Abstract
Neuronal nitric oxide synthase (nNOS) plays a modulatory role in the biology of a variety of neuroendocrine tissues and is especially relevant to gonadal function. We have previously reported the cloning and characterization of a variant of the nNOS protein, termed testis nNOS (TnNOS), the mRNA for which was restricted in expression to male gonadal tissues. To examine the cell-specificity of the testis-specific NOS regulatory regions we defined patterns of β-galactosidase expression of an insertional transgene in which the reporter gene lacZ was under the transcriptional control of the human TnNOS promoter. β-galactosidase activity was detected exclusively in the interstitial cells of the testis in transgenic mice. These cells also evidenced positive staining for nNOS protein and were identified as androgen-producing Leydig cells by staining with the Leydig cell marker, P450scc. Expression of the promoter was absent in cells of the seminiferous tubules, specifically germline cells of different stages and Sertoli cells. In contrast to the male gonad, β-galactosidase activity was not detected in ovaries of adult female mice. Activity was also not evident in organs known to express full-length nNOS, such as skeletal muscle, kidney, or cerebellum. The same pattern of β-galactosidase staining was observed in independent transgenic founders and was distinct from that observed for an endothelial NOS promoter/reporter transgene. In the testis of male adult eNOS promoter-reporter transgenic mice, β-galactosidase activity was expressed only in endothelial cells of large- and medium-sized arterial blood vessels. Transcriptional activity of the human TnNOS promoter could not be detected in a variety of cell types, including Leydig cells, using episomal promoter-reporter constructs suggesting that a nuclear environment and higher order genomic complexity are required for appropriate promoter function. The restricted expression pattern of an nNOS variant in Leydig cells of the male gonad suggests an important role in the regulation of testosterone release and represents an intriguing model with which to dissect the molecular basis of Leydig cell-specific gene expression.
Of the three known human nitric oxide synthases (NOS) isoforms, the neuronal nitric oxide synthase (nNOS) has unique properties. The nNOS (NOS1) isoform is expressed from a very complex human genomic locus spanning 240 kb at 12q24.2. 1 In contrast to the restricted endothelial cell-specific expression of the endothelial NOS (eNOS), 2 the nNOS isoform is constitutively expressed in a series of diverse cell types and tissues. 3 For instance, nNOS plays a fundamental role in the regulation of male sexual function. Lines of evidence have indicated the involvement of nNOS in penile erection, 4 in testosterone synthesis in the Leydig cells of the testis, 5,6 in sexual behavior, 7-9 and in egg activation at fertilization, 10 among others. Understanding the molecular regulation of nNOS in male sexual organs may be relevant to potential therapeutic measures in male sexual dysfunction. An important example would be the rapid clinical acceptance of the utility of Sildenifil (Viagra) for male erectile dysfunction. 11
The human gene encoding nNOS is designated NOS1 by the Human Genome Nomenclature Committee. NOS1 has recently been characterized as one of the most complex genes in the mammalian genome in terms of both structure and expression patterns. 12 At least nine unique exon 1 variants from the upstream region are used to initiate transcription in a tissue/cell-specific manner through usage of alternative promoters. 13 Interestingly, those exon 1 variants enriched in neuronal tissues are clustered in one genomic region whereas those enriched in skeletal muscle are grouped together in another genomic region 75 kb upstream, 13 indicating distinct transcriptional regulatory mechanisms. In addition, it is possible that the human nNOS gene also contains an exon 2 promoter that is analogous to the novel, calcium-responsive exon 2 promoter recently characterized in the rodent. 14
Although many of the upstream exon 1 mRNA variants are expressed in the normal human testis to various degrees, none of them is testis-specific. 13 In a recent study we reported the cloning and characterization of a novel, testis-specific nNOS mRNA transcript (TnNOS) that accounted for approximately half of the total nNOS mRNA species expressed in the testis. 15 Transcription of TnNOS initiates from a novel noncoding downstream exon 1 (Tex 1) that is localized in intron 3 of the NOS1 gene. This exon is then spliced to another novel exon (Tex2) and then to exon 4 of the full-length nNOS. Translation of the TnNOS variant transcripts produces an NH2-terminal truncated protein analogous to nNOSγ. 15,16 nNOSγ represents a 125-kd protein expressed from exon 2-deleted full-length nNOS transcripts in human 15 and mouse. 16 When stably expressed in CHO-K1 cells, the 125-kd protein encoded by TnNOS possesses NOS enzymatic activity comparable to that of the full-length nNOS (160 kd), 15 although a comprehensive understanding of the biochemistry of this NOS variant is awaited. TnNOS may have a unique biological role in the testis given that the protein domain implicated in functional interaction with the protein inhibitor of nNOS (PIN), which is highly expressed in this organ, 15,17 is removed in this NH2-deleted nNOS variant. Moreover, this protein variant lacks the PDZ protein interaction domain implicated in membrane localization.
We proposed to define the cell types of the male gonad that express the TnNOS gene and to study the molecular mechanisms responsible for its restricted expression profile. The 5′-flanking regions for this novel transcript reside within the genomic DNA representing intron 3 of the NOS1 gene, and hence would still be intact in the reported nNOS(−/−) mouse. 18 Although we previously identified multiple binding sites for both ubiquitous and testis-specific transcription factors, questions remain with respect to the activity and cell-specificity of this putative promoter. In the current study, we have examined the functional promoter activity of these regulatory regions both in vitro and in vivo. Because little is known about the molecular basis of transcriptional regulation of male gonad-specific gene expression, especially in vivo, we developed a transgenic mouse model carrying an insertional promoter-reporter gene construct. In this transgenic mouse line the expression of the reporter gene lacZ is under the transcriptional control of the putative TnNOS promoter. We demonstrate that the TnNOS promoter is a functional promoter in vivo and is exclusively expressed in the Leydig cells of the testis.
Materials and Methods
Preparation of Promoter-Reporter Constructs
DNA Construct for Transgenic Model
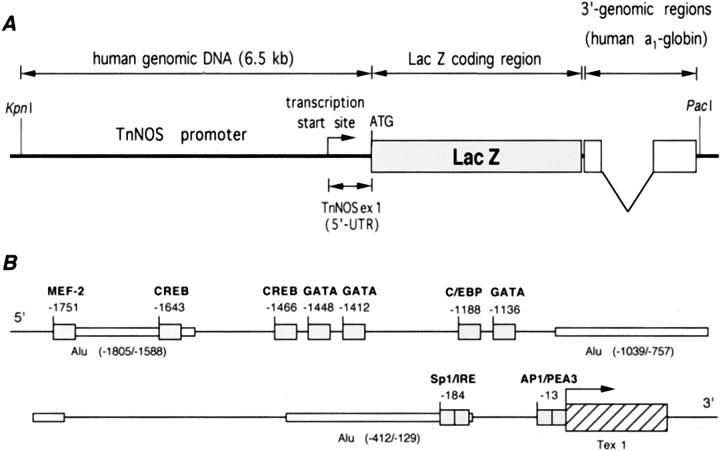
Plasmid placC (a gift from Dr. Richard D. Palmiter, University of Washington, Seattle, Washington) that contained the reporter lacZ open reading frame and eukaryotic translation initiation signal was digested with HindIII. A 516-bp HindIII fragment containing the 3′-UTR and flanking genomic sequences of the human α1-globin gene was obtained from plasmid no111paG (a gift from Dr. Vincent Raymond, Laval University, Quebec City, Quebec) and inserted into placC (placC/α1G) to enhance processing and stability of the transgene mRNA. 19 A PacI linker was inserted at the 3′ HindIII site of placC/α1G. A 6.5-kb KpnI-MunI genomic DNA fragment containing 81-bp 5′-UTR sequence and 6.5-kb 5′-flanking regions of the human TnNOS was obtained from a human genomic DNA subclone B42R/B-T1. 15 This 6.5-kb fragment containing the putative promoter for TnNOS was inserted into the KpnI-SalI site upstream of the LacZ open reading frame in placC/α1G to obtain plasmid −6500/+81 pTnNOS/lacC/α1G (Figure 1A) ▶ placing the reporter gene under the transcriptional control of the human TnNOS 5′-flanking regions. The proximal portion (1.9 kb) of the 6.5-kb KpnI-MunI fragment has been previously reported (GenBank/EMBL Data Bank accession no. U66360) and putative cis-acting DNA regulatory sequences identified using Eukaryotic Transcription Factor Data Base release, version 7.4, Genetics Computer Group sequence analysis software package (Madison, WI) (Figure 1B) ▶ .
