.1, or 1 μg/ml of VEGFR-2 antibody on days 1 and 3. Asterisks indicate a significant difference (P < 0.05, Student’s t-test) between controls and antibody treatment for each cell type except T1 fibroblasts. B: Effect of Tie-2 antibody on the growth of EC-HHV-8 was examined as above. Shown are the cell viabilities on day 5 determined by MTT. Results are the mean ± SD of triplicate experiments.
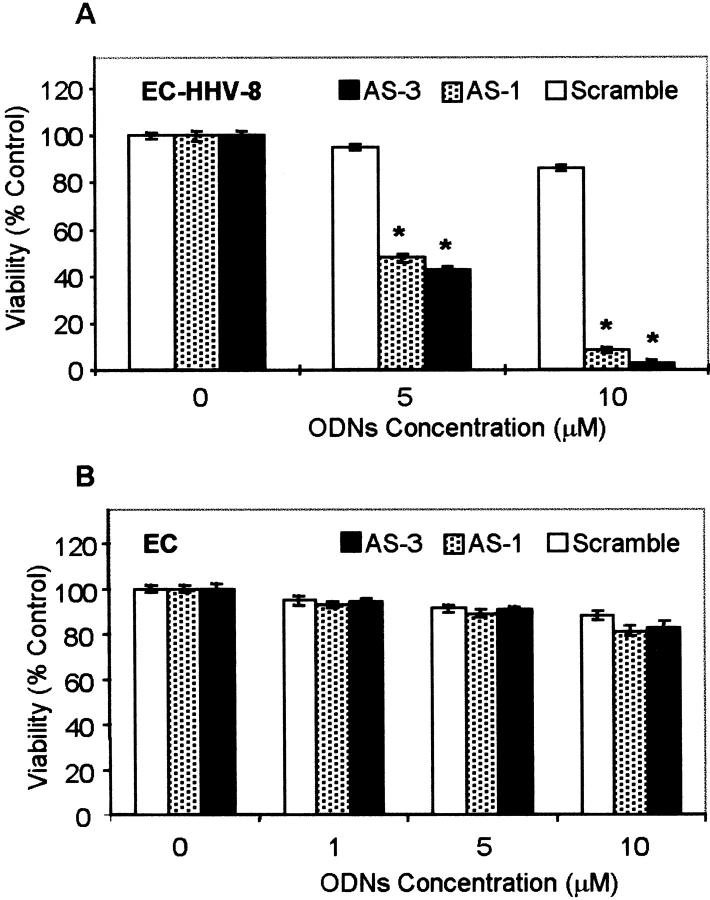
VEGF AS oligonucleotides (AS-1 and AS-3) have previously been shown to specifically inhibit VEGF expression and in turn inhibit proliferation of a number of cell types that express VEGF and VEGF receptors. 17,28 These include KS, melanoma, and ovarian carcinoma cell lines. We thus wished to determine whether inhibition of VEGF expression would similarly inhibit the proliferation of HHV-8-transformed ECs. Treatment with AS-1 and AS-3 led to a dose-dependent inhibition of EC-HHV-8 with minimal effect on ECs (Figure 3) ▶ . The IC50 of AS-1 and AS-3 oligonucleotides were less than 5 μmol/L. The scrambled oligonucleotide (base composition corresponds to AS-3 had minimal inhibitory effect on primary ECs or EC-HHV-8 (Figure 3) ▶ .
Figure 3.
Effect of VEGF AS oligonucleotides on cell growth. EC/HHV-8 (A) and ECs (B) (10 4 cells/well in 24-well plates) were treated with the phosphorothioate oligonucleotides AS-1, AS-3, and scrambled at concentrations ranging from 1 to 10 μmol/L on days 1 and 3. Cell viability was measured by MTT assay on day 5. Data represent the mean ± SD of two separate experiments performed in quadruplicate. Asterisks indicate a highly significant difference (P < 0.01, Student’s t-test) between viability in control and cells treated with either AS-1 or AS-3. No significant difference was found in the viabilities of cells treated with the scrambled oligodeoxyribonucleotide compared to untreated control (P = 0.331 and 0.066 for 5 μmol/L and 10 μmol/L, respectively).
Discussion
VEGF is a mitogen for ECs that is required for both vasculogenesis and angiogenesis. 29 The closely related VEGF-C and VEGF-D molecules have also been shown to be mitogenic for ECs, especially lymphatic ECs. 30 VEGF and VEGF-C expression has been detected in KS spindle cells in tumor samples. 22 Now we show that ECs infected with HHV-8 express VEGF, VEGF-C, VEGF-D, and PlGF, in contrast to untransformed ECs that did not express the VEGF family mRNAs in our hands.