Figure 1.
A: Structure of the DNA construct used to create the −6500/+81 TnNOS/lacC/α1G transgenic mouse. The 5′-UTR included 81 bp from TnNOS exon 1 and 39 bp from the placC plasmid. The 3′-genomic regions of the construct represented portions of the human α1-globin gene, including the last intron, and were used to facilitate RNA processing. B: Potential cis-regulatory DNA elements in the proximal region (1.9 kb) of the human TnNOS promoter. The transcription start site is numbered as position +1 and is indicated by the arrow. Only selected sites are depicted.
DNA Constructs for Transient Transfection
The aforementioned 6.5-kb KpnI-MunI fragment was inserted into the KpnI-HindIII site upstream of the luciferase open reading frame in the reporter gene vectors pGL3-basic and pGL2-basic (Promega, Madison, WI), resulting in constructs pTnNOS −6500/+81 pGL3 and pTnNOS −6500/+81 pGL2, respectively. A 2.2-kb BglII-MunI fragment representing the 3′-portion of the putative promoter for TnNOS was also inserted into the KpnI-HindIII site of the pGL3-basic and pGL2-basic, resulting in the constructs pTnNOS −2200/+81 pGL3 and pTnNOS −2200/+81 pGL2, respectively.
In Vitro Promoter-Reporter Studies
Transient expression of promoter-reporter genes in mammalian cells was performed using previously published methods. 20 Briefly, cells were cultured in 60-mm dishes and transfected at 40 to 60% confluency using the lipofectin reagent and Opti-Mem I (Life Technologies, Inc., Gaithersburg, MD). Each 60-mm dish of cells was co-transfected with 1.0 μg of promoter/reporter construct, 0.5 μg of pRSVβgal DNA, and 1.5 μg of pBluescript II SK(−) DNA with a DNA/lipofectin ratio of 2:1 (mass:mass). β-Galactosidase activity was used to control for transfection efficiency and pBluescript II SK(−) DNA was used to optimize DNA/lipofectin ratios and hence transfection efficiency. The pGL2-control/pGL3-control vectors (Promega) containing the SV40 promoter and enhancer elements were used as positive controls. The pGL2-basic/pGL3-basic vectors lacking both an eukaryotic promoter and enhancer sequences were used as negative controls. Cell extract was harvested 48 hours after transfection. Results represent determinations of the activity of multiple independent DNA preparations and were repeated at least three times. Luciferase activity was measured with a luminometer (Monolight 2010C; Analytical Luminescence Laboratory, Sparks, MD) and normalized for β-galactosidase activity and protein content. Statistical analyses were performed using the Student’s t-test.
In Vivo Promoter-Reporter Studies
The 10.5-kb KpnI-PacI fragment (Figure 1A) ▶ was prepared from plasmid −6500/+81 pTnNOS/placC/α1G by restriction digestion. After preparative gel electrophoresis in low-melting temperature agarose (SeaPlaque; FMC Products, Rockland, ME), the DNA band was recovered and digested with β-agarase (New England Biolabs, Beverly, MA) followed by CsCl2 gradient ultracentrifugation in the absence of ethidium bromide. Aliquots of the gradient were sequentially extracted and subjected to analytical agarose gel electrophoresis. Fractions containing DNA were pooled and dialyzed extensively against microinjection buffer (10 mmol/L Tris-HCl, pH 7.5, 1 mmol/L ethylenediaminetetraacetic acid). Microinjections were performed in the Transgenic Facility at the Hospital For Sick Children, Toronto, using standard protocols. Briefly, purified DNA (3 to 5 ng/μl) was microinjected into the male pronucleus of fertilized one-cell embryos derived from mating B6/SJL F1 females with B6/SJL F1 males (Charles River, Wilmington, MA). Embryos surviving microinjection were reimplanted into the oviducts of pseudopregnant CD-1 females either on the same day or after overnight culture. [minus]6500/+81 TnNOS/lacC/α1G transgene-positive mice and their copy numbers were defined by dot blot and/or Southern blot analyses using genomic DNA from tail biopsy and a [32P]dCTP-labeled, nick-translated 1.1-kb SacI-BamHI fragment from plasmid placC. Genomic DNA from a gene-targeting mouse line (heterozygous) where a lacZ reporter gene was introduced into the flk-1 locus by homologous recombination 21 was used as a single copy lacZ control. Density of the hybridization signals from Southern blots was quantified using a PhosphorImager and ImageQuant software (version 1.2; Molecular Dynamics, Sunnyvale, CA). Founder mice carrying the −6500/+81TnNOS/lacC/α1G transgene were identified and bred with wild-type B6/SJL mice to obtain hemizygous F1 progeny. F1 mice were then crossed with their negative littermates to obtain hemizygous F2 mice. Both F1 and F2 offspring were used for subsequent histological analyses. Transgenic mice used in this study were bred, housed, and monitored in accordance with the standards set by the Canadian Animal Care Committee at the Hospital for Sick Children (Toronto, Ontario).
β-Galactosidase Staining
Organs dissected from sexually mature mice (6 to 12 weeks of age), both transgene-positive and -negative, were sliced in 2-mm-thick sections, briefly rinsed with phosphate-buffered saline (PBS), and fixed in 0.2% glutaraldehyde, 1.5% formaldehyde, 5 mmol/L EGTA, and 2 mmol/L MgCl2 in 0.1 mol/L PBS, pH 7.3, at 23°C for 4 to 5 hours with gentle rocking. Fixed specimens were rinsed three times with 0.1 mol/L of PBS, pH 7.3, containing 2 mmol/L MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40. Staining was then performed in the same solution supplemented with 1 mg/ml of X-Gal (Xymotech, Toronto, Canada), 5 mmol/L potassium ferricyanide, and 5 mmol/L potassium ferrocyanide at 37°C overnight. Stained specimens were briefly rinsed with PBS and completely blot-dried before embedded in Tissue-Tek (Sakura Finetek USA, Torrance, CA) and cryosectioned to 5 -to 8-μm sections. In some experiments, fixed specimens were cryosectioned before staining and the results were identical. All slides were counterstained with neutral red. Characterization of the endothelial NOS promoter/β-galactosidase reporter transgenic mice has been previously reported. 22
Immunohistochemistry
A peroxidase anti-peroxidase-avidin-biotin-peroxidase complex amplification combination protocol was used for immunohistochemical detection of proteins on tissue sections as described previously. 23 Briefly, whole testes dissected from transgene-positive mice were fixed in Bouin’s solution at 23°C for 16 hours and embedded in paraffin after dehydration in ascending alcohol concentrations. Six-μm sections were mounted onto slides precoated with chrome-gelatin and immunolabeled with a rabbit anti-human nNOS polyclonal antibody (1:1000 dilution), 22 or a mouse anti-human nNOS monoclonal antibody (1:100 dilution) (Transduction Laboratories, Lexington, KY), or a mouse anti-rat cytochrome P450 side-chain cleavage enzyme (P450scc), a Leydig cell marker, monoclonal antibody (1:200 dilution) (Chemicon International, Hofheim, Germany). To visualize the hybridization signals, a secondary biotinylated anti-rabbit IgG or anti-mouse IgG (Dakopatts, Glostrup, Denmark) was applied (1:250 dilution) followed by a tertiary rabbit (1:200 dilution) or mouse (1:100 dilution) peroxidase anti-peroxidase complex (Dakopatts) and finally an Elite avidin-biotin-peroxidase complex (ABC) (1:250 dilution; Vector Laboratories, Burlingame, CA). The peroxidase activity was developed by means of the nickel-glucose oxidase technique. 24 For controls, sections were incubated with PBS, or rabbit or mouse serum, or rabbit or mouse IgG, instead of the primary antibodies. In previous studies the rabbit antiserum against nNOS was adsorbed with nNOS protein (20 μg/ml), resulting in negative staining of the sections. 25,26
Western Blot Analysis
Human and mouse tissue homogenates and TM3 cell lysates were electrophoresed in 6% polyacrylamide/sodium dodecyl sulfate gels and transferred by electroblotting onto nitrocellulose membranes. Blots were incubated with anti-human nNOS monoclonal antibody directed against the COOH-terminus of the protein (1:500 dilution) (Transduction Laboratories) and subsequently with horseradish peroxidase-conjugated sheep anti-mouse secondary antibody (1:20,000 dilution) (Amersham, Arlington Heights, IL), as previously described. 15 Signal detection was facilitated with enhanced chemiluminescence (ECL, Amersham).
Results
Expression of TnNOS Protein in Human and Mouse Testis
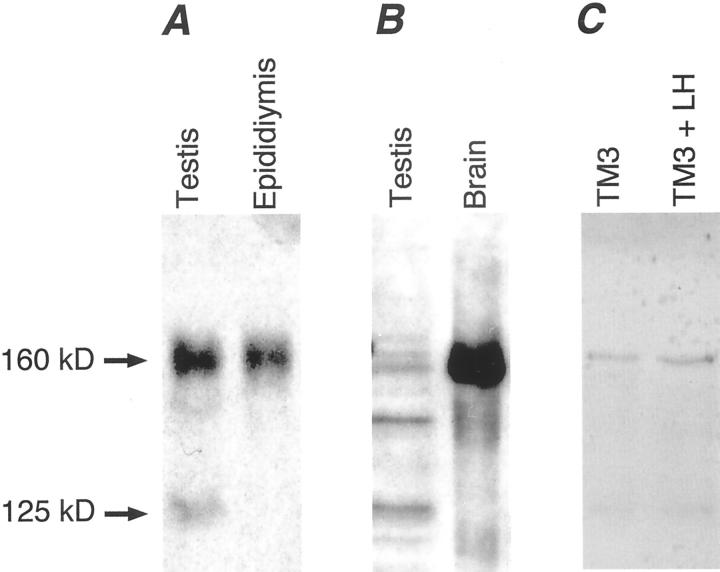
To determine whether the TnNOS mRNA variant was translated in vivo we performed immunoblotting using human and murine tissues and cells. Two nNOS-specific bands were detected in homogenates of normal human testes with Western blot analysis using a COOH-terminus nNOS-specific monoclonal antibody (Figure 2A) ▶ . A 160-kd band representing the full-length nNOS protein, and a 125-kd band consistent with the size of TnNOS were detected. TnNOS protein was not detected in homogenate of normal human epididymis where the full-length nNOS protein was easily detected (Figure 2A) ▶ . A 125-kd nNOS protein was also detected in homogenates of adult mouse testis (Figure 2B) ▶ and lysates of cultured murine Leydig cells (TM3), with and without luteinizing hormone (LH) treatment (100 ng/ml for 24 hours) (Figure 2C) ▶ . In addition to TnNOS, another molecular mechanism may underlie the presence of the 125-kd nNOS protein. Alternative usage of some of the upstream promoters in combination with the deletion of exon 2 results in mRNA transcripts that encode proteins that have a similar size. 13 However, the deletion of exon 2 exhibits tissue specificity in humans and is an uncommon species in the testis. 13 Therefore the presence of this smaller human nNOS protein variant is most consistent with the translation of TnNOS mRNA transcripts.
Figure 2.
A: Expression of nNOS proteins in human male tissues. The 160-kd full-length nNOS was detected in both tissues whereas the 125-kd TnNOS was detected only in the testis (100 μg of protein was applied per lane). B: Expression of nNOS proteins in mouse tissues. The 160-kd full-length nNOS was detected in both the brain (10 μg of protein) and testis (100 μg of protein) tissues whereas the 125-kd TnNOS was detected only in the testis. C: Expression of nNOS proteins in mouse Leydig cells (TM3 cells) after treatment with human LH (100 ng/ml, 24 hours) (15 μg protein per lane). A faint, but clear, TnNOS signal at 125 kd was detected in addition to the 160-kd full-length nNOS.
TnNOS Promoter Activity in Transient Expression Assays
To gain further insight into the transcriptional properties of the TnNOS promoter a series of cell types were transiently transfected with human TnNOS promoter-reporter luciferase constructs. We have previously reported the characterization of the human TnNOS transcription start site and sequence analysis of the 5′-flanking region. 15 As shown in Table 1 ▶ , transfection of the well-characterized murine Leydig (TM3) and Sertoli (TM4) cell lines with pTnNOS−6500/+81 pGL3 and pTnNOS−2200/+81 pGL3 (numbered with respect to transcription initiation) constructs indicated that luciferase activities were not increased above the promoterless vector (pGL3-basic) and represented <1% of the activity of a strong heterologous promoter (SV40 promoter/enhancer, pGL3-control) (n = 3, triplicate determinations). Similar findings were observed with an independent luciferase expression vector series (pGL2 vectors, data not shown).
Table 1.
Transient Expression of TnNOS Promoter in Leydig and Sertoli Cells
| Cell type | pGL3-basic | −6500/+81 TnNOSpGL3 | −2200/+81 TnNOSpGL3 | pGL3/control (SV40 promoter/enhancer)* | |
|---|---|---|---|---|---|
| TM3 | Leydig cell, murine | <1% | <1% | <1% | 1.3 ± 0.1 × 105 |
| TM4 | Sertoli cell, murine | <1% | <1% | <1% | 4.3 ± 0.1 × 104 |
*Relative light unit, mean ± SEM, n = 3.
Given the important regulatory effects of gonadotrophic hormones on testicular function we assessed the effects of LH on promoter activity in transiently transfected TM3 and TM4 cells. LH (100 ng/ml, 24 hours) failed to significantly alter promoter-reporter activity (data not shown, n = 3).
We also evaluated promoter activity in a series of 12 human and non-human cell types. As demonstrated in Table 2 ▶ , activity of the −2200/+81 TnNOSpGL3 promoter-reporter construct represented <1% activity of the pGL3-control vector (SV40 promoter/enhancer) and was not increased above the pGL3-basic promoterless vector. We take these data to indicate that the TnNOS promoter is not functionally active in transient transfection assays using episomal-based vectors in testes-derived cells and a variety of human and non-human cell types.
Table 2.
Transient Expression of TnNOS Promoter in Mammalian Cells
| Cell type | pGL3-Basic (%pGL3 Cont.) | −2200/+81 TnNOS pGL3 (%pGL3 Cont.) | pGL3-Control (SV40 promoter/enhancer)* |
|---|---|---|---|
| HeLa | |||
| Epitheloid carcinoma, human | <1% | <1% | 6.3 ± 0.4 × 105 |
| IMR-32 | |||
| Neuroblastoma, human | <1% | <1% | 1.8 ± 0.1 × 105 |
| JEG-3 | |||
| Choriocarcinoma, human | <1% | <1% | 2.7 ± 0.1 × 105 |
| NT2/D1 | |||
| Embryonal carcinoma, human | <1% | <1% | 1.6 ± 0.5 × 104 |
| WM852 | |||
| Melanoma, human | <1% | <1% | 1.6 ± 0.1 × 105 |
| DAMI | |||
| Megakaryoblast cell, human | <4% | <4% | 5.6 ± 0.3 × 104 |
| ES | |||
| Embryonic stem cell, murine | <1% | <1% | 3.5 ± 0.1 × 105 |
| F9 | |||
| Embryonal carcinoma, murine | <1% | <1% | 1.6 ± 0.01 × 105 |
| NIH3T3 | |||
| Embryo fibroblast cell, murine | <1% | <1% | 2.4 ± 0.3 × 105 |
| P19 | |||
| Embryonal carcinoma, murine | <1% | <1% | 1.9 ± 0.1 × 106 |
| BAEC | |||
| Vascular endothelial cell, bovine | <1% | <1% | 2.3 ± 0.1 × 106 |
| CHO-K1 | |||
| Chinese hamster ovary, hamster | <1% | <1% | 6.1 ± 0.3 × 106 |
*Relative light unit, mean ± SEM, n = 3.