VEGF receptors are highly restricted in their expression to ECs. 31,32 Although VEGF binds both VEGFR-1 and VEGFR-2, only binding to VEGFR-2 generates mitogenic signal in ECs. 33,34 However, binding of VEGF to VEGFR-1 seems to be necessary for high-affinity binding to VEGFR-2. The expression of both of these receptors was markedly increased in EC-HHV-8 compared to ECs. Furthermore, VEGF-C and VEGF-D bind to VEGFR-3 on lymphatic ECs. Expression of VEGFR-3 was also strongly up-regulated in EC-HHV-8 compared to primary ECs. Thus HHV-8 seems to induce the expression of both vascular and lymphatic EC-specific growth factors and their receptors.
We determined that the VEGFs secreted from the HHV-8-infected cells could act in an autocrine/paracrine manner. Blocking the binding site for the secreted VEGF (VEGFR-2) using neutralizing antibody resulted in a decrease in cell viability. In addition, blocking expression of VEGF in the EC-HHV-8 culture system by the AS-1 and AS-3 VEGF-specific AS oligonucleotides also resulted in decreased cell viability. Removal of either ligand (VEGF) or receptor (VEGFR-2) activity resulted in reduced viability thus demonstrating a functional autocrine loop in this culture system.
From the gene expression studies we conducted it is clear that HHV-8-mediated EC transformation involves the regulation of VEGF family proteins that are ligands for receptor tyrosine kinases restricted to the ECs.
In support of this, several recent reports indicate that some viral genes can regulate the expression of VEGF. The first of these is the vGPCR, which is a constitutively active broad specificity CXC chemokine receptor. Activity of this receptor can be induced by IL-8 and growth-related oncogene-α and repressed by interferon inducible protein (IP)-10 and stromal cell-derived factor-1α. 35,36 The vGPCR can transform fibroblasts, which were also tumorigenic in nude mice. 27,37 This transformation was also associated with activation of VEGF. vGPCR seems to enhance the expression of VEGF through phosphorylation of hypoxia inducible factor (HIF)-1α to in turn activate transcription of VEGF. 38 Phosphorylation of HIF-1α by both the p38 and MAPK pathways was found to be involved in the vGPCR-mediated induction of VEGF expression in this system. Further, ectopic expression of vGPCR has been found to protect human umbilical vein ECs against apoptosis induced by serum starvation; however, this was independent of VEGF. 39 In addition to its effects on VEGF expression, the vGPCR has been shown to be involved in the pathogenesis of KS because vGPCR transgenic mice develop angioproliferative lesions with the hallmarks of KS. 40
Another virally encoded gene, vIL-6, up-regulates VEGF expression when expressed ectopically in murine fibroblasts. 41 The vIL-6 has 62.2% sequence similarity to the human protein, and retains the four conserved cysteines found in all IL-6 proteins. 42,43 One important difference between the actions of vIL-6 and cellular IL-6 is that the vIL-6 signals directly through the gp130 subunit of the IL-6 receptor complex and does not first bind to the IL-6Rα subunit, which is a prerequisite of cellular IL-6 signaling through gp130. 44
Both the vGPCR and vIL-6 transcripts are present in only a small number of cells in KS lesions compared to the widespread distribution of the latent transcripts for Kaposin or LANA. 42,45,46 Therefore, it is unlikely that either vGPCR or vIL-6 directly transform spindle cells in vivo because expression as lytic genes would be transient and occurs in a cell population destined for imminent death. However, both of these viral genes could affect KS pathogenesis through contributions to angiogenesis and inflammatory cell infiltration.
Our demonstration that HHV-8 induces the VEGF family proteins and their receptors in ECs, and that a functional autocrine pathway is present, underscores the importance of VEGF in this disease. It is clear that the VEGFs and VEGF receptors are unique targets for the treatment of KS. As a result, various inhibitors of VEGF and VEGF receptors are under clinical investigation.
Footnotes
Address reprint requests to Rizwan Masood, Ph.D., USC/Norris Comprehensive Cancer Center Rm. 6330, 1441 Eastlake Ave., MC 9176, Los Angeles, CA 90033. E-mail: masood@hsc.usc.edu.
Supported in part by the National Cancer Institute (grant CA79318 to P. S. G.), the University of California (grant UARP K99 USC-054 to R. M.), The Ezralow Family Trust (to P. S. G.), and the New York University Center for AIDS Research (to O. F.).