Exclusive Expression of the PTnNOS/placC/α1G Transgene in Leydig Cells of the Testis
Given that we have provided strong evidence for the tissue-restricted expression of the human TnNOS mRNA transcript 15 and that transient transfection assays did not reveal functional promoter-reporter activity in a variety of cultured cell types we assessed the functional promoter activity of the human TnNOS 5′-flanking region using murine insertional transgenic approaches. Three positive transgenic −6500/+81 TnNOS/lacC/α1G mice (Tn3, Tn7, and Tn10) were identified with dot-blot and Southern blot analyses and they carried 6, 1, and 7 tandem copies of the transgene, respectively. Examination of these mice revealed that they were phenotypically normal under gross anatomical examination and careful histological examination of major organs. In these mice the endogenous nNOS gene was intact and its expression was not predicted to be altered by either the transgene procedure or the product of the transgene. Two of the three positive founders (Tn3 and Tn10) were capable of germline transmission and were bred for subsequent experiments. The −6500/+81 TnNOS/lacC/α1G transgene was inherited in a Mendelian distribution in these two lines as expected for a single autosomal integration event. To determine the expression pattern of the TnNOS promoter, the following organs/tissues were harvested from multiple positive F1 and F2 mice along with their negative littermates for β-galactosidase staining: whole brain, skeletal muscle, heart, kidney, adrenal gland, testis, prostate gland, seminal vesicle, vas deferens, penis, ovary, uterus, and fallopian tube. Specific β-galactosidase signal was detected only in the testis, in particular, in the interstitial cells of the testis (Figure 3, A and B) ▶ . These cells were later identified as testosterone-producing Leydig cells of the testis by staining with a Leydig cell marker P450scc (see below). Although β-galactosidase activity was absent in the cells of the seminiferous tubules, including both germline cells at different developmental stages and supporting Sertoli cells, β-galactosidase signal was consistently observed in the interstitial areas of the testis in every transgene-positive male animal examined. However, not every Leydig cell showed positive staining and signal intensity varied among those stained positive, consistent with the reported existence of subpopulations within this cell type. 5 Vascular cells within the interstitial area stained negative for β-galactosidase (Figure 3B) ▶ . There was no background β-galactosidase staining in transgene-negative testis (Figure 3C) ▶ . Endogenous β-galactosidase activity was observed in the epididymis, vas deferens, and seminal vesicle of transgene-negative mice, as reported by others. 27 Interestingly, negative staining for β-galactosidase was observed in follicular granulosa cells and theca interna cells of the ovary that are responsible for estrogen and progesterone synthesis, respectively (Figure 3D) ▶ . None of the other organs and tissues examined evidenced positive staining for β-galactosidase. Many of these are known to express robust amounts of full-length nNOS, such as skeletal muscle and cerebellum (Figure 3, E and F) ▶ . Taken together, these results indicate that the human TnNOS promoter is a Leydig cell-specific promoter that may contribute to nNOS expression in this important cell type.
Figure 3.
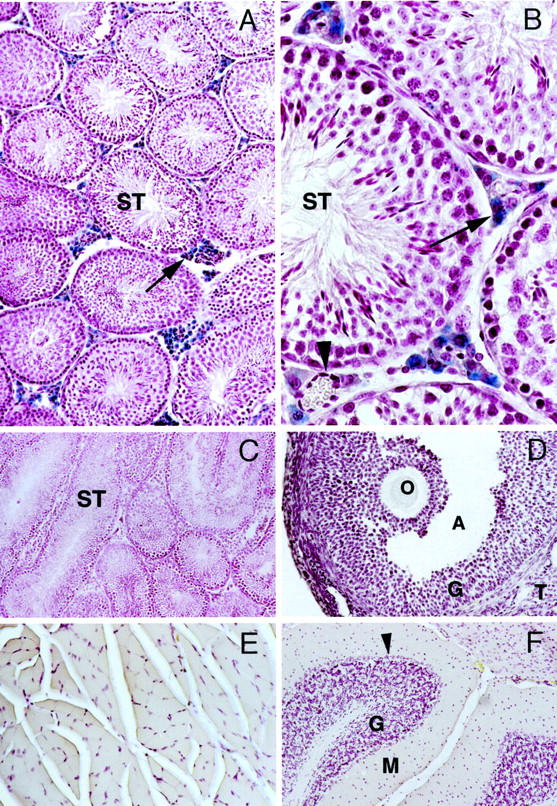
Cryosections from selective organs of −6500/+81 TnNOS/lacC/α1G transgenic mice stained for β-galactosidase enzymatic activity. Blue color indicates positive β-galactosidase staining. A–C: Testis sections. β-galactosidase activity was detected only in the Leydig cells in interstitial areas (arrows). An interstitial blood vessel (arrowhead) and all of the seminiferous tubules (ST) stained negative for β-galactosidase. A: Testis from a transgenic-positive mouse. B: Testis from a transgenic-positive mouse. C: Testis from a transgenic-negative mouse. D: Ovary from a transgenic-positive mouse. O, oocyte; A, antrum; G, granulosa cells; T, theca interna cells. E: Skeletal muscle from a transgenic-positive mouse. F: Cerebellum from a transgenic-positive mouse. G, granular layer; M, molecular layer; arrowhead indicates Purkinje cells. Original magnifications: ×150 (A, D, E, and F); ×600 (B); ×75 (C).
In the testis we also examined the cell-specific expression pattern of an eNOS promoter-reporter transgenic mouse line that we have recently reported. 2 In contrast to the Leydig cell-restricted expression of the TnNOS promoter, we found that expression of the nuclear-localized −5200/+28 Mu eNOSpromnlsLacZ was active exclusively in the nuclei of vascular endothelium of medium- and large-sized arteries (Figure 4) ▶ . Expression patterns were comparable in three independent 1, 5, and 10 copy number founders.
Figure 4.
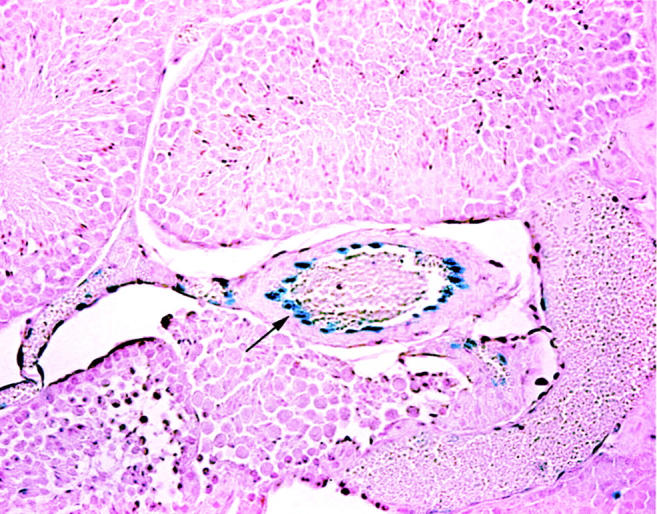
Testis cryosection from a −5200/+28 Mu eNOSpromnlsLacZ transgenic mouse stained for β-galactosidase enzymatic activity. Staining is evident only in the vascular endothelium of the medium- and larger-sized arteries. Original magnification, ×150. Arrow indicates the endothelial cells.
Native nNOS Expression in Leydig Cells
Immunohistochemical techniques were used to document the expression of native nNOS in the testis of −6500/+81 TnNOS/lacC/α1G transgenic mice. Strong to moderate nNOS-like immunoreactivity was detected in the cytoplasm of most Leydig cells (Figure 5 ▶ ; A, B, and C). Similar to that of the −6500/+81 TnNOS/lacC/α1G transgene, expression of native nNOS varied among individual Leydig cells and Leydig cell groups. This phenomenon has been observed in human testis where subpopulations of Leydig cells have also been defined, 5 as in the murine setting. In addition to the Leydig cells, weaker nNOS-like immunoreactivity was also detected in spermatogonia, spermatocytes, and spermatids, as well as Sertoli cells of the seminiferous tubules (Figure 5B) ▶ . In some seminiferous tubules distinct nNOS-like immunoreactivity was seen in the acrosomes (data not shown). We take this nNOS immunoreactivity to represent full-length nNOS transcripts, possibly derived from a number of varied exon 1-containing nNOS mRNA transcripts. Although the morphological identification of Leydig cells in the testis usually does not present difficulties, the identity of these cells were additionally verified using immunohistochemical techniques in this study. The cytochrome P450 side chain cleavage enzyme (P450scc) is responsible for the conversion of cholesterol to pregnenolone as the rate-limiting enzyme controlling steroidogenesis in the Leydig cells and has been used as a marker for these cells. 28 Strong P450scc-like immunoreactivity was detected predominantly in the cytoplasm of interstitial Leydig cells (Figure 5, D and E) ▶ . Low-intensity signals were also seen in some spermatocytes. Other structures of the testis were negative. Results from these experiments demonstrate that nNOS immunoreactivity is expressed in the interstitial Leydig cells of the mouse testis, which, at least in part, may be under the transcriptional control of the TnNOS promoter.
Figure 5.
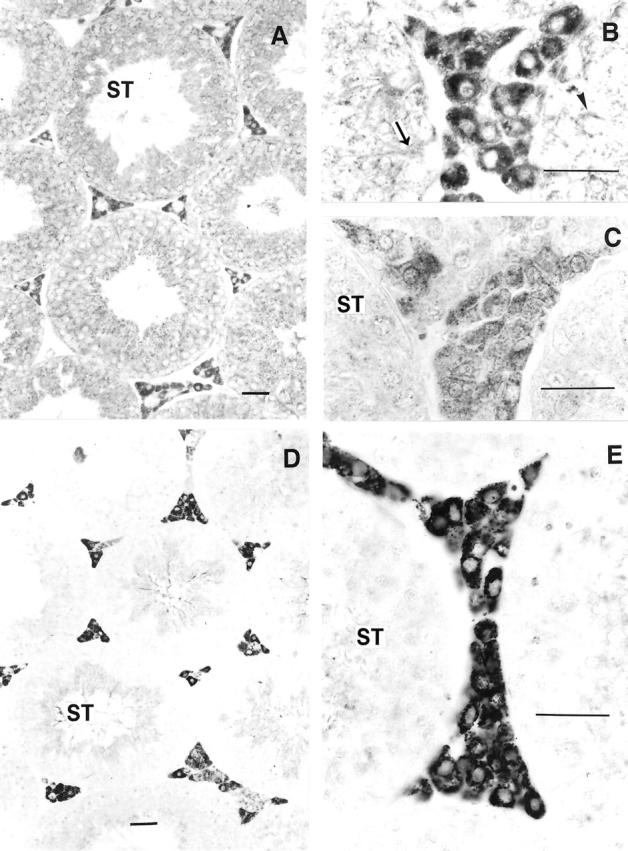
A–C: nNOS immunoreactivity in the mouse testis. Distinct staining was detected in the Leydig cells in interstitial areas. The staining appeared fine, granular, and was evenly distributed throughout the cytoplasm of these cells. Low-intensity staining was also detected in some primary spermatocytes (arrowhead), spermatids, and Sertoli cells (arrow) of the seminiferous tubules (ST). A: Section stained with a polyclonal antibody. B: Section stained with a polyclonal antibody. C: Section stained with a monoclonal antibody. D–E: Immunoreactivity for cytochrome P450scc in the testis of a transgenic-positive mouse. Strong immunoreactivity was seen in the cytoplasm of the Leydig cells. Note the granular appearance of the reaction precipitates. Low-intensity staining was also detected in some spermatocytes in the seminiferous tubules (ST). Original magnifications: ×220 (A, D); ×700 (B, C, E). Scale bars: 10 μm (A, D); 100 μm (B, C, E).
Discussion
Using a transgenic mouse model carrying an insertional promoter-reporter gene construct we have demonstrated that the human TnNOS promoter is a functional, Leydig cell-specific promoter that contributes to the regulation of the NO-signaling pathway in this important cell population. These results are in accordance with the conclusion, and further confirm previous reports, that TnNOS is a testis-specific mRNA transcript. 15
The human nNOS gene is an extremely complex locus. 12 Tissue- and cell-specific expression is controlled through usage of alternative promoters expressed from distinct 5′-flanking genomic regions at 12q24.2. 1,13 For example, a region of genomic DNA 105 kb upstream of exon 2 contains a cluster of exon 1 variants representing the transcription initiation sites for three skeletal muscle-specific promoters. Similarly, four promoters responsible for nNOS transcription in neuronal tissues are clustered in a brain/neuronal region that is ∼75 kb downstream from the aforementioned skeletal muscle region. 13 The demonstration that the TnNOS promoter functions as a Leydig cell-specific promoter identifies yet another transcriptionally active genomic region within this gene. Because this testis region is at least 50 kb further downstream from the brain/neuronal region, the entire transcription initiation machinery for the human nNOS gene spans a genomic region greater than 125 kb. A recent study in rodents has demonstrated that genomic regions immediately 5′ of exon 2 also contain a functional promoter, one that is responsible for transcriptional activation in response to calcium influx in cortical neurons. 14 Taken together it can be fairly concluded that the human nNOS gene is exceedingly complex and thus represents a unique model with which to assess the transcriptional regulation of complex genes. Unique to the promoter described in this article is that an NH2-truncated nNOS protein is produced.
The in vivo functional activity of the chromatin-based −6500/+81 nucleotide human TnNOS transgene identifies these genomic regions as a functional promoter. Although lacking canonical TATA and CCAAT boxes, the proximal portion of the TnNOS promoter contains multiple binding sites for ubiquitous transcription factors such as Sp1, AP1, AP2, NF1, and nuclear factor-κB (Figure 1B) ▶ . Among them the proximal Sp1 site at −184 may represent a core element for the general transcriptional activation of this TATA-less promoter. However, the tissue-/cell type-specific expression pattern of this promoter variant of nNOS implicates the presence of a regulatory mechanism that governs the unique Leydig cell-specific transcriptional activation of this gene.