References
- 1.Safai B, Johnson KG, Myskowski PL, Cunningham-Randles S, Godbold JH, Dupont B: The natural history of Kaposi’s sarcoma in the acquired immunodeficiency syndrome. Ann Intern Med 1985, 103:744-750 [DOI] [PubMed] [Google Scholar]
- 2.Haverkos HW, Drotman DP, Morgan M: Prevalence of Kaposi’s sarcoma among patients with AIDS. N Engl J Med 1985, 312:1518-1523 [PubMed] [Google Scholar]
- 3.Ruszczak Z, Silva AM, Orfanos CE: Kaposi’s sarcoma in AIDS. Am J Dermatopathol 1987, 9:388-398 [PubMed] [Google Scholar]
- 4.Regezi JA, Macphail LA, Daniels TE, De Souza YG, Greenspan JS, Greenspan D: Human immunodeficiency virus-associated oral Kaposi’s sarcoma: a heterogeneous cell population dominated by spindle-shaped endothelial cells. Am J Pathol 1993, 143:240-249 [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura S, Salahuddin SZ, Biberfeld P, Ensoli B, Markham PD, Wong-Staal F, Gallo RC: Kaposi’s sarcoma cells: long term culture with growth factor retrovirus-infected CD4+ T cells. Science 1988, 242:426-430 [DOI] [PubMed] [Google Scholar]
- 6.Salahuddin SZ, Nakamura S, Biberfeld P, Ensoli B, Markham PD, Larsson L, Wong-Staal F, Gallo RC: Angiogenic properties of Kaposi’s sarcoma-derived cells after long-term culture in vitro. Science 1988, 242:430-433 [DOI] [PubMed] [Google Scholar]
- 7.Ensoli B, Nakamura S, Salahuddin SZ, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo RC: AIDS Kaposi’s sarcoma derived cells express cytokines with autocrine and paracrine growth effects. Science 1989, 243:223-226 [DOI] [PubMed] [Google Scholar]
- 8.Barillari G, Buonaguro L, Fiorelli V, Hoffman J, Michaels F, Gallo RC, Ensoli B: Effects of cytokines form activated immune cells on vascular cell growth and HIV-1 gene expression: implications for AIDS-Kaposi’s sarcoma pathogenesis. J Immunol 1992, 149:3727-3734 [PubMed] [Google Scholar]
- 9.Miles SA, Rezai AR, Salazar-Gonzales JF, Vander Meyden M, Stevens RH, Logan DM, Mitsuyasu RT, Taga T, Hirano T, Kishimoto T, Martínez-Maza O: AIDS-Kaposi’s sarcoma-derived cells produce and respond to interleukin-6. Proc Natl Acad Sci USA 1990, 87:4068-4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masood R, Cai J, Tulpule A, Zheng T, Hamilton A, Sharma S, Espina BM, Smith DL, Gill PS: IL-8 is an autocrine growth factor and a surrogate marker for Kaposi’s sarcoma. Clin Cancer Res 2001, 7:2693-2702 [PubMed] [Google Scholar]
- 11.Nair BC, DeVico AL, Nakamura S, Copeland TD, Chen Y, Patel A, O’Neil T, Oroszlan S, Gallo RC, Sarngadharan MG: Identification of a major growth factor for AIDS-Kaposi’s sarcoma cells as Oncostatin M. Science 1992, 255:1430-1432 [DOI] [PubMed] [Google Scholar]
- 12.Li JJ, Huang YQ, Moscatelli D, Friedman-Kien AE: Expression of fibroblast growth factors and their receptors in acquired immunodeficiency syndrome-associated Kaposi’s sarcoma tissue and derived cells. Cancer 1993, 72:2253-2258 [DOI] [PubMed] [Google Scholar]
- 13.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang HK, Brady JN, Gallo RC: Synergy between basic fibroblast growth factor and human immunodeficiency virus type-1 Tat protein in induction of Kaposi’s sarcoma. Nature 1994, 371:674-680 [DOI] [PubMed] [Google Scholar]
- 14.Leung DW, Cachianes G, Kuang W, Goeddel DV, Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246:1306-1309 [DOI] [PubMed] [Google Scholar]
- 15.Tisher E, Mitchell R, Hartmann T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA: The human gene for vascular endothelial growth factor. J Biol Chem 1991, 266:11947-11954 [PubMed] [Google Scholar]
- 16.Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, De Water LV, Senger DR: Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med 1991, 174:1275-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masood R, Cai J, Zheng T, Smith DL, Naidu Y, Gill PS: Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci USA 1997, 94:979-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enholm B, Paavonen K, Ristimaki A. Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K: Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 1997, 14:2475–2483 [DOI] [PubMed]
- 19.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt-4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 20.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Staker SA: Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk-1) and VEGF receptor 3 (Flt-4). Proc Natl Acad Sci USA 1998, 95:548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG: Isolation of a human placental cDNA coding for a protein related to vascular permeability factor. Proc Natl Acad Sci USA 1991, 88:9267-9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skobe M, Brown LF, Tongnazzi K, Ganju RK, Dezube BJ, Alitalo K, Detmar M: Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi’s sarcoma. J Invest Dermatol 1999, 113:1047-1053 [DOI] [PubMed] [Google Scholar]