Genes whose expression is restricted specifically to Leydig cells are uncommon. However, transcripts that are selectively enriched in Leydig cells have been described. For example, the steroidogenic acute regulatory (StAR) protein, P450scc, and the 3β-hydroxysteroid dehydrogenase (3β-HSD), the three key components in testosterone synthesis pathway, are somewhat Leydig cell-specific although they are also expressed in extra-testicular tissues such as the ovary and brain. 29-31 Research in recent years has begun to identify a group of cis-regulatory elements that play critical roles in transcriptional activation of genes that are important for Leydig cell biology and, intriguingly, many of them are present within the proximal portion of the TnNOS promoter. The most noticeable among them all are the two steroidogenic factor 1 (SF-1) AGGTCA 32,33 binding sites at −1803 and +193 (Figure 1) ▶ . An SF-1 site is required for the protein-DNA interaction in a critical region of the StAR promoter and for the maximal activity of the promoter in Leydig cells. 34-36 It is also required for transcription in Leydig cells of aromatase, 32,37 luteinizing hormone receptor, 35 anti-Mullerian hormone receptor/Mullerian-inhibiting substance type II receptor, 33,38 the Leydig insulin-like gene (Ley I-L), 39 P450scc, 40 and 3β-HSD. 41 Other cis-acting DNA elements present within the proximal portion of the TnNOS promoter have been implicated in transcriptional regulation within Leydig cells. Multiple potential GATA binding sites which, through interaction with GATA-1 and GATA-4, participate in the transcription of StAR, 34 inhibin α-subunit, and inhibin/activin β-b-subunit 42 are evident. Additionally, a CCAAT/enhancer-binding protein (C/EBP) element is evident. Interaction of C/EBPβ with such sites is required for transactivation of the StAR gene. 34,36,43 Two copies of the ATF/CRE-like motif are present and are known to be involved in the transcriptional activation of a number of genes in the Leydig cells including those encoding aromatase, 37 steroid 11 β-hydroxylase, 44 and P450scc, 45 among others. In summary, although the functional relevance of the cis-acting DNA elements for both ubiquitous and Leydig-related transcription factors as discussed warrants further investigation, the linear structure of the proximal portion of the TnNOS promoter corresponds to a Leydig cell-specific promoter.
An intriguing finding of this study was that the TnNOS promoter was inactive in transient transfection assays of varied cell types, including Leydig cells either in the presence or absence of LH. Clearly, the assessed region was sufficient to direct cell type-specific expression of the reporter gene in transgenic mice. It is possible that paracrine or endocrine mediators are necessary for transcriptional competency of the TnNOS promoter in the in vivo setting. In this respect juxtaposition to the gonadal supporting cells may be crucial. Also, there is a growing appreciation of the importance of nuclear architecture in the control of gene expression. 46 When discrepancies have been observed between the in vitro and in vivo activities of transcriptional regions a number of alternatives are observed. In some examples, expression of the reporter gene is promiscuous in a broad spectrum of cultured cells types in transient transfection assays but cell type-specific expression is observed only when the DNA construct is introduced into the germline of transgenic mice. Examples include the genes encoding the human K14 keratin, 47 the mouse N-myc, 48 the mouse Crp/SmLim, 49 and the human complement receptor type 2. 50 In the latter case, incorporation of the DNA construct into the genome via stable transfection of cultured cells had the same effects as germline transmission in transgenic mice. In contrast, some elements may have no transcriptional activity in transient transfection assays of cultured cells but are strong promoters when integrated into the genome of transgenic mice. This is the case in the current study for TnNOS and is reported for a number of genes such as the mouse metallothionein I 51 and the mouse α1 collagen genes. 52 In this regard, this is the first example of a Leydig cell-specific gene with these properties. These examples, together with our findings, have led to the realization that nuclear architecture at multiple levels is important for stringent transcriptional regulation of eukaryotic genes. In addition to the primary linear order of cis-regulatory elements, chromatin structure, nucleosome organization, epigenetic pathways, and nuclear matrix all contribute to the appropriate expression of a gene. 53-57
NO, produced in Leydig cells, plays multiple roles in testicular biology. 5,25,58 Leydig cells, located in the interstitium of the testis, function as the primary site for the synthesis of testosterone. Exogenous NO is a dual modulator of testosterone synthesis and release, being stimulatory at low and inhibitory at high concentrations. 58,59 Pharmacological inhibition of endogenous NO in cultured Leydig cells increases both basal and gonadotropin-stimulated testosterone production. 60,61 It is believed that NO inhibits the synthesis and release of testosterone from Leydig cells through the inhibition of steroidogenic enzymes, most likely P450scc, in a cGMP-independent manner. 6,62 Our current work adds newer perspective to the concept that NO derived from nNOS expressed in Leydig cells may be acting in an autocrine manner to regulate Leydig cell function. Specifically, NO produced from TnNOS-positive Leydig cells may act on these same cells as an intercellular and intracellular signaling molecule. 5
The gene encoding nNOS was disrupted via targeted replacement of exon 2 obviating synthesis of full-length transcripts. Given the importance of NO in penile erectile function, 5,59,60 it could be argued that the ability of the nNOS(−/−) mice to reproduce was somewhat surprising. When the nNOS(−/−) mice were studied they evidenced loss of 85% of nNOS activity, rather than the complete abrogation of activity. 7,9 Residual activity reflects, in part, splicing around exon 2 and production of nNOSβ and nNOSγ. 16,63 We have reported the existence of these variants in the human setting. 13,15 The TnNOS variant was most likely expressed in these nNOS(−/−) mice because the gene-targeting strategy disrupted upstream exon 2 genomic regions, leaving the TnNOS promoter unaltered. 18 The male nNOS(−/−) mice demonstrate aggressive and inappropriate sexual behavior relative to wild-type mice 7,9 and selective nNOS inhibitors elicit a similar increase in male impulsive aggressive behavior. 64 Recent evidence implicates selective decreases in serotonic (5-HT) turnover and deficient 5-HT1a and 5-HT1b receptor function in these mice. 65-67 Of great interest for the current work, this aggressive behavior required testosterone, but serum testosterone levels did not vary between the (+/+) and (−/−) mice. It is appreciated that although testosterone is required for normal erectile function, it is permission and does not play a direct role. Given the strong evidence that Leydig cell-derived NO modulates testosterone release, the failure to document increases in serum testosterone levels in male (−/−) mice is even more puzzling given that systemic NOS inhibition is argued to increase testosterone levels in some settings. 68,69 Therefore, it is possible that TnNOS compensated for the loss of full-length nNOS to maintain normal testosterone production. This needs to be confirmed by further understanding the role of TnNOS in male sexual function.
In summary we take these newer findings to indicate that TnNOS contributes to overall nNOS expression in Leydig cells, a key cell type in male reproductive biology. Given its conserved expression across different species, TnNOS is likely an indispensable component of the NO-signaling pathway functioning in the testis, especially considering the fact that this NH2-terminal truncated NOS is resistant to inhibition by PIN that is highly active in the testis. 15,17 We have identified the TnNOS promoter and demonstrate its in vivo expression profile. The 6.5-kb promoter region directs exquisite organ- and cell-specificity to a β-galactosidase reporter gene and accurately recapitulates the native TnNOS expression profile. We conclude that the TnNOS gene is a valuable model to study Leydig cell biology and male sexual function, especially as it relates to identifying and characterizing newer facets of Leydig cell-specific gene expression.
Acknowledgments
We thank Dr. R. Palmiter for the plasmid placC; Dr. V. Raymond for the plasmid no111paG; Drs. R. Middendorff and D. Müller for critical suggestions and help; Mrs. M. Kohler for her technical assistance; and the National Disease Research Interchange, Philadelphia, PA, for human tissue procurement.
Footnotes
Address reprint requests to Philip A. Marsden, M.D., Rm 7358, Medical Sciences Building, University of Toronto, 1 King’s College Circle, Toronto, Ontario M5S 1A8. E-mail: p.marsden@utoronto.ca.
Supported by a grant from the Heart and Stroke Foundation of Canada (T-3668), the Deutsche Forschungsgemeinschaft (Da 459/1-1), a Centennial Fellowship Award from the Canadian Institutes of Health Research (to Y. W.), a Career Investigator Award from the Heart and Stroke Foundation of Canada (to P. A. M.), and a Canadian Institutes of Health Research doctoral research award (to D. C. N.).