- 23.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS: Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266:1865-1869 [DOI] [PubMed] [Google Scholar]
- 24.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, Marck E, Salmon D, Gorin I, Escande JP: Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci USA 1999, 96:4546-4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, Cesarman E: Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature 1998, 394:588-592 [DOI] [PubMed] [Google Scholar]
- 26.Masood R, Husain SR, Rhaman A, Gill P: Potentiation of cytotoxicity of Kaposi’s sarcoma related to immunodeficiency syndrome (AIDS) by liposome-encapsulated doxorubicin. AIDS Res Hum Retroviruses 1993, 9:741-746 [DOI] [PubMed] [Google Scholar]
- 27.Bais C, Santomasso B, Coso O, Arvanitakis L, Geras-Raaka E, Gutkind JS, Arch AS, Cesarman E, Gershengorn MC, Mesri E: G-protein coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 1998, 391:86-89 [DOI] [PubMed] [Google Scholar]
- 28.Masood R, Cai J, Zheng T, Smith DL, Hinton DR, Gill PS: Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor positive human tumors. Blood 2001, 98:1904-1913 [DOI] [PubMed] [Google Scholar]
- 29.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hilan KJ, Moore MW: Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380:438-442 [DOI] [PubMed] [Google Scholar]
- 30.Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K: Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem 1998, 273:8413-8418 [DOI] [PubMed] [Google Scholar]
- 31.DeVries C, Escobedo JA, Ueno H, Houck KA, Ferrara N, Williams LT: The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255:989-991 [DOI] [PubMed] [Google Scholar]
- 32.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P: Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 1992, 187:1579-1586 [DOI] [PubMed] [Google Scholar]
- 33.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A: High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72:835-846 [DOI] [PubMed] [Google Scholar]
- 34.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH: Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1994, 269:26988-26995 [PubMed] [Google Scholar]
- 35.Gershengorn MC, Geras-Raaka E, Verma A, Clark-Lewis I: Chemokines activate Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J Clin Invest 1998, 102:1469-1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenkilde MM, Kledal TN, Brauner-Osborne H, Schwartz TW: Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogenes product, ORF-74. J Biol Chem 1998, 274:956-961 [DOI] [PubMed] [Google Scholar]
- 37.Arvanitakis L, Geras-Raaka E, Gershengorn MC, Cerarman E: Human herpesvirus KSHV encodes a constitutively active G protein-coupled receptor linked to cell proliferation. Nature 1997, 385:347-350 [DOI] [PubMed] [Google Scholar]
- 38.Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS: The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res 2000, 60:4873-4880 [PubMed] [Google Scholar]
- 39.Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS: The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res 2001, 61:2641-2648 [PubMed] [Google Scholar]
- 40.Yang T-Y, Chen S-C, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh C-H, Narula SK, Chensue SW, Lira SA: Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med 2000, 191:445-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G: Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood 1999, 93:4034-4043 [PubMed] [Google Scholar]
- 42.Moore PS, Boshoff C, Weiss RA, Chang Y: Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 1996, 274:1739-1744 [DOI] [PubMed] [Google Scholar]
- 43.Nicholas J, Ruvolo VR, Burns WH, Sandford G, Wan X, Ciufo D, Hendrickson SB, Guo HG, Hayward GS, Reitz MS: Kaposi’s sarcoma-associated herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med 1997, 3:287-292 [DOI] [PubMed] [Google Scholar]
- 44.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA: A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem 1997, 272:19625-19631 [DOI] [PubMed] [Google Scholar]
- 45.Kirshner JR, Staskus K, Haase A, Lagunoff M, Ganem D: Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi’s sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J Virol 1999, 73:6006-6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarid R, Wiezorek JS, Moore PS, Chang Y: Characterization and cell cycle regulation of the major Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J Virol 1999, 73:1438-1446 [DOI] [PMC free article] [PubMed] [Google Scholar]