References
- 1.Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA: Structural organization of the human neuronal nitric oxide synthase gene (NOS1). J Biol Chem 1994, 269:33082-33090 [PubMed] [Google Scholar]
- 2.Teichert AM, Miller TL, Tai SC, Wang Y, Bei X, Robb GB, Phillips MJ, Marsden PA: In vivo expression profile of an endothelial nitric oxide synthase promoter-reporter transgene. Am J Physiol 2000, 278:H1352-H1361 [DOI] [PubMed] [Google Scholar]
- 3.Christopherson KS, Bredt DS: Nitric oxide in excitable tissues: physiological roles and disease. J Clin Invest 1997, 100:2424-2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH: Nitric oxide: a physiologic mediator of penile erection. Science 1992, 257:401-403 [DOI] [PubMed] [Google Scholar]
- 5.Davidoff MS, Middendorff R, Mayer B, Holstein AF: Nitric oxide synthase (NOS-I) in Leydig cells of the human testis. Arch Histol Cytol 1995, 58:17-30 [DOI] [PubMed] [Google Scholar]
- 6.Del Punta K, Charreau EH, Pignataro OP: Nitric oxide inhibits Leydig cell steroidogenesis. Endocrinology 1996, 137:5337-5343 [DOI] [PubMed] [Google Scholar]
- 7.Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH: Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 1995, 378:383-386 [DOI] [PubMed] [Google Scholar]
- 8.Demas GE, Eliasson MJ, Dawson TM, Dawson VL, Kriegsfeld LJ, Nelson RJ, Snyder SH: Inhibition of neuronal nitric oxide synthase increases aggressive behavior in mice. Mol Med 1997, 3:610-616 [PMC free article] [PubMed] [Google Scholar]
- 9.Kriegsfeld LJ, Dawson TM, Dawson VL, Nelson RJ, Snyder SH: Aggressive behavior in male mice lacking the gene for neuronal nitric oxide synthase requires testosterone. Brain Res 1997, 769:66-70 [DOI] [PubMed] [Google Scholar]
- 10.Kuo RC, Baxter GT, Thompson SH, Stricker SA, Patton C, Bonaventura J, Epel D: NO is necessary and sufficient for egg activation at fertilization. Nature 2000, 406:633-636 [DOI] [PubMed] [Google Scholar]
- 11.Bivalacqua TJ, Champion HC, Hellstrom WJ, Kadowitz PJ: Pharmacotherapy for erectile dysfunction. Trends Pharmacol Sci 2000, 21:484-489 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Newton DC, Marsden PA: Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol 1999, 13:21-24 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Newton DC, Robb GB, Kau CL, Miller TL, Cheung AH, Hall AV, VanDamme S, Wilcox JN, Marsden PA: RNA diversity has profound effects on the translation of neuronal nitric oxide synthase. Proc Natl Acad Sci USA 1999, 96:12150-12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM: Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci USA 2000, 97:8617-8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Goligorsky MS, Lin M, Wilcox JN, Marsden PA: A novel, testis-specific mRNA transcript encoding an NH2-terminal truncated nitric-oxide synthase. J Biol Chem 1997, 272:11392-11401 [PubMed] [Google Scholar]
- 16.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS: Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α 1-syntrophin mediated by PDZ domains. Cell 1996, 84:757-767 [DOI] [PubMed] [Google Scholar]
- 17.Jaffrey SR, Snyder SH: PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 1996, 274:774-777 [DOI] [PubMed] [Google Scholar]
- 18.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC: Targeted disruption of the neuronal nitric oxide synthase gene. Cell 1993, 75:1273-1286 [DOI] [PubMed] [Google Scholar]
- 19.Palmiter RD, Sandgren EP, Avarbock MR, Allen DD, Brinster RL: Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci USA 1991, 88:478-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karantzoulis-Fegaras F, Antoniou H, Lai SL, Kulkarni G, D’Abreo C, Wong GK, Miller TL, Chan Y, Atkins J, Wang Y, Marsden PA: Characterization of the human endothelial nitric-oxide synthase promoter. J Biol Chem 1999, 274:3076-3093 [DOI] [PubMed] [Google Scholar]
- 21.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC: Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376:62-66 [DOI] [PubMed] [Google Scholar]
- 22.Mayer B, John M, Bohme E: Purification of a Ca2+/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor-role of tetrahydrobiopterin. FEBS Lett 1990, 277:215-219 [DOI] [PubMed] [Google Scholar]
- 23.Davidoff M, Schulze W: Combination of the peroxidase anti-peroxidase (PAP)- and avidin-biotin-peroxidase complex (ABC)-techniques: an amplification alternative in immunocytochemical staining. Histochemistry 1990, 93:531-536 [DOI] [PubMed] [Google Scholar]
- 24.Zaborszky L, Leranth C: Simultaneous ultrastructural demonstration of retrogradely transported horseradish peroxidase and choline acetyltransferase immunoreactivity. Histochemistry 1985, 82:529-537 [DOI] [PubMed] [Google Scholar]
- 25.Davidoff MS, Middendorff R, Mayer B, deVente J, Koesling D, Holstein AF: Nitric oxide/cGMP pathway components in the Leydig cells of the human testis. Cell Tissue Res 1997, 287:161-170 [DOI] [PubMed] [Google Scholar]
- 26.Middendorff R, Muller D, Wichers S, Holstein AF, Davidoff MS: Evidence for production and functional activity of nitric oxide in seminiferous tubules and blood vessels of the human testis. J Clin Endocrinol Metab 1997, 82:4154-4161 [DOI] [PubMed] [Google Scholar]
- 27.Wassarman PM, DePamphilis ML: Guide to techniques in mouse development. Methods in Enzymology. 1993, Academic Press, San Diego
- 28.Mayerhofer A, Lahr G, Seidl K, Eusterschulte B, Christoph A, Gratzl M: The neural cell adhesion molecule (NCAM) provides clues to the development of testicular Leydig cells. J Androl 1996, 17:223-230 [PubMed] [Google Scholar]
- 29.Gradi A, Tang-Wai R, McBride HM, Chu LL, Shore GC, Pelletier J: The human steroidogenic acute regulatory (StAR) gene is expressed in the urogenital system and encodes a mitochondrial polypeptide. Biochim Biophys Acta 1995, 1258:228-233 [DOI] [PubMed] [Google Scholar]
- 30.Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y: Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3beta-hydroxysteroid dehydrogenase in the rat brain. J Neurochem 1998, 71:2231-2238 [DOI] [PubMed] [Google Scholar]
- 31.Mamluk R, Wolfenson D, Meidan R: LH receptor mRNA and cytochrome P450 side-chain cleavage expression in bovine theca and granulosa cells luteinized by LH or forskolin. Domest Anim Endocrinol 1998, 15:103-114 [DOI] [PubMed] [Google Scholar]
- 32.Lynch JP, Lala DS, Peluso JJ, Luo W, Parker KL, White BA: Steroidogenic factor 1, an orphan nuclear receptor, regulates the expression of the rat aromatase gene in gonadal tissues. Mol Endocrinol 1993, 7:776-786 [DOI] [PubMed] [Google Scholar]
- 33.Barbara PS, Moniot B, Poulat F, Boizet B, Berta P: Steroidogenic factor-1 regulates transcription of the human anti-Mullerian hormone receptor. J Biol Chem 1998, 273:29654-29660 [DOI] [PubMed] [Google Scholar]
- 34.Wooton-Kee CR, Clark BJ: Steroidogenic factor-1 influences protein-deoxyribonucleic acid interactions within the cyclic adenosine 3,5-monophosphate-responsive regions of the murine steroidogenic acute regulatory protein gene. Endocrinology 2000, 141:1345-1355 [DOI] [PubMed] [Google Scholar]
- 35.Manna PR, Kero J, Tena-Sempere M, Pakarinen P, Stocco DM, Huhtaniemi IT: Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology 2001, 142:319-331 [DOI] [PubMed] [Google Scholar]
- 36.Reinhart AJ, Williams SC, Clark BJ, Stocco DM: SF-1 (steroidogenic factor-1) and C/EBP beta (CCAAT/enhancer binding protein-beta) cooperate to regulate the murine StAR (steroidogenic acute regulatory) promoter. Mol Endocrinol 1999, 13:729-741 [DOI] [PubMed] [Google Scholar]
- 37.Young M, McPhaul MJ: A steroidogenic factor-1-binding site and cyclic adenosine 3′,5′-monophosphate response element-like elements are required for the activity of the rat aromatase promoter in rat Leydig tumor cell lines. Endocrinology 1998, 139:5082-5093 [DOI] [PubMed] [Google Scholar]
- 38.Teixeira J, Kehas DJ, Antun R, Donahoe PK: Transcriptional regulation of the rat Mullerian inhibiting substance type II receptor in rodent Leydig cells. Proc Natl Acad Sci USA 1999, 96:13831-13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann S, Schwarzler A, Buth S, Engel W, Adham IM: Transcription of the Leydig insulin-like gene is mediated by steroidogenic factor-1. Mol Endocrinol 1998, 12:706-713 [DOI] [PubMed] [Google Scholar]
- 40.Chau YM, Crawford PA, Woodson KG, Polish JA, Olson LM, Sadovsky Y: Role of steroidogenic-factor 1 in basal and 3′,5′-cyclic adenosine monophosphate-mediated regulation of cytochrome P450 side-chain cleavage enzyme in the mouse. Biol Reprod 1997, 57:765-771 [DOI] [PubMed] [Google Scholar]
- 41.Leers-Sucheta S, Morohashi K, Mason JI, Melner MH: Synergistic activation of the human type II 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase promoter by the transcription factor steroidogenic factor-1/adrenal 4-binding protein and phorbol ester. J Biol Chem 1997, 272:7960-7967 [DOI] [PubMed] [Google Scholar]
- 42.Feng ZM, Wu AZ, Zhang Z, Chen CL: GATA-1 and GATA-4 transactivate inhibin/activin beta-B-subunit gene transcription in testicular cells. Mol Endocrinol 2000, 14:1820-1835 [DOI] [PubMed] [Google Scholar]
- 43.Nalbant D, Williams SC, Stocco DM, Khan SA: Luteinizing hormone-dependent gene regulation in Leydig cells may be mediated by CCAAT/enhancer-binding protein-beta. Endocrinology 1998, 139:272-279 [DOI] [PubMed] [Google Scholar]
- 44.Mouw AR, Rice DA, Meade JC, Chua SC, White PC, Schimmer BP, Parker KL: Structural and functional analysis of the promoter region of the gene encoding mouse steroid 11 beta-hydroxylase. J Biol Chem 1989, 264:1305-1309 [PubMed] [Google Scholar]
- 45.Hum DW, Staels B, Black SM, Miller WL: Basal transcriptional activity and cyclic adenosine 3′,5′-monophosphate responsiveness of the human cytochrome P450scc promoter transfected into MA-10 Leydig cells. Endocrinology 1993, 132:546-552 [DOI] [PubMed] [Google Scholar]
- 46.Cremer T, Cremer C: Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2001, 2:292-301 [DOI] [PubMed] [Google Scholar]
- 47.Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E: Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci USA 1989, 86:1563-1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmerman K, Legouy E, Stewart V, Depinho R, Alt FW: Differential regulation of the N-myc gene in transfected cells and transgenic mice. Mol Cell Biol 1990, 10:2096-2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yet SF, Folta SC, Jain MK, Hsieh CM, Maemura K, Layne MD, Zhang D, Marria PB, Yoshizumi M, Chin MT, Perrella MA, Lee ME: Molecular cloning, characterization, and promoter analysis of the mouse Crp2/SmLim gene. Preferential expression of its promoter in the vascular smooth muscle cells of transgenic mice. J Biol Chem 1998, 273:10530-10537 [DOI] [PubMed] [Google Scholar]
- 50.Makar KW, Pham CT, Dehoff MH, O’Connor SM, Jacobi SM, Holers VM: An intronic silencer regulates B lymphocyte cell- and stage-specific expression of the human complement receptor type 2 (CR2, CD21) gene. J Immunol 1998, 160:1268-1278 [PubMed] [Google Scholar]
- 51.Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD: Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA 1988, 85:836-840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krempen K, Grotkopp D, Hall K, Bache A, Gillan A, Rippe RA, Brenner DA, Breindl M: Far upstream regulatory elements enhance position-independent and uterus-specific expression of the murine alpha1(I) collagen promoter in transgenic mice. Gene Expr 1999, 8:151-163 [PMC free article] [PubMed] [Google Scholar]
- 53.Stein GS, van Wijnen AJ, Stein JL, Lian JB, Pockwinse SH, McNeil S: Implications for interrelationships between nuclear architecture and control of gene expression under microgravity conditions. FASEB J 1999, 13:S157-S166 [DOI] [PubMed] [Google Scholar]
- 54.Ciejek EM, Tsai MJ, O’Malley BW: Actively transcribed genes are associated with the nuclear matrix. Nature 1983, 306:607-609 [DOI] [PubMed] [Google Scholar]
- 55.Getzenberg RH: Nuclear matrix and the regulation of gene expression: tissue specificity. J Cell Biochem 1994, 55:22-31 [DOI] [PubMed] [Google Scholar]
- 56.Brown K: Nuclear structure, gene expression and development. Crit Rev Eukaryot Gene Expr 1999, 9:203-212 [DOI] [PubMed] [Google Scholar]
- 57.Stein GS, van Wijnen AJ, Stein JL, Lian JB, McNeil S, Pockwinse SM: Transcriptional control within the three-dimensional context of nuclear architecture: requirements for boundaries and direction. J Cell Biochem 1999, (Suppl) 75:S24–S31 [DOI] [PubMed]
- 58.Valenti S, Cuttica CM, Giusti M, Giordano G: Nitric oxide modulates Leydig cell function in vitro: is this a way of communication between the immune and endocrine system in the testis? Ann N Y Acad Sci 1999, 876:298-300 [DOI] [PubMed] [Google Scholar]
- 59.Valenti S, Cuttica CM, Fazzuoli L, Giordano G, Giusti M: Biphasic effect of nitric oxide on testosterone and cyclic GMP production by purified rat Leydig cells cultured in vitro. Int J Androl 1999, 22:336-341 [DOI] [PubMed] [Google Scholar]
- 60.Welch C, Watson ME, Poth M, Hong T, Francis GL: Evidence to suggest nitric oxide is an interstitial regulator of Leydig cell steroidogenesis. Metabolism 1995, 44:234-238 [DOI] [PubMed] [Google Scholar]
- 61.Kostic T, Andric S, Kovacevic R, Maric D: The involvement of nitric oxide in stress-impaired testicular steroidogenesis. Eur J Pharmacol 1998, 346:267-273 [DOI] [PubMed] [Google Scholar]
- 62.Pomerantz DK, Pitelka V: Nitric oxide is a mediator of the inhibitory effect of activated macrophages on production of androgen by the Leydig cell of the mouse. Endocrinology 1998, 139:922-931 [DOI] [PubMed] [Google Scholar]
- 63.Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH: Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci USA 1997, 94:3396-3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demas GE, Eliasson MJ, Dawson TM, Dawson VL, Kriegsfeld LJ, Nelson RJ, Snyder SH: Inhibition of neuronal nitric oxide synthase increases aggressive behavior in mice. Mol Med 1997, 3:610-616 [PMC free article] [PubMed] [Google Scholar]
- 65.Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ: Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci USA 2001, 98:1277-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R: Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 1994, 265:1875-1878 [DOI] [PubMed] [Google Scholar]
- 67.Benelli A, Bertolini A, Poggioli R, Cavazzuti E, Calza L, Giardino L, Arletti R: Nitric oxide is involved in male sexual behavior of rats. Eur J Pharmacol 1995, 294:505-510 [DOI] [PubMed] [Google Scholar]
- 68.Welch C, Watson ME, Poth M, Hong T, Francis GL: Evidence to suggest nitric oxide is an interstitial regulator of Leydig cell steroidogenesis. Metabolism 1995, 44:234-238 [DOI] [PubMed] [Google Scholar]
- 69.Adams LA, Vician L, Clifton DK, Steiner RA: Testosterone regulates pro-opiomelanocortin gene expression in the primate brain. Endocrinology 1991, 128:1881-1886 [DOI] [PubMed] [Google Scholar]